Physiological and Microbial Community Dynamics in Does During Mid-Gestation to Lactation and Their Impact on the Growth, Immune Function, and Microbiome Transmission of Offspring Kids
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Does Feeding Experiment and Sampling
2.2. Serum Parameters
2.3. DNA Extraction, 16S rRNA Sequencing, and Data Analysis
2.4. Volatile Fatty Acids Determination
2.5. Statistical Analysis
3. Results
3.1. Changes in the Serum Physiological Indices of Does During Mid-Gestation and Lactation
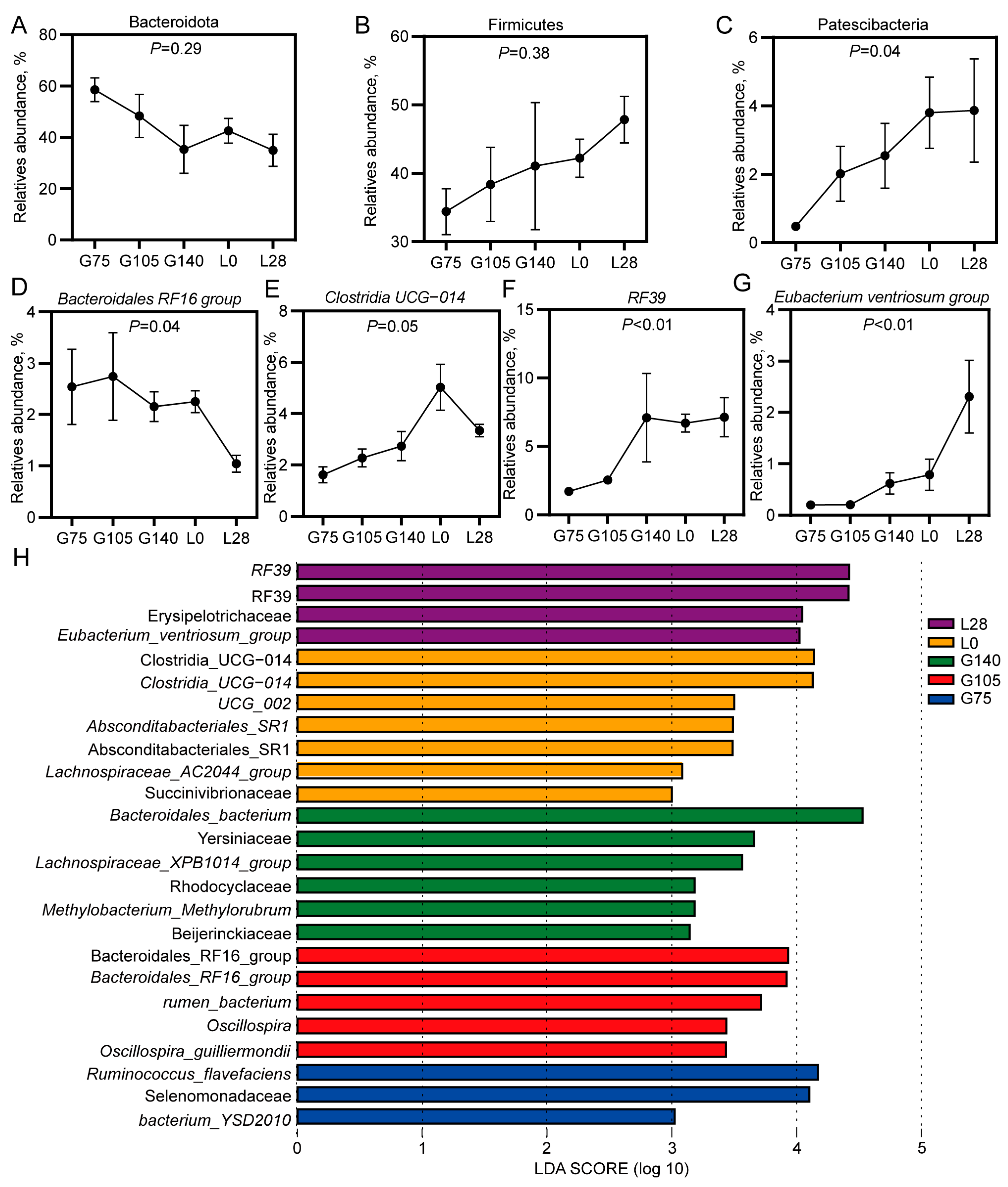
3.2. Microbiological Analysis of the Rumen Microbiota in Does During Mid-Gestation and Lactation
3.3. Changes in the Dominant Bacterial Genera in the Rumen of Does During Mid-Gestation and Lactation
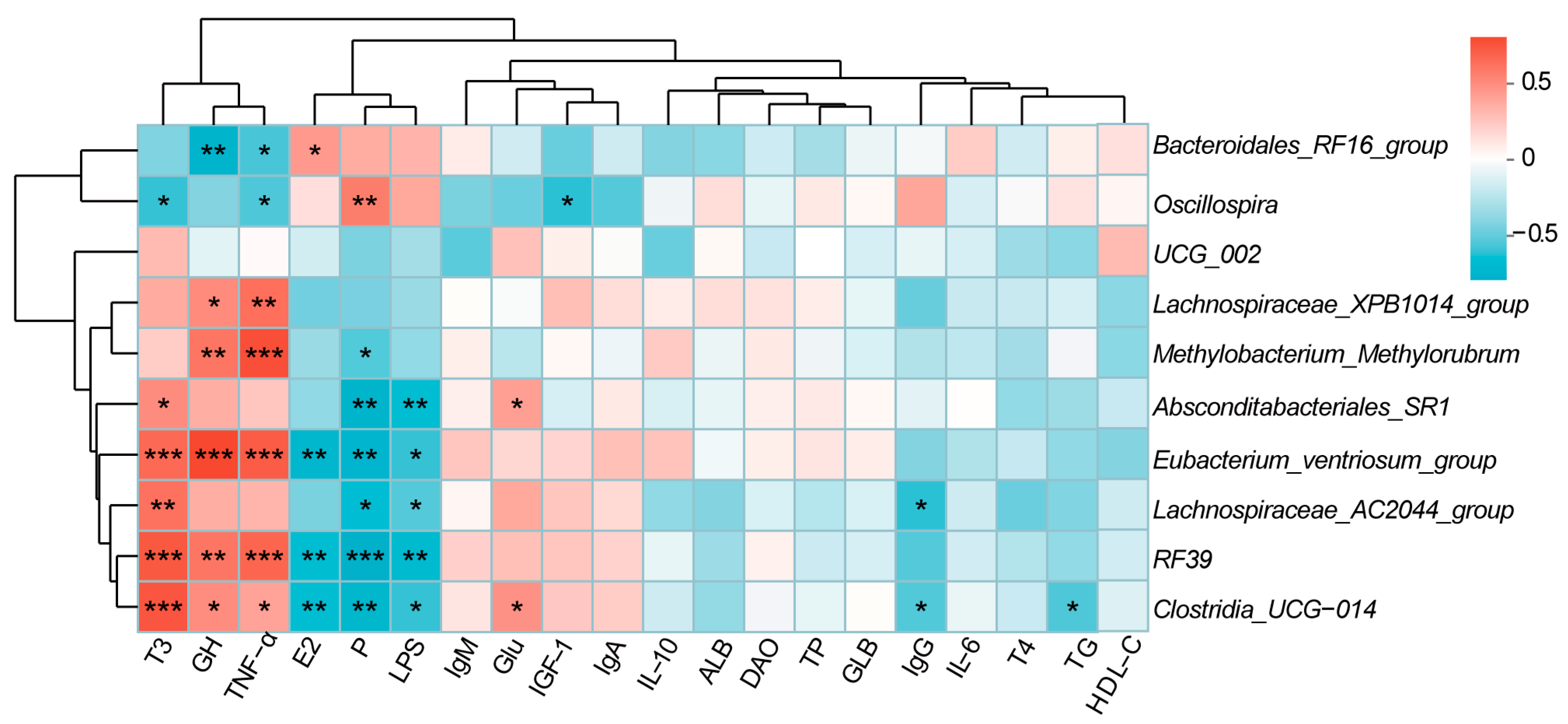
3.4. Correlation Analysis Between Phenotypic Indicators and Microorganisms in Does
3.5. Changes in the Level of Volatile Fatty Acids in the Rumen of Doe During Mid-Gestation and Lactation
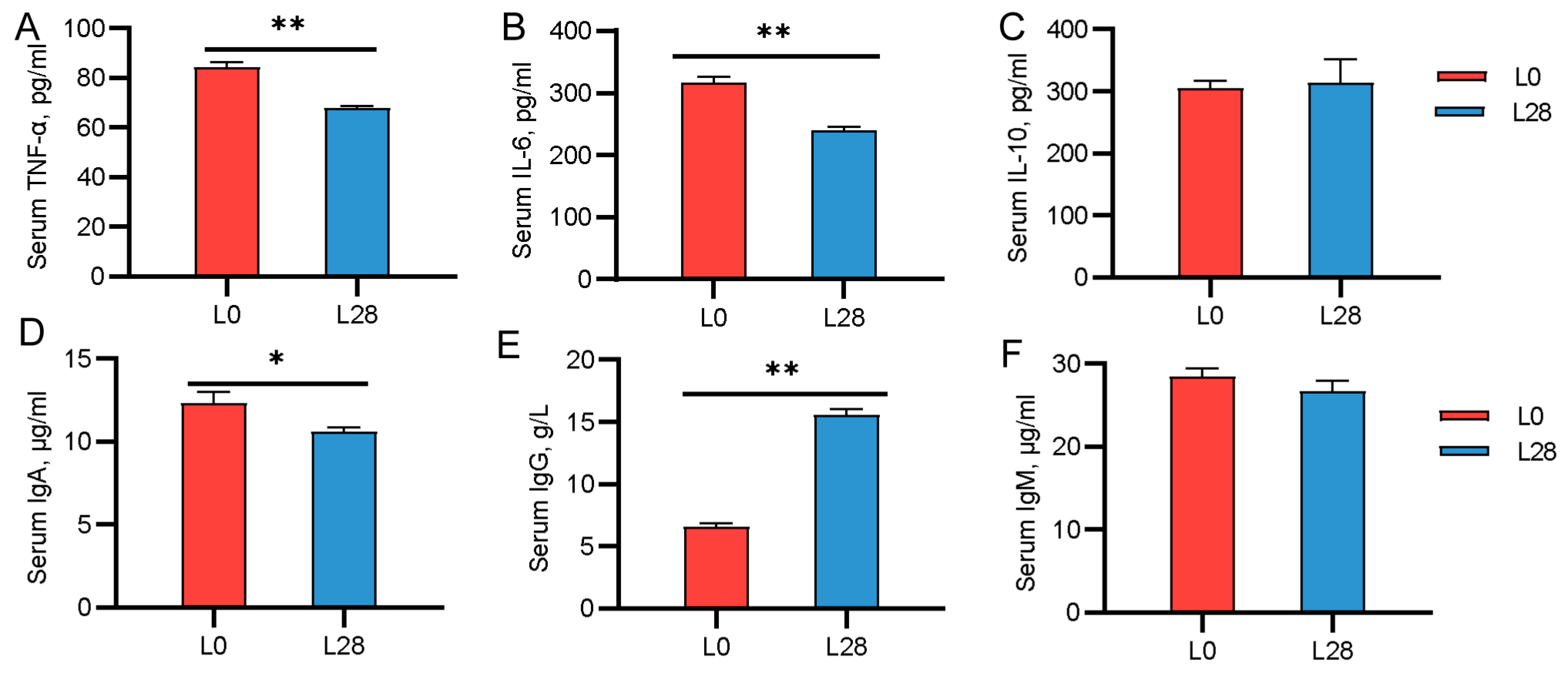
3.6. Changes in the Serum Immune Indicators of Goat Kids
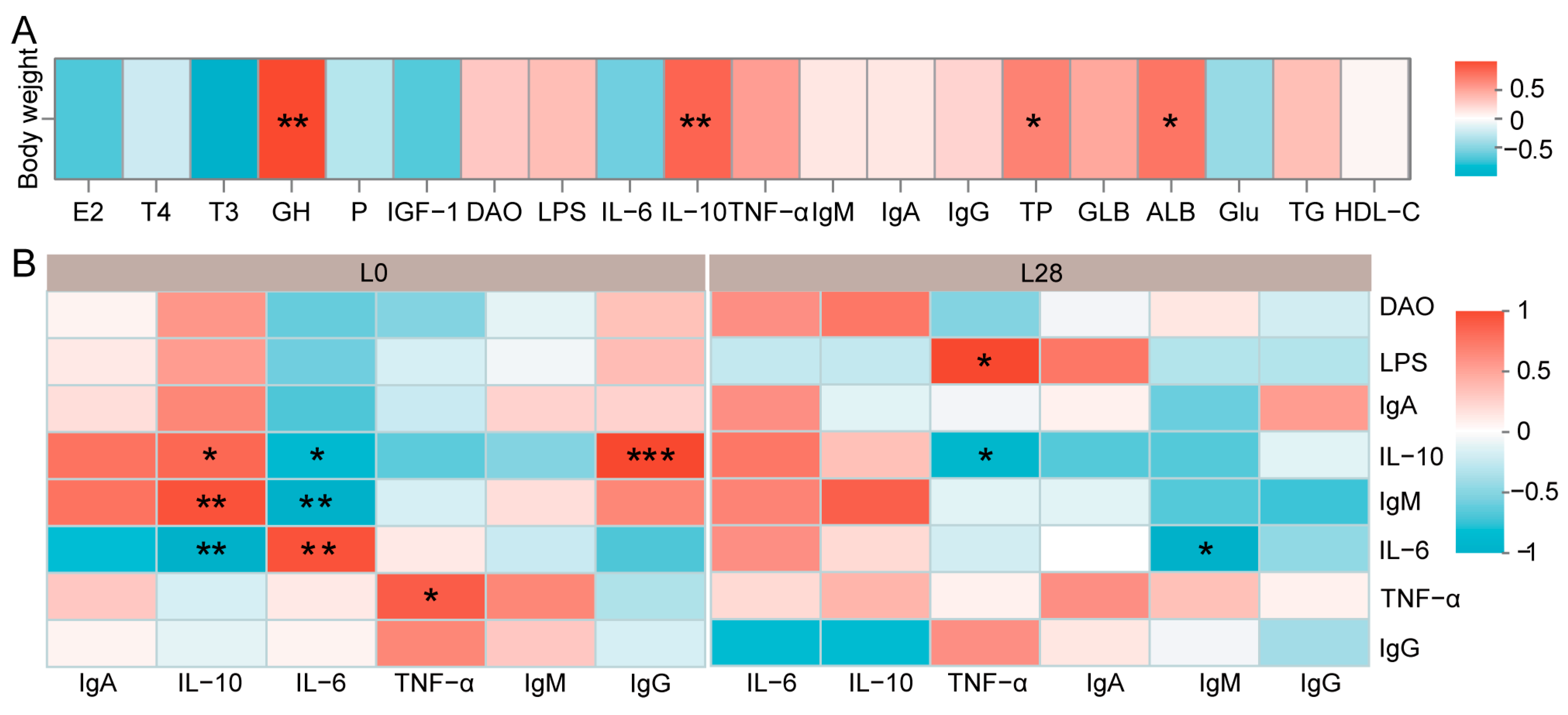
3.7. The Maternal Physiological Condition Affects the Growth and Health of the Offspring
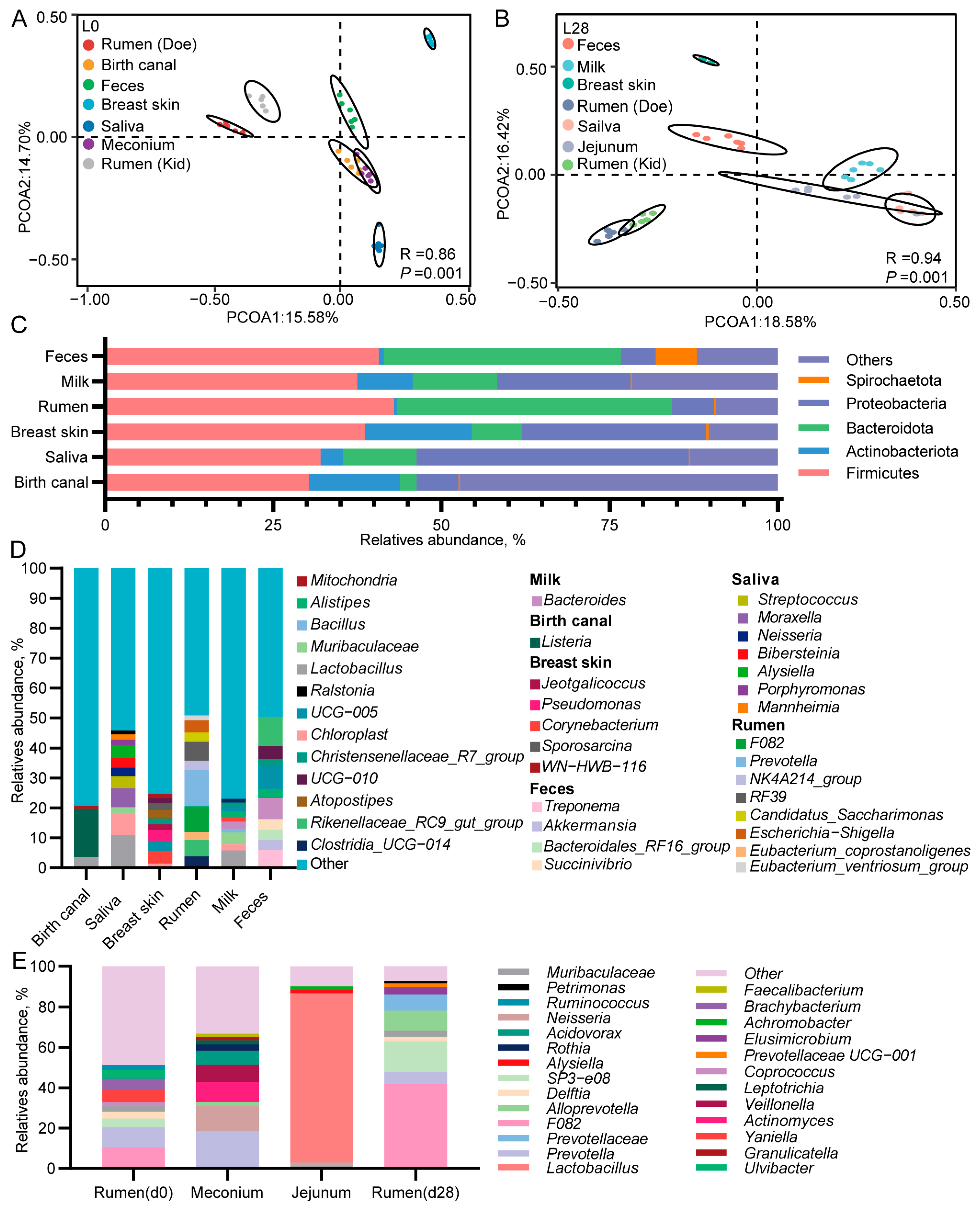
3.8. The Microbial Composition of Multiple Body Sites of Does and Goat Kids
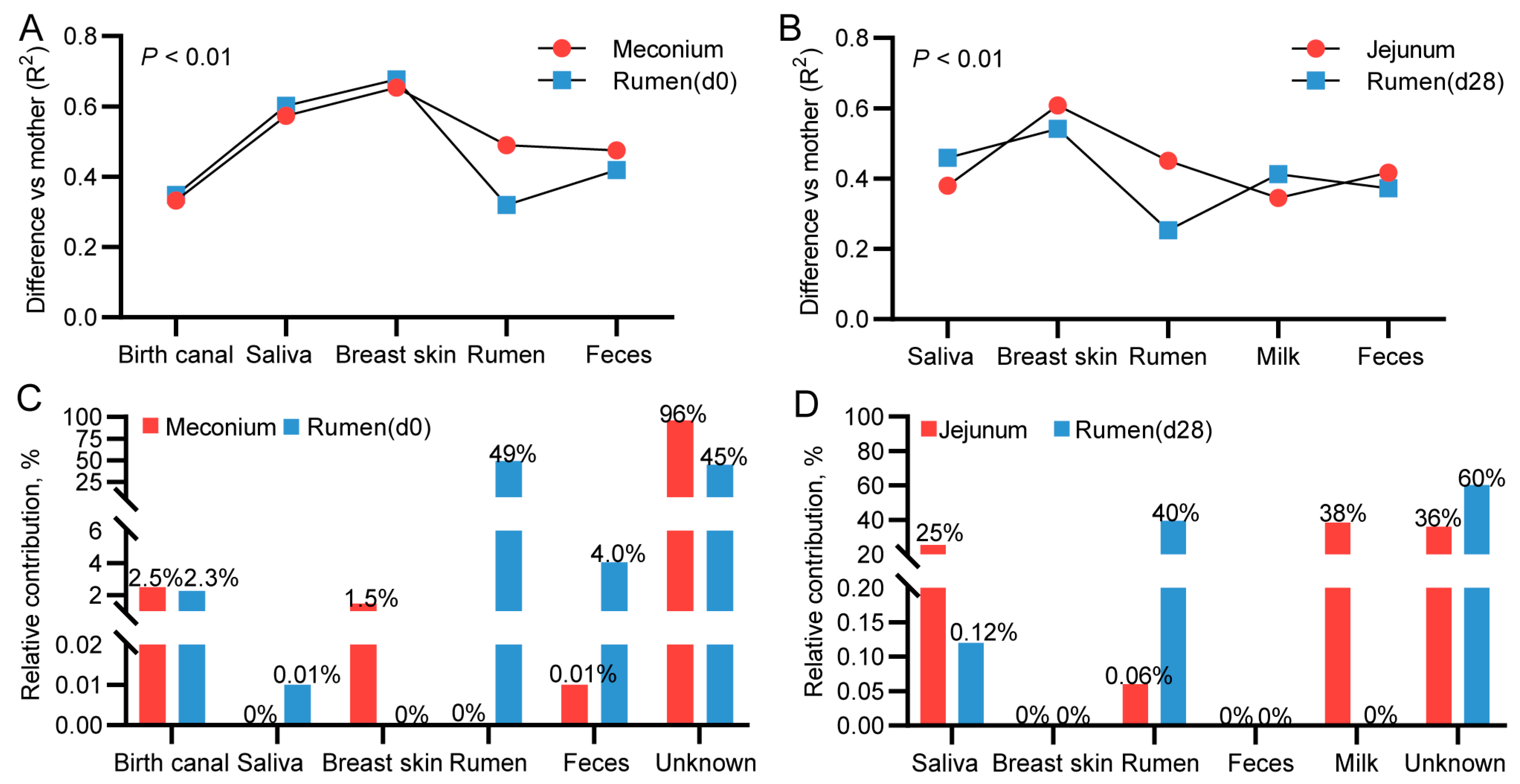
3.9. Mother-to-Infant Microbial Transmission from Different Body Sites
3.10. Correlation Analysis Between Immunological Indicators and Microorganisms in Goat Kids
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, B.; Zhu, X.; Cui, Y.; Wang, W.; Liu, H.; Li, Z.; Guo, Z.; Ma, S.; Li, D.; Wang, C. Consumption of dietary fiber from different sources during pregnancy alters sow gut microbiota and improves performance and reduces inflammation in sows and piglets. Msystems 2021, 6, e00591-20. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Wu, S.; Liu, S.; Sun, L.; Feng, Y.; Cao, Y.; Chai, S.; Zhang, G.; Yao, J. From maternal grazing to barn feeding during pre-weaning period: Altered gastrointestinal microbiota contributes to change the development and function of the rumen and intestine of yak calves. Front. Microbiol. 2020, 11, 485–499. [Google Scholar] [CrossRef] [PubMed]
- Neville, T.L.; Reynolds, L.P.; Vonnahme, K.A.; Redmer, D.A.; Perry, G.A.; Caton, J.S. Maternal dietary intake alters organ mass and endocrine and metabolic profiles in pregnant ewe lambs. Anim. Reprod. Sci. 2013, 141, 131–141. [Google Scholar] [CrossRef]
- Hove, K.; Blom, A.K. Plasma insulin and growth hormone concentrations in pregnant sheep II: Post-absorptive levels in mid—and late pregnancy. Acta Endocrinol. 1976, 82, 553–560. [Google Scholar] [CrossRef]
- Harding, J.E.; Evans, P.C.; Gluckman, P.D. Maternal growth hormone treatment increases placental diffusion capacity but not fetal or placental growth in sheep. Endocrinology 1997, 138, 5352–5358. [Google Scholar] [CrossRef] [PubMed]
- Eerdekens, A.; Verhaeghe, J.; Darras, V.; Naulaers, G.; Van den Berghe, G.; Langouche, L.; Vanhole, C. The placenta in fetal thyroid hormone delivery: From normal physiology to adaptive mechanisms in complicated pregnancies. J. Matern. Fetal Neonatal Med. 2020, 33, 3857–3866. [Google Scholar] [CrossRef]
- Santarosa, B.P.; Ferreira, D.O.L.; Hooper, H.B.; Sinzato, Y.K.; Damasceno, D.C.; Polizel, D.M.; Fioratti, E.G.; Santos, V.H.D.; da Silva, A.A.; Gonçalves, R.C. Endocrine-metabolic adaptations in Dorper ewes: Comparison between single and twin pregnancies during gestation, parturition, and postpartum. Trop. Anim. Health Prod. 2022, 54, 307. [Google Scholar] [CrossRef]
- Vejrazkova, D.; Vcelak, J.; Vankova, M.; Lukasova, P.; Bradnova, O.; Halkova, T.; Kancheva, R.; Bendlova, B. Steroids and insulin resistance in pregnancy (Review). J. Steroid Biochem. Mol. Biol. 2014, 139, 122–129. [Google Scholar] [CrossRef]
- Barrett, H.L.; Dekker Nitert, M.; D’Emden, M.; Lingwood, B.; de Jersey, S.; McIntyre, H.D.; Callaway, L.K. Capillary triglycerides in late pregnancy—Challenging to measure, hard to Interpret: A cohort study of practicality. Nutrients 2021, 13, 1266. [Google Scholar] [CrossRef]
- Griffith, R.J.; Alsweiler, J.; Moore, A.E.; Brown, S.; Middleton, P.; Shepherd, E.; Crowther, C.A. Interventions to prevent women from developing gestational diabetes mellitus: An overview of Cochrane Reviews. Cochrane Database Syst. Rev. 2020, 6, CD012394. [Google Scholar] [CrossRef]
- Andjelić, B.; Djoković, R.; Cincović, M.; Bogosavljević-Bošković, S.; Petrović, M.; Mladenović, J.; Čukić, A. Relationships between Milk and Blood Biochemical Parameters and Metabolic Status in Dairy Cows during Lactation. Metabolites 2022, 12, 733. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishna, K.P.; Hand, T.W. Influence of maternal milk on the neonatal intestinal microbiome. Nutrients 2020, 12, 823. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Bauer, L.L.; Chen, X.; Wang, M.; Kuhlenschmidt, T.B.; Kuhlenschmidt, M.S.; Fahey, G.C.; Donovan, S.M. Microbial composition and in vitro fermentation patterns of human milk oligosaccharides and prebiotics differ between formula-fed and sow-reared piglets. J. Nutr. 2012, 142, 681–689. [Google Scholar] [CrossRef]
- Zou, X.; Liu, G.; Meng, F.; Hong, L.; Li, Y.; Lian, Z.; Yang, Z.; Luo, C.; Liu, D. Exploring the rumen and cecum microbial community from fetus to adulthood in goat. Animals 2020, 10, 1639. [Google Scholar] [CrossRef] [PubMed]
- Sha, Y.; Liu, X.; Li, X.; Wang, Z.; Shao, P.; Jiao, T.; He, Y.; Zhao, S. Succession of rumen microbiota and metabolites across different reproductive periods in different sheep breeds and their impact on the growth and development of offspring lambs. Microbiome 2024, 12, 172–193. [Google Scholar] [CrossRef]
- Antwis, R.E.; Edwards, K.L.; Unwin, B.; Walker, S.L.; Shultz, S. Rare gut microbiota associated with breeding success, hormone metabolites and ovarian cycle phase in the critically endangered eastern black rhino. Microbiome 2019, 7, 27. [Google Scholar] [CrossRef]
- Koren, O.; Goodrich, J.K.; Cullender, T.C.; Spor, A.; Laitinen, K.; Bäckhed, H.K.; Gonzalez, A.; Werner, J.J.; Angenent, L.T.; Knight, R. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 2012, 150, 470–480. [Google Scholar] [CrossRef]
- Nuriel-Ohayon, M.; Neuman, H.; Ziv, O.; Belogolovski, A.; Barsheshet, Y.; Bloch, N.; Uzan, A.; Lahav, R.; Peretz, A.; Frishman, S.; et al. Progesterone Increases Bifidobacterium Relative Abundance during Late Pregnancy. Cell Rep. 2019, 27, 730–736.e3. [Google Scholar] [CrossRef]
- Kimura, I.; Miyamoto, J.; Ohue-Kitano, R.; Watanabe, K.; Yamada, T.; Onuki, M.; Aoki, R.; Isobe, Y.; Kashihara, D.; Inoue, D. Maternal gut microbiota in pregnancy influences offspring metabolic phenotype in mice. Science 2020, 367, eaaw8429. [Google Scholar] [CrossRef]
- Mor, G.; Aldo, P.; Alvero, A.B. The unique immunological and microbial aspects of pregnancy. Nat. Rev. Immunol. 2017, 17, 469–482. [Google Scholar] [CrossRef]
- Adams Waldorf, K.; Rubens, C.E.; Gravett, M.G. Use of nonhuman primate models to investigate mechanisms of infection-associated preterm birth. BJOG: BJOG 2011, 118, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Racicot, K.; Kwon, J.Y.; Aldo, P.; Abrahams, V.; El-Guindy, A.; Romero, R.; Mor, G. Type I interferon regulates the placental inflammatory response to bacteria and is targeted by virus: Mechanism of polymicrobial infection-induced preterm birth. Am. J. Reprod. Immunol. 2016, 75, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; He, Y.; Kang, Z.; Chen, S.; Sun, W.; Wang, J.; Lai, S. Comparison of fecal microbiota communities between primiparous and multiparous cows during non-pregnancy and pregnancy. Animals 2023, 13, 869. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Liu, G.; Wu, Y.; Zhang, T.; Guo, M.; Lei, Y.; Cao, X.; Suo, L.; Brugger, D.; Wang, X. Gut microbial succession patterns and metabolic profiling during pregnancy and lactation in a goat model. Microbiol. Spectr. 2023, 11, e02955-22. [Google Scholar] [CrossRef]
- Klein-Jöstl, D.; Quijada, N.M.; Dzieciol, M.; Feldbacher, B.; Wagner, M.; Drillich, M.; Schmitz-Esser, S.; Mann, E. Microbiota of newborn calves and their mothers reveals possible transfer routes for newborn calves’ gastrointestinal microbiota. PLoS ONE 2019, 14, e0220554. [Google Scholar] [CrossRef]
- Sha, Y.; Liu, X.; Pu, X.; He, Y.; Wang, J.; Zhao, S.; Shao, P.; Wang, F.; Xie, Z.; Chen, X. Characterizing the dynamics of the rumen microbiota, its metabolites, and blood metabolites across reproductive stages in Small-tailed Han sheep. Microbiol. Spectr. 2023, 11, e02867-23. [Google Scholar] [CrossRef]
- Li, X.; Bi, R.; Xiao, K.; Roy, A.; Zhang, Z.; Chen, X.; Peng, J.; Wang, R.; Yang, R.; Shen, X. Hen raising helps chicks establish gut microbiota in their early life and improve microbiota stability after H9N2 challenge. Microbiome 2022, 10, 14–26. [Google Scholar] [CrossRef]
- Sindi, A.S.; Geddes, D.T.; Wlodek, M.E.; Muhlhausler, B.S.; Payne, M.S.; Stinson, L.F. Can we modulate the breastfed infant gut microbiota through maternal diet? FEMS Microbiol. Rev. 2021, 45, fuab011. [Google Scholar] [CrossRef]
- Gong, H.; Wang, T.; Wu, M.; Chu, Q.; Lan, H.; Lang, W.; Zhu, L.; Song, Y.; Zhou, Y.; Wen, Q. Maternal effects drive intestinal development beginning in the embryonic period on the basis of maternal immune and microbial transfer in chickens. Microbiome 2023, 11, 41–60. [Google Scholar] [CrossRef]
- Linehan, K.; Dempsey, E.M.; Ryan, C.A.; Ross, R.P.; Stanton, C. First encounters of the microbial kind: Perinatal factors direct infant gut microbiome establishment. Microbiome Res. Rep. 2022, 1, 10. [Google Scholar] [CrossRef]
- Ignacio, A.; Czyz, S.; Mccoy, K.D. Early life microbiome influences on development of the mucosal innate immune system. Semin. Immunol. 2024, 73, 101885–101897. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Lai, G.C.; Yao, L.J.; Aung, T.T.; Shental, N.; Rotter-Maskowitz, A.; Shepherdson, E.; Singh, G.S.N.; Pai, R.; Shanti, A.; et al. Microbial exposure during early human development primes fetal immune cells. Cell 2021, 184, 3394–3409.e3320. [Google Scholar] [CrossRef] [PubMed]
- Agüero, M.G.d.; Ganal-Vonarburg, S.C.; Fuhrer, T.; Rupp, S.; Uchimura, Y.; Li, H.; Steinert, A.; Heikenwalder, M.; Hapfelmeier, S.; Sauer, U.; et al. The maternal microbiota drives early postnatal innate immune development. Science 2016, 351, 1296–1302. [Google Scholar] [CrossRef] [PubMed]
- NY/T 4048-2021; Nutrient Requirements of Cashmere Goats. Ministry of Agriculture of the People’s Republic of China: Beijing, China, 2021. Available online: https://hbba.sacinfo.org.cn/ (accessed on 11 February 2025).
- Priyadarshini, M.; Navarro, G.; Reiman, D.J.; Sharma, A.; Xu, K.; Lednovich, K.; Manzella, C.R.; Khan, M.W.; Garcia, M.S.; Allard, S. Gestational insulin resistance is mediated by the gut microbiome–indoleamine 2, 3-dioxygenase axis. Gastroenterology 2022, 162, 1675–1689.e11. [Google Scholar] [CrossRef]
- Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Bresnitz, W.; Lorca, R.A. Potassium channels in the uterine vasculature: Role in healthy and complicated pregnancies. Int. J. Mol. Sci. 2022, 23, 9446. [Google Scholar] [CrossRef]
- Tal, R.; Taylor, H.S. Endocrinology of Pregnancy; Endotext MDText.com, Inc.: South Dartmouth, MA, USA, 2021. [Google Scholar]
- Patel, B.; Elguero, S.; Thakore, S.; Dahoud, W.; Bedaiwy, M.; Mesiano, S. Role of nuclear progesterone receptor isoforms in uterine pathophysiology. Hum. Reprod. Update 2015, 21, 155–173. [Google Scholar] [CrossRef]
- Albrecht, E.D.; Robb, V.A.; Pepe, G.J. Regulation of placental vascular endothelial growth/permeability factor expression and angiogenesis by estrogen during early baboon pregnancy. J. Clin. Endocrinol. Metab. 2004, 89, 5803–5809. [Google Scholar] [CrossRef]
- Fuhler, G. The immune system and microbiome in pregnancy. Best. Pract. Res. Clin. Gastroenterol. 2020, 44, 101671–101701. [Google Scholar] [CrossRef]
- Gluckman, P.D.; Pinal, C.S. Maternal-placental-fetal interactions in the endocrine regulation of fetal growth: Role of somatotrophic axes. Endocrine 2002, 19, 81–89. [Google Scholar] [CrossRef]
- Johns, L.E.; Ferguson, K.K.; Cantonwine, D.E.; Mukherjee, B.; Meeker, J.D.; McElrath, T.F. Subclinical changes in maternal thyroid function parameters in pregnancy and fetal growth. J. Clin. Endocrinol. Metab. 2018, 103, 1349–1358. [Google Scholar] [CrossRef] [PubMed]
- Graves, B.W.; Haley, M.M. Newborn transition. J. Midwifery Womens Health 2013, 58, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.N.; Phelan, J.P. Pregnancy-Induced Physiologic Alterations. Crit. Care Obstet. 2024, 4, 49–75. [Google Scholar] [CrossRef]
- Wang, Q.; Würtz, P.; Auro, K.; Mäkinen, V.P.; Kangas, A.J.; Soininen, P.; Tiainen, M.; Tynkkynen, T.; Jokelainen, J.; Santalahti, K. Metabolic profiling of pregnancy: Cross-sectional and longitudinal evidence. BMC Med. 2016, 14, 205. [Google Scholar] [CrossRef]
- Eppel, D.; Feichtinger, M.; Lindner, T.; Kotzaeridi, G.; Rosicky, I.; Yerlikaya-Schatten, G.; Eppel, W.; Husslein, P.; Tura, A.; Göbl, C.S. Association between maternal triglycerides and disturbed glucose metabolism in pregnancy. Acta Diabetol. 2021, 58, 459–465. [Google Scholar] [CrossRef]
- Herrera, E.; Ortega-Senovilla, H. Disturbances in lipid metabolism in diabetic pregnancy–are these the cause of the problem? Best. Pract. Res. Clin. Endocrinol. Metab. 2010, 24, 515–525. [Google Scholar] [CrossRef]
- Luan, L.; Patil, N.K.; Guo, Y.; Hernandez, A.; Bohannon, J.K.; Fensterheim, B.A.; Wang, J.; Xu, Y.; Enkhbaatar, P.; Stark, R.; et al. Comparative Transcriptome Profiles of Human Blood in Response to the Toll-like Receptor 4 Ligands Lipopolysaccharide and Monophosphoryl. Lipid A Sci. Rep. 2017, 7, 40050–40066. [Google Scholar] [CrossRef]
- Awadalla, A.; Mahdi, M.R.; Zahran, M.H.; Abdelbaset-Ismail, A.; El-Dosoky, M.; Negm, A. Baicalein and alpha-tocopherol inhibit toll-like receptor pathways in cisplatin-induced nephrotoxicity. Molecules 2022, 27, 2179. [Google Scholar] [CrossRef]
- Wang, X.; Nelin, L.D.; Kuhlman, J.R.; Meng, X.; Welty, S.E.; Liu, Y. The role of MAP kinase phosphatase-1 in the protective mechanism of dexamethasone against endotoxemia. Life Sci. 2008, 83, 671–680. [Google Scholar] [CrossRef]
- Nimmerjahn, F.; Ravetch, J.V. Antibody-mediated modulation of immune responses. Immunol. Rev. 2010, 236, 265–275. [Google Scholar] [CrossRef]
- Liu, H.; Hou, C.; Li, N.; Zhang, X.; Zhang, G.; Yang, F.; Zeng, X.; Liu, Z.; Qiao, S. Microbial and metabolic alterations in gut microbiota of sows during pregnancy and lactation. FASEB J. 2019, 33, 4490–4501. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Chen, H.; Zhang, M.; Wu, T.; Liu, R. Altered short chain fatty acid profiles induced by dietary fiber intervention regulate AMPK levels and intestinal homeostasis. Food Funct. 2019, 10, 7174–7187. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, B.; Lan, F.; Zhong, C.; Jin, J.; Li, X.; Zhou, Q.; Li, J.; Yang, N.; Wen, C. Host genetics and gut microbiota jointly regulate blood biochemical indicators in chickens. Appl. Microbiol. Biotechnol. 2023, 107, 7601–7620. [Google Scholar] [CrossRef]
- Wang, Y.; Nan, X.; Zhao, Y.; Jiang, L.; Wang, H.; Zhang, F.; Hua, D.; Liu, J.; Yao, J.; Yang, L. Dietary supplementation of inulin ameliorates subclinical mastitis via regulation of rumen microbial community and metabolites in dairy cows. Microbiol. Spectr. 2021, 9, e00105–e00121. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, Y.; Wen, Z.; Liu, W.; Meng, L.; Huang, H. Oscillospira-a candidate for the next-generation probiotics. Gut Microbes 2021, 13, 1987783–1987801. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.J.; Ji, S.K.; Duan, C.H.; Tian, P.Z.; Ju, S.S.; Hui, Y.; Zhang, Y.J.; Liu, Y.Q. The succession of fecal bacterial community and its correlation with the changes of serum immune indicators in lambs from birth to 4 months. J. Integr. Agric. 2023, 22, 537–550. [Google Scholar] [CrossRef]
- Zhong, Y.; Xue, M.Y.; Sun, H.Z.; Valencak, T.G.; Guan, L.L.; Liu, J. Rumen and hindgut bacteria are potential indicators for mastitis of mid-lactating holstein dairy cows. Microorganisms 2020, 8, 2042. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, J.M.; Zhou, Y.L.; Almeida, A.; Finn, R.D.; Danchin, A.; He, L.S. Phylogenomics of expanding uncultured environmental Tenericutes provides insights into their pathogenicity and evolutionary relationship with Bacilli. BMC Genom. 2020, 21, 408. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, Y.; Huang, G.; Zheng, N.; Zhao, S.; Wang, J. Ruminal bacterial community is associated with the variations of total milk solid content in Holstein lactating cows. Anim. Nutr. 2022, 9, 175–183. [Google Scholar] [CrossRef]
- Krautkramer, K.A.; Kreznar, J.H.; Romano, K.A.; Vivas, E.I.; Barrett-Wilt, G.A.; Rabaglia, M.E.; Keller, M.P.; Attie, A.D.; Rey, F.E.; Denu, J.M. Diet-Microbiota interactions mediate global epigenetic programming in multiple host tissues. Mol. Cell 2016, 64, 982–992. [Google Scholar] [CrossRef]
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Zhu, L. Short-Chain Fatty Acids and Their Association with Signalling Pathways in Inflammation, Glucose and Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 6356. [Google Scholar] [CrossRef] [PubMed]
- Bergman, E.N. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol. Rev. 1990, 70, 567–590. [Google Scholar] [CrossRef] [PubMed]
- Tygesen, M.P.; Nielsen, M.O.; Nørgaard, P.; Ranvig, H.; Harrison, A.P.; Tauson, A.H. Late gestational nutrient restriction: Effects on ewes’ metabolic and homeorhetic adaptation, consequences for lamb birth weight and lactation performance. Arch. Anim. Nutr. 2008, 62, 44–59. [Google Scholar] [CrossRef]
- Ramos-Lobo, A.M.; Furigo, I.C.; Teixeira, P.D.; Zampieri, T.T.; Wasinski, F.; Buonfiglio, D.C.; Donato Jr, J. Maternal metabolic adaptations are necessary for normal offspring growth and brain development. Physiol. Rep. 2018, 6, e13643–e13658. [Google Scholar] [CrossRef]
- Darby, M.G.; Chetty, A.; Mrjden, D.; Rolot, M.; Smith, K.; Mackowiak, C.; Sedda, D.; Nyangahu, D.; Jaspan, H.; Toellner, K.M. Pre-conception maternal helminth infection transfers via nursing long-lasting cellular immunity against helminths to offspring. Sci. Adv. 2019, 5, eaav3058–eaav3067. [Google Scholar] [CrossRef]
- Xie, Q.; Cui, D.; Zhu, Q.; Qin, X.; Ren, D.; Xu, X. Supplementing maternal diet with milk oligosaccharides and probiotics helps develop the immune system and intestinal flora of offsprings. Food Sci. Nutr. 2023, 11, 6868–6877. [Google Scholar] [CrossRef]
- Okouchi, R.; Shuang, E.; Yamamoto, K.; Ota, T.; Seki, K.; Imai, M.; Ota, R.; Asayama, Y.; Nakashima, A.; Suzuki, K. Simultaneous intake of euglena gracilis and vegetables exerts synergistic anti-obesity and anti-inflammatory effects by modulating the gut microbiota in diet-induced obese mice. Nutrients 2019, 11, 204. [Google Scholar] [CrossRef]
- Guo, W.; Mao, B.; Tang, X.; Zhang, Q.; Zhao, J.; Hao, Z.; Chen, W.; Cui, S. Improvement of inflammatory bowel disease by lactic acid bacteria-derived metabolites: A review. Crit. Rev. Food Sci. Nutr. 2023, 65, 1261–1278. [Google Scholar] [CrossRef]
- Macpherson, A.J.; Agüero, M.G.; Ganal-Vonarburg, S.C. How nutrition and the maternal microbiota shape the neonatal immune system. Nat. Rev. Immunol. 2017, 17, 508–517. [Google Scholar] [CrossRef]
- Dreisbach, C.; Morgan, H.; Cochran, C.; Gyamfi, A.; Henderson, W.A.; Prescott, S. Metaboliand microbial changes associated with diet and obesity during pregnancy: What can we learn from animal studies? Front. Cell Infect. Microbiol. 2022, 11, 795924–795931. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wei, S.; Liu, B.; Wang, F.; Lu, Z.; Jin, M.; Wang, Y. Maternal consumption of a fermented diet protects offspring against intestinal inflammation by regulating the gut microbiota. Gut Microbes 2022, 14, 2057779–2057802. [Google Scholar] [CrossRef] [PubMed]
- Bauer, E.; Williams, B.A.; Smidt, H.; Verstegen, M.W.; Mosenthin, R. Influence of the gastrointestinal microbiota on development of the immune system in young animals. Curr. Issues Intest. Microbiol. 2006, 7, 35–52. [Google Scholar] [PubMed]
- Perez-Muñoz, M.E.; Arrieta, M.-C.; Ramer-Tait, A.E.; Walter, J. A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: Implications for research on the pioneer infant microbiome. Microbiome 2017, 5, 48. [Google Scholar] [CrossRef]
- Jin, S.; Zhang, Z.; Zhang, G.; He, B.; Qin, Y.; Yang, B.; Yu, Z.; Wang, J. Maternal rumen bacteriota shapes the offspring rumen bacteriota, affecting the development of young ruminants. Microbiol. Spectr. 2023, 11, e0359022. [Google Scholar] [CrossRef]
- Sarkar, A.; Harty, S.; Johnson, K.V.A.; Moeller, A.H.; Archie, E.A.; Schell, L.D.; Carmody, R.N.; Clutton-Brock, T.H.; Dunbar, R.I.; Burnet, P.W. Microbial transmission in animal social networks and the social microbiome. Nat. Ecol. Evol. 2020, 4, 1020–1035. [Google Scholar] [CrossRef]
- Amin, N.; Schwarzkopf, S.; Kinoshita, A.; Tröscher-Mußotter, J.; Dänicke, S.; Camarinha-Silva, A.; Huber, K.; Frahm, J.; Seifert, J. Evolution of rumen and oral microbiota in calves is influenced by age and time of weaning. Anim. Microbiome 2021, 3, 31–46. [Google Scholar] [CrossRef]
- Zhuang, Y.; Liu, S.; Gao, D.; Xu, Y.; Jiang, W.; Hou, G.; Li, S.; Zhao, X.; Chen, T.; Li, S.; et al. Maternal gastrointestinal microbiome shapes gut microbial function and resistome of newborns in a cow-to-calf model. Microbiome 2024, 12, 216–234. [Google Scholar] [CrossRef]
- Alawneh, J.I.; Ramay, H.; Olchowy, T.; Allavena, R.; Soust, M.; Jassim, R.A. Effectof a lactobacilli-based direct-fed microbial product on gut microbiota and gastrointestinal morphological changes. Animals 2024, 14, 693. [Google Scholar] [CrossRef]
- Lv, X.; Chai, J.; Diao, Q.; Huang, W.; Zhuang, Y.; Zhang, N. The Signature Microbiota Drive Rumen Function Shifts in Goat Kids Introduced to Solid Diet Regimes. Microorganisms 2019, 7, 516. [Google Scholar] [CrossRef]
- García Mantrana, I.; Selma Royo, M.; González Solares, S.; Parra Llorca, A.; Martínez Costa, C.; Collado, M.C. Distinct maternal microbiota clusters are associated with diet during pregnancy: Impact on neonatal microbiota and infant growth during the first 18 months of life. Gut Microbes 2020, 11, 962–978. [Google Scholar] [CrossRef] [PubMed]
- Firth, M.A.; Shewen, P.E.; Hodgins, D.C. Passive and active components of neonatal innate immune defenses. Anim. Health Res. Rev. 2005, 6, 143–158. [Google Scholar] [CrossRef] [PubMed]
- Kamdar, S.; Hutchinson, R.; Laing, A.; Stacey, F.; Ansbro, K.; Millar, M.R.; Costeloe, K.; Wade, W.G.; Fleming, P.; Gibbons, D.L. Perinatal inflammation influences but does not arrest rapid immune development in preterm babies. Nat. Commun. 2020, 11, 1284–1298. [Google Scholar] [CrossRef]
- Tett, A.; Pasolli, E.; Masetti, G.; Ercolini, D.; Segata, N. Prevotella diversity, niches and interactions with the human host. Nat. Rev. Microbiol. 2021, 19, 585–599. [Google Scholar] [CrossRef]
- Criss, A.K.; Seifert, H.S. A bacterial siren song: Intimate interactions between Neisseria and neutrophils. Nat. Rev. Microbiol. 2012, 10, 178–190. [Google Scholar] [CrossRef]
- Mar, J.S.; Ota, N.; Pokorzynski, N.D.; Peng, Y.; Jaochico, A.; Sangaraju, D.; Skippington, E.; Lekkerkerker, A.N.; Rothenberg, M.E.; Tan, M.W. IL-22 alters gut microbiota composition and function to increase aryl hydrocarbon receptor activity in mice and humans. Microbiome 2023, 11, 47–64. [Google Scholar] [CrossRef]



| Items | G75 | G105 | G140 | L0 | L28 | p-Value |
|---|---|---|---|---|---|---|
| Acetate (mmol/L) | 55.64 ± 3.84 c | 74.91 ± 1.41 ab | 62.4 ± 10.12 bc | 74.01 ± 4.79 ab | 78.83 ± 8.93 a | 0.03 |
| Propionate (mmol/L) | 17.27 ± 0.50 | 18.59 ± 3.67 | 14.92 ± 1.20 | 21.41 ± 2.87 | 20.81 ± 3.71 | 0.19 |
| Butyrate (mmol/L) | 2.98 ± 0.41 | 3.14 ± 0.62 | 2.3 ± 0.19 | 2.76 ± 0.43 | 3.14 ± 0.47 | 0.36 |
| Isobutyrate (mmol/L) | 6.13 ± 1.45 | 5.58 ± 0.85 | 6.36 ± 0.96 | 5.88 ± 0.55 | 7.18 ± 1.09 | 0.61 |
| valerate (mmol/L) | 1.05 ± 0.23 | 0.89 ± 0.06 | 0.87 ± 0.03 | 0.88 ± 0.04 | 0.94 ± 0.06 | 0.51 |
| Isovalerate (mmol/L) | 4.21 ± 1.25 | 4.04 ± 0.88 | 4.64 ± 0.84 | 4.18 ± 0.53 | 5.19 ± 0.93 | 0.72 |
| TVFA (mmol/L) | 94.03 ± 7.61 | 107.15 ± 4.99 | 84.73 ± 1.96 | 109.13 ± 15.89 | 116.08 ± 22.45 | 0.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, H.; Li, K.; Guo, W.; Na, M.; Zhang, J.; Zhang, J.; Na, R. Physiological and Microbial Community Dynamics in Does During Mid-Gestation to Lactation and Their Impact on the Growth, Immune Function, and Microbiome Transmission of Offspring Kids. Animals 2025, 15, 954. https://doi.org/10.3390/ani15070954
Du H, Li K, Guo W, Na M, Zhang J, Zhang J, Na R. Physiological and Microbial Community Dynamics in Does During Mid-Gestation to Lactation and Their Impact on the Growth, Immune Function, and Microbiome Transmission of Offspring Kids. Animals. 2025; 15(7):954. https://doi.org/10.3390/ani15070954
Chicago/Turabian StyleDu, Haidong, Kenan Li, Wenliang Guo, Meila Na, Jing Zhang, Jing Zhang, and Renhua Na. 2025. "Physiological and Microbial Community Dynamics in Does During Mid-Gestation to Lactation and Their Impact on the Growth, Immune Function, and Microbiome Transmission of Offspring Kids" Animals 15, no. 7: 954. https://doi.org/10.3390/ani15070954
APA StyleDu, H., Li, K., Guo, W., Na, M., Zhang, J., Zhang, J., & Na, R. (2025). Physiological and Microbial Community Dynamics in Does During Mid-Gestation to Lactation and Their Impact on the Growth, Immune Function, and Microbiome Transmission of Offspring Kids. Animals, 15(7), 954. https://doi.org/10.3390/ani15070954







