Hair Dehydroepiandrosterone Sulfate (DHEA(S)) and Cortisol/DHEA(S) Ratio as Long-Lasting Biomarkers of Clinical Syndromes Exhibited by Piglets Early in Life
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
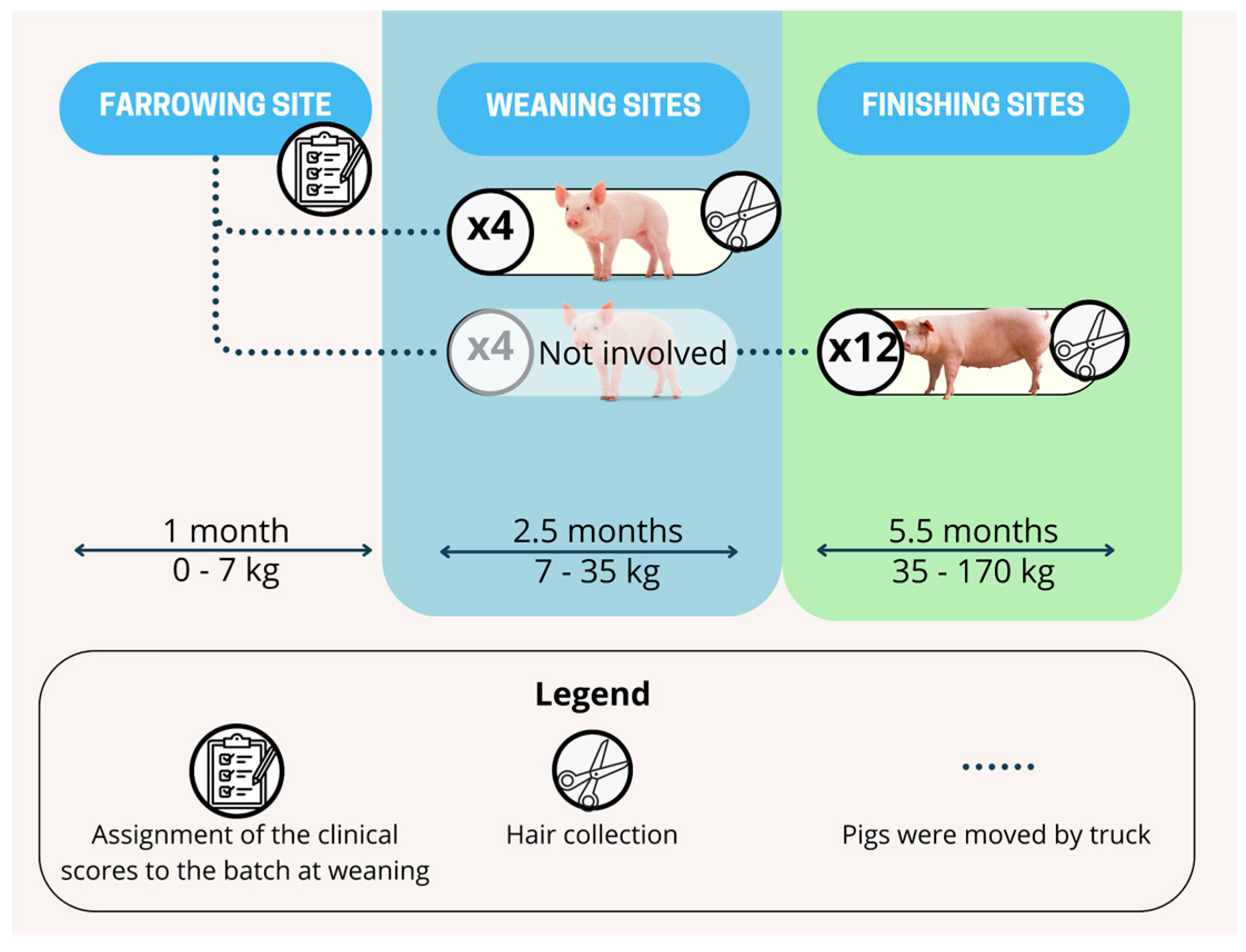
2.1. Clinical Scores
2.2. Hair Sampling and Analysis
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maes, D.; Verbeke, W.; Vicca, J.; Verdonck, M.; de Kruif, A. Benefit to cost of vaccination against Mycoplasma hyopneumoniae in pig herds under Belgian market conditions from 1996 to 2000. Livest. Prod. Sci. 2003, 83, 85–93. [Google Scholar]
- Martínez, J.; Jaro, P.J.; Aduriz, G.; Gómez, E.A.; Peris, B.; Corpa, J.M. Carcass condemnation causes of growth retard–ed pigs at slaughter. Vet. J. 2007, 174, 160–164. [Google Scholar]
- Kilbride, A.L.; Mendl, M.; Statham, P.; Held, S.; Harris, M.; Cooper, S.; Green, L.E. A cohort study of preweaning piglet mortality and farrowing accommodation on 112 commercial pig farms in England. Prev. Vet. Med. 2012, 104, 281–291. [Google Scholar] [PubMed]
- Quiniou, N.; Dagorn, J.; Gaudré, D. Variation of piglets’ birth weight and consequences on subsequent performance. Livest. Prod. Sci. 2002, 78, 63–70. [Google Scholar]
- Wolter, B.F.; Ellis, M.; Corrigan, B.P.; DeDecker, J.M. The effect of birth weight and feeding of supplemental milk replacer to piglets during lactation on preweaning and postweaning growth performance and carcass characteristics. J. Anim. Sci. 2002, 80, 301–308. [Google Scholar]
- Larriestra, A.J.; Wattanaphansak, S.; Neumann, E.J.; Bradford, J.; Morrison, R.B.; Deen, J. Pig characteristics associated with mortality and light exit weight for the nursery phase. Can. Vet. J. 2006, 47, 560. [Google Scholar] [PubMed]
- Fix, J.S.; Cassady, J.P.; Herring, W.O.; Holl, J.W.; Culbertson, M.S.; See, M.T. Effect of piglet birth weight on body weight, growth, backfat, and longissimus muscle area of commercial market swine. Livest. Sci. 2010, 127, 51–59. [Google Scholar]
- Cabrera, R.A.; Boyd, R.D.; Jungst, S.B.; Wilson, E.R.; Johnston, M.E.; Vignes, J.L.; Odle, J. Impact of lactation length and piglet weaning weight on long–term growth and viability of progeny. J. Anim. Sci. 2010, 88, 2265–2276. [Google Scholar]
- Douglas, S.L.; Edwards, S.A.; Kyriazakis, I. Management strategies to improve the performance of low birth weight pigs to weaning and their long–term consequences. J. Anim. Sci. 2014, 92, 2280–2288. [Google Scholar]
- Neal, S.M.; Irvin, K.M. The effects of crossfostering pigs on survival and growth. J. Anim. Sci. 1991, 69, 41–46. [Google Scholar]
- Tuchscherer, M.; Puppe, B.; Tuchscherer, A.; Tiemann, U. Early identification of neonates at risk: Traits of newborn piglets with respect to survival. Theriogenology 2000, 54, 371–388. [Google Scholar] [CrossRef]
- Deen, M.G.H.; Bilkei, G. Cross fostering of low–birthweight piglets. Livest. Prod. Sci. 2004, 90, 279–284. [Google Scholar] [CrossRef]
- Maes, D.G.D.; Janssens, G.P.J.; Delputte, P.; Lammertyn, A.; de Kruif, A. Back fat measurements in sows from three commercial pig herds: Relationship with reproductive efficiency and correlation with visual body condition scores. Livest. Prod. Sci. 2004, 91, 57–67. [Google Scholar] [CrossRef]
- Koketsu, Y.; Takenobu, S.; Nakamura, R. Preweaning mortality risks and recorded causes of death associated with production factors in swine breeding herds in Japan. J. Vet. Med. Sci. 2006, 68, 821–826. [Google Scholar] [CrossRef]
- Kritas, S.K.; Morrison, R.B. Relationships between tail biting in pigs and disease lesions and condemnations at slaughter. Vet. Rec. 2007, 160, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Fraile, L.; Alegre, A.; López–Jiménez, R.; Nofrarías, M.; Segalés, J. Risk factors associated with pleuritis and cranio–ventral pulmonary consolidation in slaughter–aged pigs. Vet. J. 2010, 184, 326–333. [Google Scholar] [CrossRef]
- Calderón Díaz, J.A.; Boyle, L.A.; Diana, A.; Leonard, F.C.; Moriarty, J.P.; McElroy, M.C.; McGettrick, S.; Kelliher, D.; García Manzanilla, E. Early life indicators predict mortality, illness, reduced welfare and carcass characteristics in finisher pigs. Prev. Vet. Med. 2017, 146, 94–102. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Interacting mediators of allostasis and allostatic load: Towards an understanding of resilience in aging. Metabolism 2003, 52, 10–16. [Google Scholar] [CrossRef]
- Charney, D.S. Psychobiological mechanisms of resilience and vulnerability: Implications for successful adaptations to extreme stress. Am. J. Psychiatr. 2004, 161, 195–216. [Google Scholar] [CrossRef]
- Peric, T.; Corazzin, M.; Romanzin, A.; Bovolenta, S.; Prandi, A.; Montillo, M.; Comin, A. Cortisol and DHEA concentrations in the hair of dairy cows managed indoor or on pasture. Livest. Sci. 2017, 202, 39–43. [Google Scholar] [CrossRef]
- Logan, J.G.; Barksdale, D.J. Allostasis and allostatic load: Expanding the discourse on stress and cardiovascular disease. J. Clin. Nurs. 2008, 17, 201–208. [Google Scholar] [PubMed]
- Maninger, N.; Wolkowitz, O.M.; Reus, V.I.; Epel, E.S.; Mellon, S.H. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS). Front. Neuroendocrinol. 2009, 30, 65–91. [Google Scholar] [CrossRef]
- Saczawa, M.E.; Graber, J.A.; Brooks–Gunn, J.; Warren, M.P. Methodological considerations in use of the cortisol/DHEA(S) ratio in adolescent populations. Psychoneuroendocrinology 2013, 38, 2815–2819. [Google Scholar] [CrossRef]
- Gabai, G.; Mongillo, P.; Giaretta, E.; Marinelli, L. Do Dehydroepiandrosterone (DHEA) and Its Sulfate (DHEAS) Play a Role in the Stress Response in Domestic Animals? Front. Vet. Sci. 2020, 7, 588835. [Google Scholar]
- Kamin, H.S.; Kertes, D.A. Cortisol and DHEA in development and psychopathology. Horm. Behav. 2017, 89, 69–85. [Google Scholar]
- Wingfield, J.C. The concept of allostasis: Coping with a capricious environment. J. Mammal. 2005, 86, 248–254. [Google Scholar]
- Dickens, M.J.; Romero, L.M. A consensus endocrine profile for chronically stressed wild animals does not exist. Gen. Comp. Endocr. 2013, 191, 177–189. [Google Scholar] [PubMed]
- Miller, G.; Chen, E.; Zhou, E. If it goes up, must it come down? Chronic stress and the hypothalamic–pituitary–adrenocortical axis in humans. Psychol. Bull. 2007, 133, 25–45. [Google Scholar] [PubMed]
- Wielebnowski, N. Stress and distress: Evaluating their impact for the well–being of zoo animals. J. Am. Vet. Med. Assoc. 2003, 223, 973–977. [Google Scholar]
- Guilliams, T.G.; Edwards, L. Chronic stress and the HPA axis: Clinical assessment and therapeutic considerations. Standard 2010, 9, 1–12. [Google Scholar]
- Colditz, I.; Hine, B. Resilience in farm animals: Biology, management, breeding and implications for animal welfare. Anim. Prod. Sci. 2016, 56, 1961–1983. [Google Scholar]
- Bergamin, C.; Comin, A.; Corazzin, M.; Faustini, M.; Peric, T.; Scollo, A.; Gottardo, F.; Montillo, M.; Prandi, A. Cortisol, DHEA, and sexual steroid concentrations in fattening pigs’ hair. Animals 2019, 9, 345. [Google Scholar] [CrossRef] [PubMed]
- Montillo, M.; Rota Nodari, S.; Peric, T.; Polloni, A.; Corazzin, M.; Bergamin, C.; Balestrieri, A.; Prandi, A.; Comin, A. Steroids in pig hair and welfare evaluation systems: Combined approaches to improve management in pig breeding? Vet. Ital. 2020, 56, 177–184. [Google Scholar]
- Peric, T.; Mazzoni, C.; Quai, F.; Cotticelli, A.; Pividori, I.; Corazzin, M.; Comin, A.; Bresciani, C.; Prandi, A. Sow’s pre– and post–delivery in different confinement systems evaluated by hair hormones concentrations. Livest. Sci. 2023, 272, 105235. [Google Scholar]
- Schurr, M.J.; Fabian, T.C.; Croce, M.A.; Varnavas, L.E.; Proctor, K.G. Dehydroepiandrosterone, an endogenous immune modulator, after traumatic shock. Shock 1997, 7, 55–59. [Google Scholar]
- Council Directive 2008/120/EC of 18 December 2008 Laying Down Minimum Standards for the Protection of Pigs (Codified Version). Available online: https://eur-lex.europa.eu/eli/dir/2008/120/oj/eng (accessed on 31 March 2025).
- Carr, J.; Howells, M.; Hersey, W.; McKelvey, R.; Couto, S. Capturing batching options in pig farms. Livestock 2023, 28, 168–171. [Google Scholar]
- Scollo, A.; Levallois, P.; Fourichon, C.; Motta, A.; Mannelli, A.; Lombardo, F.; Ferrari, P. Monitoring Means and Results of Biosecurity in Pig Fattening Farms: Systematic Assessment of Measures in Place and Exploration of Biomarkers of Interest. Animals 2022, 12, 2655. [Google Scholar] [CrossRef]
- Park, C.; Kang, B. Studies on exudative epidermitis in pigs; isolation and some properties of Staphylococcus hyicus subsp. hyicusfrom diseased and healthy pigs. Kor. J. Vet. Res. 1986, 26, 251–257. [Google Scholar]
- Levallois, P.; Leblanc–Maridor, M.; Lehébel, A.; Gavaud, S.; Lieubeau, B.; Hervé, J.; Fourichon, C.; Belloc, C. Hair cortisol concentration in finishing pigs on commercial farms: Variability between pigs, batches, and farms. Front. Vet. Sci. 2024, 10, 1298756. [Google Scholar]
- Fusi, J.; Comin, A.; Faustini, M.; Prandi, A.; Veronesi, M.C. The usefulness of claws collected without invasiveness for cortisol and dehydroepiandrosterone (sulfate) monitoring in healthy newborn puppies after birth. Theriogenology 2018, 122, 137–143. [Google Scholar]
- Tsekouras, N.; Meletis, E.; Kostoulas, P.; Labronikou, G.; Athanasakopoulou, Z.; Christodoulopoulos, G.; Billinis, C.; Papatsiros, V.G. Detection of enterotoxigenic escherichia coli and clostridia in the aetiology of neonatal piglet diarrhoea: Important factors for their prevention. Life 2023, 13, 1092. [Google Scholar] [CrossRef]
- Segura, M.; Aragon, V.; Brockmeier, S.L.; Gebhart, C.; Greeff, A.D.; Kerdsin, A.; O’Dea, M.A.; Okura, M.; Saléry, M.; Schultsz, C.; et al. Update on Streptococcus suis Research and Prevention in the Era of Antimicrobial Restriction: 4th International Workshop on S. suis. Pathogens 2020, 9, 374. [Google Scholar] [CrossRef]
- Victor, I.; Akwuobu, C.A.; Akinleye, O.A.; Tyagher, J.A.; Buba, E. Management of exudative epidermitis (greasy pig disease) in 4 week old piglets. J. Vet. Med. Anim. Health 2013, 5, 180–185. [Google Scholar]
- Zoric, M.; Schmidt, U.; Wallenbeck, A.; Wallgren, P. Lameness in piglets–should pain killers be included at treatment? Porc. Health Manag. 2016, 2, 8. [Google Scholar]
- Russell, E.; Koren, G.; Rieder, M.; Van Uum, S. Hair cortisol as a biological marker of chronic stress: Current status, future directions and unanswered questions. Psychoneuroendocrinology 2012, 37, 589–601. [Google Scholar] [PubMed]
- Bechshøft, T.Ø.; Sonne, C.; Dietz, R.; Born, E.W.; Novak, M.A.; Henchey, E.; Meyer, J.S. Cortisol levels in hair of East Greenland polar bears. Sci. Total Environ. 2011, 409, 831–834. [Google Scholar] [PubMed]
- Caslini, C.; Comin, A.; Peric, T.; Prandi, A.; Pedrotti, L.; Mattiello, S. Use of hair cortisol analysis for comparing population status in wild red deer (Cervus elaphus) living in areas with di erent characteristics. Eur. J. Wildl. Res. 2016, 62, 713–723. [Google Scholar]
- Meyer, J.S.; Novak, M.A. Minireview: Hair cortisol: A novel biomarker of hypothalamic–pituitary–adrenocortical activity. Endocrinology 2012, 153, 4120–4127. [Google Scholar]
- Mowafy, M.; Cassens, R.G. Hair growth in the domestic pig. Quantitative aspects. J. Am. Leather Chem. Assoc. 1976, 71, 71–78. [Google Scholar]
- Watson, S.A.; Moore, G.P. Postnatal development of the hair cycle in the domestic pig. J. Anat. 1990, 170, 1–9. [Google Scholar]
- Whitham, J.C.; Bryant, J.L.; Miller, L.J. Beyond glucocorticoids: Integrating dehydroepiandrosterone (DHEA) into animal welfare research. Animals 2020, 10, 1381. [Google Scholar] [CrossRef] [PubMed]
- Herdt, T.H.; Wisnieski, L.; Buchweitz, J. Random-effects linear model application to herd-level assessment of bovine hepatic trace mineral concentrations. J. Vet. Diagn. Investig. 2021, 33, 469–478. [Google Scholar]
- Sapolsky, R.M.; Krey, L.C.; Mc Ewen, B.S. The neuroendocrinology of stress and aging: The glucocorticoid cascade hypothesis. Endocr. Rev. 1986, 7, 284–301. [Google Scholar] [PubMed]
- Luz, C.; Dornelles, F.; Preissler, T.; Collaziol, D.; da Cruz, I.M.; Bauer, M.E. Impact of psychological and endocrine factors on cytokine production of healthy elderly people. Mech. Ageing Dev. 2003, 124, 887–895. [Google Scholar]
- Sapolsky, R.M.; Altmann, J. Incidence of hypercortisolism and dexamethasone resistance increases with age among wild baboons. Biol. Psychiatry 1991, 30, 1008–1016. [Google Scholar]
- O’Brien, J.K.; Steinman, K.J.; Fetter, G.A.; Robeck, T.R. Androgen and glucocorticoid production in the male killer whale (Orcinus orca): Influence of age, maturity, and environmental factors. Andrology 2017, 5, 180–190. [Google Scholar]
- Peric, T.; Comin, A.; Montillo, M.; Spigarelli, C.; Corazzin, M.; Cotticelli, A.; Prandi, A. Postnatal and postweaning endocrine setting in dairy calves through hair cortisol, dehydroepiandrosterone and dehydroepiandrosterone sulphate. Agric. Nat. Resour. 2022, 56, 867–876. [Google Scholar]
- Uetake, K.; Akiyama, K.; Tanaka, T. Relationship between stress levels of the antepartum cow and her newborn calf. Anim. Sci. J. 2014, 85, 81–84. [Google Scholar]
- Strong, R.A.; Silva, E.B.; Cheng, H.W.; Eicher, S.D. Acute brief heat stress in late gestation alters neonatal calf innate immune functions. J. Dairy Sci. 2015, 98, 7771–7783. [Google Scholar]
- Tang, X.; Xiong, K.; Fang, R.; Li, M. Weaning stress and intestinal health of piglets: A review. Front. Immunol. 2022, 13, 1042778. [Google Scholar]
- Vardaxis, N.J.; Brans, T.A.; Boon, M.E.; Kreis, R.W.; Marres, L.M. Confocal laser scanning microscopy of porcine skin: Implications for human wound healing studies. J. Anat. 1997, 190, 601–611. [Google Scholar]
- Fustini, M.; Galeati, G.; Gabai, G.; Mammi, L.E.; Bucci, D.; Baratta, M.; Accorsi, P.A.; Formigoni, A. Overstocking dairy cows during the dry period a ects dehydroepiandrosterone and cortisol secretion. J. Dairy Sci. 2017, 100, 620–628. [Google Scholar]
- Lennartsson, A.K.; Kushnir, M.M.; Bergquist, J.; Jonsdottir, I.H. DHEA and DHEA–S response to acute psychosocial stress in healthy men and women. Biol. Psychol. 2012, 90, 143–149. [Google Scholar] [PubMed]
- Skarlandtová, H.; Bičíková, M.; Neužil, P.; Mlček, M.; Hrachovina, V.; Svoboda, T.; Medová, E.; Kudlička, J.; Dohnalová, A.; Havránek, Š.; et al. Are there any dierences between stress hormone levels in non–stress conditions and in potentional stress overload (heart catheterisation) in sows? Physiol. Res. 2014, 63, 733–741. [Google Scholar] [CrossRef]
- Straub, R.H.; Lehle, K.; Herfarth, H.; Weber, M.; Falk, W.; Preuner, J.; Scholmerich, J. Dehydroepiandrosterone in relation to other adrenal hormones during an acute inflammatory stressful disease state compared with chronic inflammatory disease: Role of interleukin–6 and tumour necrosis factor. Eur. J. Endocrinol. 2002, 146, 365–374. [Google Scholar] [PubMed]
- Sugaya, N.; Izawa, S.; Ogawa, N.; Shirotsuki, K.; Kobayashi, H.; Yamada, K.C.; Tsumura, H.; Nomura, S.; Shimada, H. Effect of day–to–day variations in adrenal cortex hormone levels on abdominal symptoms. Biopsychosoc. Med. 2010, 4, 2. [Google Scholar] [CrossRef]
- Sosvorova, L.; Hill, M.; Mohapl, M.; Vitku, J.; Hampl, R. Steroid hormones in prediction of normal pressure hydrocephalus. J. Steroid Biochem. Mol. Biol. 2015, 152, 124–132. [Google Scholar] [PubMed]
- Seele, J.; Tauber, S.C.; Bunkowski, S.; Baums, C.G.; Valentin–Weigand, P.; de Buhr, N.; Beineke, A.; Iliev, A.I.; Brück, W.; Nau, R. The inflammatory response and neuronal injury in Streptococcus suis meningitis. BMC Infect. Dis. 2018, 18, 297. [Google Scholar]
- Dimitrova, A.; Yordanov, S. Exudative epidermitis in pigs/Greasy pig disease. Životnov Dni Nauki 2022, 59, 63–69. [Google Scholar]
- Rutkowski, K.; Sowa, P.; Rutkowska–Talipska, J.; Kuryliszyn–Moskal, A.; Rutkowski, R. Dehydroepiandrosterone (DHEA): Hypes and hopes. Drugs 2014, 74, 1195–1207. [Google Scholar] [CrossRef]
- Nouveau, S.; Bastien, P.; Baldo, F.; de Lacharriere, O. Effects of topical DHEA on aging skin: A pilot study. Maturitas 2008, 59, 174–181. [Google Scholar]
- Tagliolatto, S.; Alchorne, M.; Enokihara, M. Sebaceous hyperplasia: A pilot study to correlate this skin disease with circulating androgen levels. An. Bras. Dermatol. 2011, 86, 917–923. [Google Scholar] [PubMed]
- O’Driscoll, K.; McCabe, M.; Earley, B. Differences in leukocyte profile, gene expression, and metabolite status of dairy cows with or without sole ulcers. J. Dairy Sci. 2015, 98, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- O’Driscoll, K.; McCabe, M.; Earley, B. Leukocyte profile, gene expression, acute phase response, and metabolite status of cows with sole hemorrhages. J. Dairy Sci. 2017, 100, 9382–9391. [Google Scholar] [CrossRef]
- Sprecher, D.J.; Hostetler, D.E.; Kaneene, J. A lameness scoring system that uses posture and gait to predict dairy cattle reproductive performance. Theriogenology 1997, 47, 1179–1187. [Google Scholar] [PubMed]
- Jurkovich, V.; Bakony, M.; Laky, E.; Ruff, F.; Kézér, F.L.; Bende, A.; Kovács, L. Cardiac vagal tone, plasma cortisol, and dehydroepiandrosterone response to an ACTH challenge in lame and nonlame dairy cows. Domest. Anim. Endocrinol. 2020, 71, 106388. [Google Scholar]
- Ytrehus, B.; Carlson, C.S.; Lundeheim, N.; Mathisen, L.; Reinholt, F.P.; Teige, J.; Ekman, S. Vascularisation and osteochondrosis of the epiphyseal growth cartilage of the distal femur in pigs—Development with age, growth rate, weight and joint shape. Bone 2004, 34, 454–465. [Google Scholar]
- Lanci, A.; Mariella, J.; Ellero, N.; Faoro, A.; Peric, T.; Prandi, A.; Freccero, F.; Castagnetti, C. Hair Cortisol and DHEA–S in Foals and Mares as a Retrospective Picture of Feto–Maternal Relationship under Physiological and Pathological Conditions. Animals 2022, 12, 1266. [Google Scholar] [CrossRef]
- Comin, A.; Peric, T.; Corazzin, M.; Veronesi, M.C.; Meloni, T.; Zufferli, V.; Cornacchia, G.; Prandi, A. Hair cortisol as a marker of hypothalamic–pituitary–adrenal axis activation in Friesian dairy cows clinically or physiologically compromised. Livest. Sci. 2013, 152, 36–41. [Google Scholar]
- Wu, T.T.; Chen, Y.; Zhou, Y.; Adi, D.; Zheng, Y.Y.; Liu, F.; Ma, Y.T.; Xie, X. Prognostic value of dehydroepiandrosterone sulfate for patients with cardiovascular disease: A systematic review and meta-analysis. J. Am. Heart Assoc. 2017, 6, e004896. [Google Scholar]

| Clinical Syndrome | Laboratory Analyses and Diagnosis | Threshold to Classify the Batch as Clinically Affected (Score 1) |
|---|---|---|
| Enteric | Bacteriology on rectal samples isolated Escherichia coli. Colonies surrounded by a zone of lysis after overnight growth at 37 °C on blood agar were classified as haemolytic, and detection of virulence factor genes F18, STa, and STb was obtained by PCR | More than 10% of piglets with profuse yellowish diarrhoea |
| Neurological | Bacteriological analysis of brain samples (cultured on blood agar supplemented with NAD and Gassner agar) led to the isolation of Streptococcus suis | More than 0.6% of piglets with neurological signs |
| Cutaneous | Bacteriology from skin wounds or pus (cultured on MacConkey agar) allowed to isolate Staphylococcus spp. The identification of S. hyicus was obtained by PCR (diagnosis: exudative epidermitis or “Greasy pig disease” [39]) | More than 5% of piglets with exudative epidermitis |
| Locomotor | Bacteriology from intra–articular fluid (cultured on MacConkey agar) allowed to isolate Staphylococcus spp. The identification of S. hyicus was obtained by PCR | More than 1.5% of piglets with at least one leg with signs of inflammation without traumas. |
| Clinical Index | – | Presence of more than one clinical syndrome scored 1 |
| Factors | Cortisol, pg/mg | DHEA(S), pg/mg | Cortisol/DHEA(S) Ratio × 100 | ||||
|---|---|---|---|---|---|---|---|
| Weaning | Finishing | Weaning | Finishing | Weaning | Finishing | ||
| Enteric score | 0 | 17.18 ± 1.05 | 8.28 ± 1.05 | 23.15 ± 1.54 * | 23.88 ± 1.34 | 73.73 ± 1.08 | 41.07 ± 1.07 * |
| 1 | 13.30 ± 1.04 | 9.33 ± 1.02 | 13.84 ± 1.16 * | 17.93 ± 0.64 | 105.62 ± 1.05 | 64.96 ± 1.03 * | |
| Neurological score | 0 | 14.68 ± 1.03 | 9.05 ± 1.02 | 18.41 ± 1.01 | 20.44 ± 0.68 | 86.57 ± 1.05 ** | 57.03 ± 1.03 |
| 1 | 14.35 ± 1.08 | 9.39 ± 1.04 | 10.47 ± 2.43 | 15.35 ± 1.09 | 138.53 ± 1.13 ** | 68.14 ± 1.05 | |
| Cutaneous score | 0 | 14.69 ± 1.04 | 9.23 ± 1.03 | 15.83 ± 1.07 * | 22.13 ± 0.82 | 102.13 ± 1.05 *** | 52.06 ± 1.04 |
| 1 | 14.47 ± 1.06 | 9.08 ± 1.03 | 22.11 ± 1.88 * | 16.24 ± 0.82 | 66.89 ± 1.10 *** | 67.85 ± 1.04 | |
| Locomotor score | 0 | 15.03 ± 1.05 | 9.34 ± 1.03 | 18.34 ± 1.48 | 16.52 ± 0.78 * | 87.87 ± 1.08 | 68.54 ± 1.04 ** |
| 1 | 14.40 ± 1.04 | 8.83 ± 1.03 | 16.69 ± 1.19 | 22.35 ± 0.86 * | 95.22 ± 1.06 | 49.92 ± 1.04 ** | |
| Clinical index | 0 | 18.67 ± 1.06 | 7.99 ± 1.02 | 21.27 ± 1.89 | 22.64 ± 1.53 | 92.96 ± 1.10 | 40.52 ± 1.08 |
| 1 | 13.51 ± 1.04 | 9.33 ± 1.02 | 16.02 ± 1.06 | 18.41 ± 0.62 | 92.09 ± 1.05 | 63.62 ± 1.03 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scollo, A.; Cotticelli, A.; Peric, T.; Perrucci, A.; Prandi, A.; Ferrari, P. Hair Dehydroepiandrosterone Sulfate (DHEA(S)) and Cortisol/DHEA(S) Ratio as Long-Lasting Biomarkers of Clinical Syndromes Exhibited by Piglets Early in Life. Animals 2025, 15, 1032. https://doi.org/10.3390/ani15071032
Scollo A, Cotticelli A, Peric T, Perrucci A, Prandi A, Ferrari P. Hair Dehydroepiandrosterone Sulfate (DHEA(S)) and Cortisol/DHEA(S) Ratio as Long-Lasting Biomarkers of Clinical Syndromes Exhibited by Piglets Early in Life. Animals. 2025; 15(7):1032. https://doi.org/10.3390/ani15071032
Chicago/Turabian StyleScollo, Annalisa, Alessio Cotticelli, Tanja Peric, Alice Perrucci, Alberto Prandi, and Paolo Ferrari. 2025. "Hair Dehydroepiandrosterone Sulfate (DHEA(S)) and Cortisol/DHEA(S) Ratio as Long-Lasting Biomarkers of Clinical Syndromes Exhibited by Piglets Early in Life" Animals 15, no. 7: 1032. https://doi.org/10.3390/ani15071032
APA StyleScollo, A., Cotticelli, A., Peric, T., Perrucci, A., Prandi, A., & Ferrari, P. (2025). Hair Dehydroepiandrosterone Sulfate (DHEA(S)) and Cortisol/DHEA(S) Ratio as Long-Lasting Biomarkers of Clinical Syndromes Exhibited by Piglets Early in Life. Animals, 15(7), 1032. https://doi.org/10.3390/ani15071032






