A Review of Nitrogen Use Efficiency of Dairy Replacement Heifers: Improving Management Practices and Minimizing Nitrogen Losses
Simple Summary
Abstract
1. Introduction
2. Nitrogen Pollution of the Dairy Herd and the Role of Dairy Replacement Heifers
3. Growth Targets of Dairy Heifers
4. Nutritional Management of Dairy Heifers
4.1. Physiological Basis and Nitrogen Metabolism
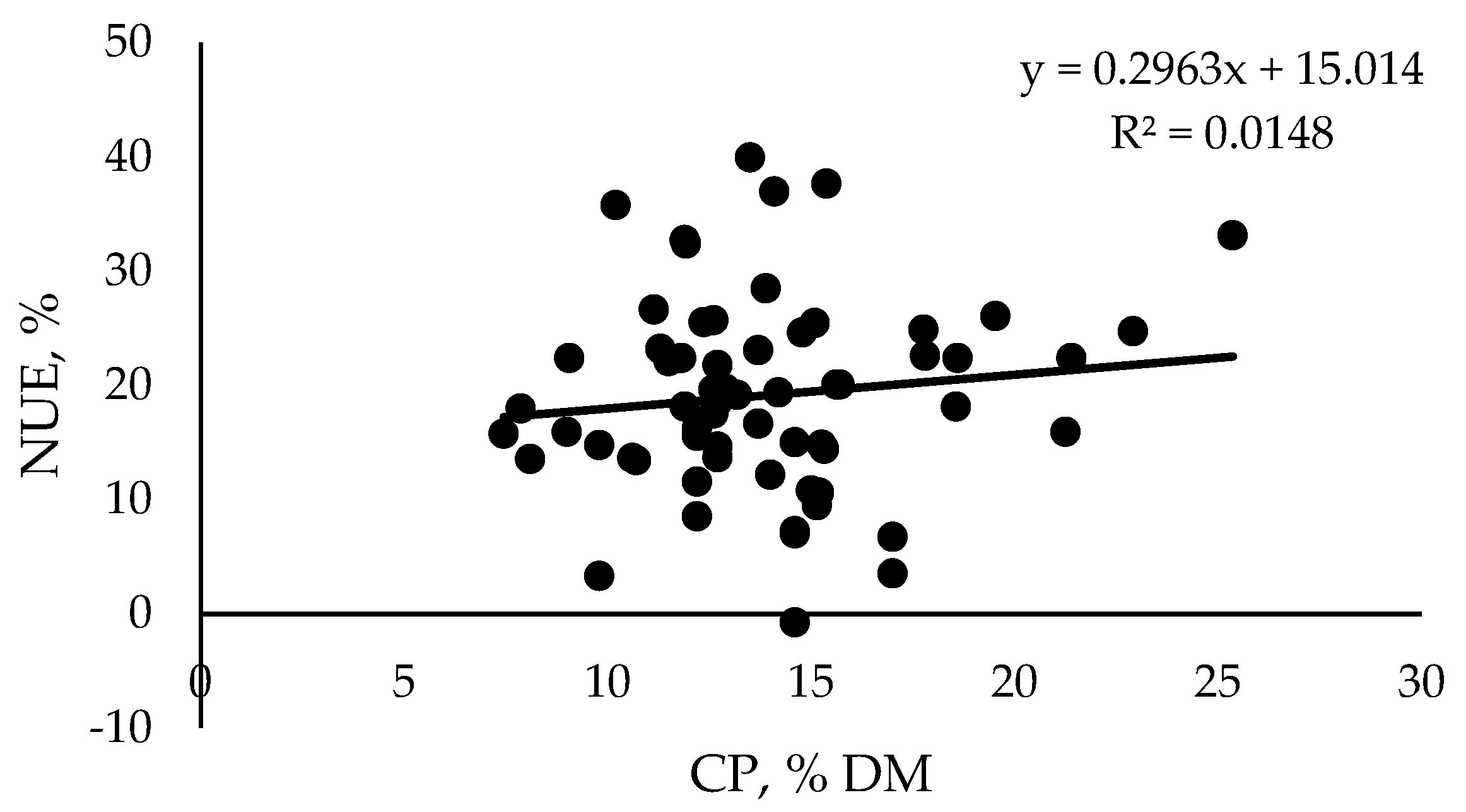
4.2. Effects of Crude Protein and Rumen Degradable Protein Level on Nitrogen Use Efficiency of Replacement Heifers
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Erisman, J.W.; Galloway, J.N.; Seitzinger, S.; Bleeker, A.; Dise, N.B.; Petrescu, A.M.R.; Leach, A.M.; de Vries, W. Consequences of human modification of the global nitrogen cycle. Philos. Trans. R. Soc. B 2013, 368, 20130116. [Google Scholar] [CrossRef]
- Sutton, M.A.; van Grinsven, H.; Billen, G.; Bleeker, A.; Bouwman, A.F.; Bull, K.; Erisman, J.W.; Grennfelt, P.; Grizzetti, B.; Howard, C.M.; et al. Summary for policy makers. In The European Nitrogen Assessment: Sources, Effects and Policy Perspectives; Bleeker, A., Grizzetti, B., Howard, C.M., Billen, G., van Grinsven, H., Erisman, J.W., Sutton, M.A., Grennfelt, P., Eds.; Cambridge University Press: Cambridge, UK, 2011; pp. xxiv–xxxiv. [Google Scholar]
- Galloway, J.N.; Aber, J.D.; Erisman, J.W.; Seitzinger, S.P.; Howarth, R.W.; Cowling, E.B.; Cosby, B.J. The Nitrogen cascade. BioScience 2003, 53, 341–356. [Google Scholar] [CrossRef]
- Fowler, D.; Coyle, M.; Skiba, U.; Sutton, M.A.; Cape, J.N.; Reis, S.; Sheppard, L.J.; Jenkins, A.; Grizzetti, B.; Galloway, J.N.; et al. The global nitrogen cycle in the twenty-first century. Philos. Trans. R. Soc. B 2013, 368, 20130164. [Google Scholar] [CrossRef] [PubMed]
- Uwizeye, A.; de Boer, I.J.M.; Opio, C.I.; Schulte, R.P.O.; Falcucci, A.; Tempio, G.; Teillard, F.; Casu, F.; Rulli, M.; Galloway, J.N.; et al. Nitrogen emissions along global livestock supply chains. Nat. Food 2020, 1, 437–446. [Google Scholar] [CrossRef]
- Dijkstra, J.; Oenema, O.; van Groenigen, J.W.; Spek, J.W.; van Vuuren, A.M.; Bannink, A. Diet effects on urine composition of cattle and N2O emissions. Animal 2013, 7, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Firkins, J.L.; Reynolds, C.K. Whole-animal nitrogen balance in cattle. In Nitrogen and Phosphorus Nutrition of Cattle: Reducing the Environmental Impact of Cattle Operations; Pfeffer, E., Hristov, A.N., Eds.; CAB International: Wallingford, UK, 2005; pp. 167–186. [Google Scholar] [CrossRef]
- Van Amburgh, M.E.; Collao-Saenz, E.A.; Higgs, R.J.; Ross, D.A.; Recktenwald, E.B.; Raffrenato, E.; Chase, L.E.; Overton, T.R.; Mills, J.K.; Foskolos, A. The Cornell Net Carbohydrate and Protein System: Updates to the model and evaluation of version 6.5. J. Dairy Sci. 2015, 98, 6361–6380. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Villegas, H.A.; Larson, R.A.; Sharara, M.A. Anaerobic digestion, solid-liquid separation, and drying of dairy manure: Measuring constituents and modeling emission. Sci. Total Environ. 2019, 696, 134059. [Google Scholar] [CrossRef]
- Behera, S.N.; Sharma, M.; Aneja, V.P.; Balasubramanian, R. Ammonia in the atmosphere: A review on emission sources, atmospheric chemistry and deposition on terrestrial bodies. Environ. Sci. Pollut. Res. 2013, 20, 8092–8131. [Google Scholar] [CrossRef]
- Malherbe, L.; German, R.; Couvidat, F.; Zanatta, L.; Blannin, L.; James, A.; Lètinois, L.; Schucht, S.; Berthelot, B.; Raoult, J. Emissions of ammonia and methane from the agricultural sector. In Emissions from Livestock Farming (ETC HE Report 2022/21); European Topic Centre on Human Health and the Environment: Cornwall, UK, 2022. [Google Scholar]
- Calsamiglia, S.; Ferret, A.; Reynolds, C.K.; Kristensen, N.B.; van Vuuren, A.M. Strategies for optimizing nitrogen use by ruminants. Animal 2010, 4, 1184–1196. [Google Scholar] [CrossRef]
- Foskolos, A.; Moorby, J.M. Evaluating lifetime nitrogen use efficiency of dairy cattle: A modelling approach. PLoS ONE 2018, 13, e0201638. [Google Scholar] [CrossRef]
- Boulton, A.C.; Rushton, J.; Wathes, D.C. An empirical analysis of the cost of rearing dairy heifers from birth to first calving and the time taken to repay these costs. Animal 2017, 11, 1372–1380. [Google Scholar] [CrossRef] [PubMed]
- Hristov, A.N.; Hanigan, M.; Cole, A.; Todd, R.; McAllister, T.A.; Ndegwa, P.M.; Rotz, A. Review: Ammonia emissions from dairy farms and beef feedlots. Can. J. Anim. Sci. 2011, 91, 1–35. [Google Scholar] [CrossRef]
- Bussink, D.W.; Oenema, O. Ammonia volatilization from dairy farming systems in temperate areas: A review. Nutr. Cycl. Agroecosyst. 1998, 51, 19–33. [Google Scholar] [CrossRef]
- Bougouin, A.; Hristov, A.; Dijkstra, J.; Aguerre, M.J.; Ahvenjärvi, S.; Arndt, C.; Bannink, A.; Bayat, A.R.; Benchaar, C.; Boland, T.; et al. Prediction of nitrogen excretion from data on dairy cows fed a wide range of diets compiled in an intercontinental database: A meta-analysis. J. Dairy Sci. 2022, 105, 7462–7481. [Google Scholar] [CrossRef]
- Bougouin, A.; Leytem, A.; Dijkstra, J.; Dungan, R.S.; Kebreab, E. Nutritional and environmental effects on ammonia emissions from dairy cattle housing: A meta-analysis. J. Environ. Qual. 2016, 45, 1123–1132. [Google Scholar] [CrossRef]
- Powell, J.M.; Misselbrook, T.H.; Casler, M.D. Season and bedding impacts on ammonia emissions from tie-stall dairy barns. J. Environ. Qual. 2008, 37, 7–15. [Google Scholar] [CrossRef]
- Lascano, G.J.; Zanton, G.I.; Moody, M.L.; Topper, P.A.; Wheeler, E.F.; Heinrichs, A.J. Short communication: Effect of changing the ratio of forage to concentrate on ammonia emissions by dairy heifers. J. Dairy Sci. 2008, 91, 4301–4306. [Google Scholar] [CrossRef]
- Powell, J.M.; Broderick, G.A.; Misselbrook, T.H. Seasonal diet affects ammonia emissions from tie-stall dairy barns. J. Dairy Sci. 2008, 91, 857–869. [Google Scholar] [CrossRef]
- Marini, J.C.; Van Amburgh, M.E. Nitrogen metabolism and recycling in Holstein heifers. J. Anim. Sci. 2003, 81, 545–552. [Google Scholar] [CrossRef]
- Erisman, J.W.; Leach, A.; Bleeker, A.; Atwell, B.; Cattaneo, L.; Galloway, J. An integrated approach to a nitrogen use efficiency (NUE) indicator for the food production–consumption chain. Sustainability 2018, 10, 925. [Google Scholar] [CrossRef]
- Huhtanen, P.; Hristov, A.N. A meta-analysis of the effects of dietary protein concentration and degradability on milk protein yield and milk N efficiency in dairy cows. J. Dairy Sci. 2009, 92, 3222–3232. [Google Scholar] [CrossRef] [PubMed]
- Marini, J.C.; Van Amburgh, M.E. Partition of nitrogen excretion in urine and the feces of holstein replacement heifers. J. Dairy Sci. 2005, 88, 1778–1784. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, P.C.; Esser, N.M.; Bauman, L.M.; Denzine, S.L.; Engstrom, M.; Chester-Jones, H. Short communication: Effect of dietary protein on growth and nitrogen balance of holstein heifers. J. Dairy Sci. 2001, 84, 843–847. [Google Scholar] [CrossRef]
- Gabler, M.T.; Heinrichs, A.J. Dietary protein to metabolizable energy ratios on feed efficiency and structural growth of prepubertal Holstein heifers. J. Dairy Sci. 2003, 86, 268–274. [Google Scholar] [CrossRef] [PubMed]
- USDA. Dairy 2014, “Trends in Dairy Cattle Health and Management Practices in the United States, 1991–2014”; USDA–APHIS–VS–CEAH-NAHMS: Fort Collins, CO, USA, 2021.
- Schillo, K.K.; Hall, J.B.; Hileman, S.M. Effects of nutrition and season on the onset of puberty in the beef heifer. J. Anim. Sci. 1992, 70, 3994–4005. [Google Scholar] [CrossRef]
- Funston, R.N.; Martin, J.L.; Larson, D.M.; Roberts, A.J. Physiology and endocrinology symposium: Nutritional aspects of developing replacement heifers. J. Anim. Sci. 2012, 90, 1166–1171. [Google Scholar] [CrossRef]
- Heinrichs, A.J.; Zanton, G.I.; Lascano, G.J.; Jones, C.M. A 100-Year Review: A century of dairy heifer research. J. Dairy Sci. 2017, 100, 10173–10188. [Google Scholar] [CrossRef]
- Le Cozler, Y.; Lollivier, V.; Lacasse, P.; Disenhaus, C. Rearing strategy and optimizing first-calving targets in dairy heifers: A review. Animal 2008, 2, 1393–1404. [Google Scholar] [CrossRef]
- Ettema, J.F.; Santos, J.E.P. Impact of age at calving on lactation, reproduction, health, and income in first-parity Holsteins on commercial farms. J. Dairy Sci. 2004, 87, 2730–2742. [Google Scholar] [CrossRef]
- Eastham, N.T.; Coates, A.; Cripps, P.; Richardson, H.; Smith, R.; Oikonomou, G. Associations between age at first calving and subsequent lactation performance in UK Holstein and Holstein-Friesian dairy cows. PLoS ONE 2018, 13, e0197764. [Google Scholar] [CrossRef]
- Hutchison, J.L.; VanRaden, P.M.; Null, D.J.; Cole, J.B.; Bickhart, D.M. Genomic evaluation of age at first calving. J. Dairy Sci. 2017, 100, 6853–6861. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, P.C. Feeding management of the dairy heifer from 4 months to calving. In Large Dairy Herd Management, 3rd ed.; Beede, D.K., Ed.; American Dairy Science Association: Champaign, IL, USA, 2017; pp. 431–443. [Google Scholar]
- Coffey, M.P.; Hickey, J.; Brotherstone, S. Genetic aspects of growth of Holstein-Friesian dairy cows from birth to maturity. J. Dairy Sci. 2006, 89, 322–329. [Google Scholar] [CrossRef]
- Handcock, R.C.; Lopez-Villalobos, N.; McNaughton, L.R.; Back, P.J.; Edwards, G.R.; Hickson, R.E. Live weight and growth of Holstein-Friesian, Jersey and crossbred dairy heifers in New Zealand. N. Z. J. Agric. Res. 2019, 62, 173–183. [Google Scholar] [CrossRef]
- Van Amburgh, M.E.; Galton, D.M.; Bauman, D.E.; Everett, R.W.; Fox, D.G.; Chase, L.E.; Erb, H.N. Effects of three prepubertal body growth rates on performance of holstein heifers during first lactation. J. Dairy Sci. 1998, 81, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Fox, D.G.; Van Amburgh, M.E.; Tylutki, T.P. Predicting requirements for growth, maturity, and body reserves in dairy cattle. J. Dairy Sci. 1999, 82, 1968–1977. [Google Scholar] [CrossRef]
- Fox, D.G.; Sniffen, C.J.; O’Connor, J.D.; Russell, J.B.; Van Soest, P.J. A net carbohydrate and protein system for evaluating cattle diets: III. Cattle requirements and diet adequacy. J. Anim. Sci. 1992, 70, 3578–3596. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Dairy Cattle, 7th ed.; The National Academies Press: Washington, DC, USA, 2001; p. 405.
- Hoffman, P.C. Optimum body size of Holstein replacement heifers. J. Anim. Sci. 1997, 75, 836–845. [Google Scholar] [CrossRef]
- Van Amburgh, M.E.; Soberon, F.; Meyer, M.J.; Molano, R.A. Symposium review: Integration of postweaning nutrient requirements and supply with composition of growth and mammary development in modern dairy heifers. J. Dairy Sci. 2019, 102, 3692–3705. [Google Scholar] [CrossRef]
- NASEM. Nutrient Requirements of Dairy Cattle, 8th ed.; The National Academies Press: Washington, DC, USA, 2021. [Google Scholar]
- Ferrell, C.L. Effects of postweaning rate of gain on onset of puberty and productive performance of heifers of different breeds. J. Anim. Sci. 1982, 55, 1272–1283. [Google Scholar] [CrossRef]
- Wathes, D.C.; Brickell, J.S.; Bourne, N.E.; Swali, A.; Cheng, Z. Factors influencing heifer survival and fertility on commercial dairy farms. Animal 2008, 2, 1135–1143. [Google Scholar] [CrossRef]
- Archbold, H.; Shalloo, L.; Kennedy, E.; Pierce, K.M.; Buckley, F. Influence of age, body weight and body condition score before mating start date on the pubertal rate of maiden Holstein–Friesian heifers and implications for subsequent cow performance and profitability. Animal 2012, 6, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Handcock, R.C.; Lopez-Villalobos, N.; McNaughton, L.R.; Back, P.J.; Edwards, G.R.; Hickson, R.E. Body weight of dairy heifers is positively associated with reproduction and stayability. J. Dairy Sci. 2020, 103, 4466–4474. [Google Scholar] [CrossRef] [PubMed]
- Handcock, R.C.; Lopez-Villalobos, N.; McNaughton, L.R.; Back, P.J.; Edwards, G.R.; Hickson, R.E. Positive relationships between body weight of dairy heifers and their first-lactation and accumulated three-parity lactation production. J. Dairy Sci. 2019, 102, 4577–4589. [Google Scholar] [CrossRef]
- Han, L.; Heinrichs, A.J.; De Vries, A.; Dechow, C.D. Relationship of body weight at first calving with milk yield and herd life. J. Dairy Sci. 2021, 104, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Lauber, M.R.; Fricke, P.M. The association between insemination eligibility and reproductive performance of nulliparous heifers on subsequent body weight and milk production of primiparous Holstein cows. JDS Commun. 2023, 4, 428–432. [Google Scholar] [CrossRef]
- Bach, A.; Calsamiglia, S.; Stern, M.D. Nitrogen metabolism in the rumen. J. Dairy Sci. 2005, 88, E9–E21. [Google Scholar] [CrossRef]
- Huhtanen, P.; Ahvenjärvi, S. Review: Problems in determining metabolisable protein value of dairy cow diets and the impact on protein feeding. Animal 2022, 16, 100539. [Google Scholar] [CrossRef]
- Storm, E.; Orskov, E.R. The nutritive value of rumen micro-organisms in ruminants. 1. Large-scale isolation and chemical composition of rumen micro-organisms. Br. J. Nutr. 1983, 50, 463–470. [Google Scholar] [CrossRef]
- Lapierre, H.; Larsen, M.; Sauvant, D.; Van Amburgh, M.E.; Van Duinkerken, G. Review: Converting nutritional knowledge into feeding practices: A case study comparing different protein feeding systems for dairy cows. Animal 2018, 12, s457–s466. [Google Scholar] [CrossRef]
- Wickersham, T.A.; Titgemeyer, E.C.; Cochran, R.C. Methodology for concurrent determination of urea kinetics and the capture of recycled urea nitrogen by ruminal microbes in cattle. Animal 2009, 3, 372–379. [Google Scholar] [CrossRef]
- Batista, E.D.; Detmann, E.; Valadares Filho, S.C.; Titgemeyer, E.C.; Valadares, R.F.D. The effect of CP concentration in the diet on urea kinetics and microbial usage of recycled urea in cattle: A meta-analysis. Animal 2017, 11, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, P.J. Nutritional Ecology of the Ruminant, 2nd ed.; Cornell University Press: Ithaca, NY, USA, 1994. [Google Scholar]
- Merchen, N.R.; Titgemeyer, E.C. Manipulation of amino acid supply to the growing ruminant. J. Anim. Sci. 1992, 70, 3238–3247. [Google Scholar] [CrossRef]
- Lascano, G.J.; Koch, L.E.; Heinrichs, A.J. Precision-feeding dairy heifers a high rumen-degradable protein diet with different proportions of dietary fiber and forage-to-concentrate ratios. J. Dairy Sci. 2016, 99, 7175–7190. [Google Scholar] [CrossRef] [PubMed]
- Firkins, J.L. Maximizing microbial protein synthesis in the rumen. J. Nutr. 1996, 126, 1347S–1354S. [Google Scholar] [CrossRef]
- Hoover, W.H.; Stokes, S.R. Balancing carbohydrates and proteins for optimum rumen microbial yield. J. Dairy Sci. 1991, 74, 3630–3644. [Google Scholar] [CrossRef]
- Russell, J.B.; O’Connor, J.D.; Fox, D.G.; Van Soest, P.J.; Sniffen, C.J. A net carbohydrate and protein system for evaluating cattle diets: I. Ruminal fermentation. J. Anim. Sci. 1992, 70, 3551–3561. [Google Scholar] [CrossRef] [PubMed]
- Higgs, R.J.; Chase, L.E.; Ross, D.A.; Van Amburgh, M.E. Updating the Cornell Net Carbohydrate and Protein System feed library and analyzing model sensitivity to feed inputs. J. Dairy Sci. 2015, 98, 6340–6360. [Google Scholar] [CrossRef]
- Green, T.C.; Jago, J.G.; Macdonald, K.A.; Waghorn, G.C. Relationships between residual feed intake, average daily gain, and feeding behavior in growing dairy heifers. J. Dairy Sci. 2013, 96, 3098–3107. [Google Scholar] [CrossRef]
- Robles, V.; González, L.A.; Ferret, A.; Manteca, X.; Calsamiglia, S. Effects of feeding frequency on intake, ruminal fermentation, and feeding behavior in heifers fed high-concentrate diets. J. Anim. Sci. 2007, 85, 2538–2547. [Google Scholar] [CrossRef]
- Tedeschi, L.O.; Fox, D.G.; Fonseca, M.A.; Cavalcanti, L.F.L. Models of protein and amino acid requirements for cattle. Rev. Bras. Zootec. 2015, 44, 109–132. [Google Scholar] [CrossRef]
- Van Amburgh, M.E.; Fox, D.G.; Galton, D.M.; Bauman, D.E.; Chase, L.E. Evaluation of National Research Council and Cornell Net Carbohydrate and Protein Systems for predicting requirements of Holstein heifers. J. Dairy Sci. 1998, 81, 509–526. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Dairy Cattle; The National Academies Press: Washington, DC, USA, 1989.
- NRC. Nutrient Requirements of Beef Cattle, 7th ed.; The National Academies Press: Washington, DC, USA, 1996.
- Zanton, G.I.; Heinrichs, A.J. Efficiency and rumen responses in younger and older Holstein heifers limit-fed diets of differing energy density. J. Dairy Sci. 2016, 99, 2825–2836. [Google Scholar] [CrossRef]
- Chapman, C.E.; Hill, T.M.; Elder, D.R.; Erickson, P.S. Nitrogen utilization, preweaning nutrient digestibility, and growth effects of Holstein dairy calves fed 2 amounts of a moderately high protein or conventional milk replacer. J. Dairy Sci. 2017, 100, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Lobley, G.E. Species comparisons of tissue protein metabolism: Effects of age and hormonal action. J. Nutr. 1993, 123, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Blome, R.M.; Drackley, J.K.; McKeith, F.K.; Hutjens, M.F.; McCoy, G.C. Growth, nutrient utilization, and body composition of dairy calves fed milk replacers containing different amounts of protein. J. Anim. Sci. 2003, 81, 1641–1655. [Google Scholar] [CrossRef] [PubMed]
- Amado, L.; Leal, L.N.; Berends, H.; van Keulen, P.; Martín-Tereso, J.; Gerrits, W.J.J. Responses to incremental nutrient supply on energy and protein metabolism in preweaning dairy calves. J. Dairy Sci. 2024, 107, 5616–5625. [Google Scholar] [CrossRef]
- Corea, E.E.; Castro-Montoya, J.; Mendoza, M.V.; López, F.M.; Martinez, A.; Alvarado, M.E.; Moreno, C.; Broderick, G.A.; Dickhoefer, U. Effect of forage source and dietary rumen-undegradable protein on nutrient use and growth in dairy heifers. Anim. Feed Sci. Technol. 2020, 269, 114658. [Google Scholar] [CrossRef]
- Johansen, M.; Madsen, P.A.; Brask-Pedersen, D.N.; Lund, P. Effect of age and dietary crude protein level on nitrogen excretion in dairy heifers. Livest. Sci. 2022, 264, 105058. [Google Scholar] [CrossRef]
- Silva, A.L.; Detmann, E.; Rennó, L.N.; Pedroso, A.M.; Fontes, M.M.S.; Morais, V.C.; Sguizzato, A.L.L.; Abreu, M.B.; Rotta, P.P.; Marcondes, M.I. Effects of rumen undegradable protein on intake, digestibility and rumen kinetics and fermentation characteristics of dairy heifers. Anim. Feed Sci. Technol. 2018, 244, 1–10. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, C.; Liu, H.; Liu, J.; Liu, H. Effects of dietary protein level on growth performance and nitrogen excretion of dairy heifers. Asian-Australas. J. Anim. Sci. 2017, 30, 386–391. [Google Scholar] [CrossRef]
- Zanton, G.I.; Heinrichs, A.J. Digestion and nitrogen utilization in dairy heifers limit-fed a low or high forage ration at four levels of nitrogen intake. J. Dairy Sci. 2009, 92, 2078–2094. [Google Scholar] [CrossRef] [PubMed]
- Zanton, G.I.; Gabler, M.T.; Heinrichs, A.J. Manipulation of soluble and rumen-undegradable protein in diets fed to postpubertal dairy heifers. J. Dairy Sci. 2007, 90, 978–986. [Google Scholar] [CrossRef]
- Hoffman, P.C.; Simson, C.R.; Wattiaux, M. Limit feeding of gravid Holstein heifers: Effect on growth, manure nutrient excretion, and subsequent early lactation performance. J. Dairy Sci. 2007, 90, 946–954. [Google Scholar] [CrossRef]
- Adachi, N.; Kusuhara, T.; Nonaka, I.; Terada, F. Effect of close-up dry period protein level on preparturiental nitrogen balance and lactating performance of primigravid and multiparous Holstein cows. Asian-Australas. J. Anim. Sci. 2006, 19, 831–836. [Google Scholar] [CrossRef]
- Hill, S.R.; Knowlton, K.F.; James, R.E.; Pearson, R.E.; Bethard, G.L.; Pence, K.J. Nitrogen and phosphorus retention and excretion in late-gestation dairy heifers. J. Dairy Sci. 2007, 90, 5634–5642. [Google Scholar] [CrossRef]
- Tamminga, S. A review on environmental impacts of nutritional strategies in ruminants. J. Anim. Sci. 1996, 74, 3112–3124. [Google Scholar] [CrossRef]
- Davidson, S.; Hopkins, B.A.; Diaz, D.E.; Bolt, S.M.; Brownie, C.; Fellner, V.; Whitlow, L.W. Effects of amounts and degradability of dietary protein on lactation, nitrogen utilization, and excretion in early lactation Holstein cows. J. Dairy Sci. 2003, 86, 1681–1689. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, D.L.; James, R.E.; Bethard, G.L.; McGilliard, M.L. Influence of undegradability of protein in the diet on intake, daily gain, feed efficiency, and body composition of Holstein heifers. J. Dairy Sci. 1997, 80, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Broderick, G.A.; Huhtanen, P.; Ahvenjärvi, S.; Reynal, S.M.; Shingfield, K.J. Quantifying ruminal nitrogen metabolism using the omasal sampling technique in cattle—A meta-analysis. J. Dairy Sci. 2010, 93, 3216–3230. [Google Scholar] [CrossRef]
- Foskolos, A.; Plomaritou, A. Guest editorial: Holistic Nutritional Management as a tool to mitigate nitrogen pollution from dairy cattle farming. J. Agric. Sci. 2023, 161, 313–317. [Google Scholar] [CrossRef]
| MBW (%) | |||||
|---|---|---|---|---|---|
| Stage | Hoffman [36] | Van Amburgh et al. [39] | Fox et al. [40] | NRC, 2001 [42] | NASEM, 2021 [45] |
| Breeding | 55 | 55–60 | - | 55 | 55 |
| Prepartum | 94 | - | - | - | 91 |
| 1st Calving | - | 82 | 85 | 82 | 82 |
| 2nd Calving | - | - | 92 | 92 | 92 |
| 3rd Calving | - | - | 96 | 100 | 100 |
| Age (Months) | BW (kg) | SD | CP (% DM) | SD | NUE (%) | SD | Reference |
|---|---|---|---|---|---|---|---|
| Preweaning | - | - | 22.9 | 5.1 | 50.7 | 9.3 | [73,75,76] |
| 5–7 | 221 | 52 | 14.2 | 3.8 | 19.4 | 2.1 | [25,77] |
| 8–9 | 261 | 15 | 14.4 | 3.2 | 19.6 | 9.7 | [22,78,79,80] |
| 10–12 | 372 | 16 | 14.5 | 5.3 | 17.9 | 8.1 | [26,78,81] |
| 15–18 | 466 | 17 | 12.9 | 1.7 | 20.2 | 10.3 | [61,78,82,83] |
| 20–24 | 576 | 69 | 13.5 | 2.4 | 17.8 | 9.3 | [78,84,85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plomaritou, A.; Hanlon, M.; Kantas, D.; Georgakoudis, K.; Dovolou, E.; Foskolos, A. A Review of Nitrogen Use Efficiency of Dairy Replacement Heifers: Improving Management Practices and Minimizing Nitrogen Losses. Animals 2025, 15, 1031. https://doi.org/10.3390/ani15071031
Plomaritou A, Hanlon M, Kantas D, Georgakoudis K, Dovolou E, Foskolos A. A Review of Nitrogen Use Efficiency of Dairy Replacement Heifers: Improving Management Practices and Minimizing Nitrogen Losses. Animals. 2025; 15(7):1031. https://doi.org/10.3390/ani15071031
Chicago/Turabian StylePlomaritou, Antigoni, Mikenzie Hanlon, Dimitrios Kantas, Konstantinos Georgakoudis, Eleni Dovolou, and Andreas Foskolos. 2025. "A Review of Nitrogen Use Efficiency of Dairy Replacement Heifers: Improving Management Practices and Minimizing Nitrogen Losses" Animals 15, no. 7: 1031. https://doi.org/10.3390/ani15071031
APA StylePlomaritou, A., Hanlon, M., Kantas, D., Georgakoudis, K., Dovolou, E., & Foskolos, A. (2025). A Review of Nitrogen Use Efficiency of Dairy Replacement Heifers: Improving Management Practices and Minimizing Nitrogen Losses. Animals, 15(7), 1031. https://doi.org/10.3390/ani15071031






