Divergent Embryo Responses to Chemical Cues in Two Freshwater Fishes with Different Parental Care Strategies
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Test Subjects
2.2. Reproduction of Zebrafish and Nile Tilapia
2.3. Cultivation of Embryos
2.4. Treatment Preparation
2.5. Determination of Embryo Heart Rate in Zebrafish
2.6. Determination of Embryo Hatching Performance in Zebrafish
2.7. Determination of Embryo Heart Rate and Hatching Performance in Nile Tilapia
2.8. Statistical Analysis
3. Results
3.1. Zebrafish Embryonic Heart Rate Responses to Chemical Cues
3.2. Zebrafish Hatching Performance Responses to Chemical Cues
3.3. Nile Tilapia Embryonic Heart Rate Responses to Chemical Cues
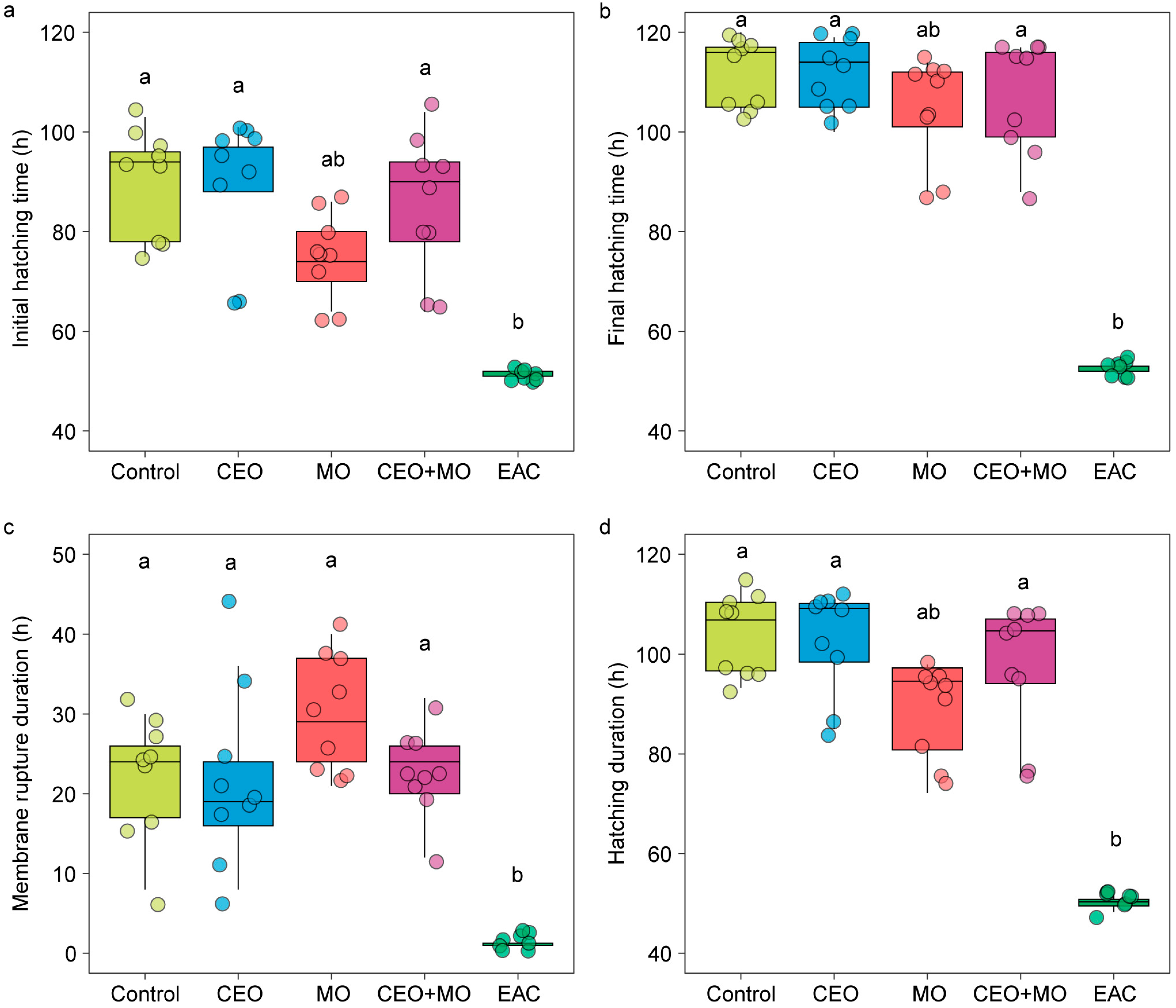
3.4. Nile Tilapia Hatching Performance Responses to Chemical Cues
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bradbury, J.W.; Vehrencamp, S.L. Principles of Animal Communication; Sinauer: Sunderland, MA, USA, 1998. [Google Scholar]
- Johansson, B.G.; Jones, T.M. The role of chemical communication in mate choice. Biol. Rev. 2007, 82, 265–289. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Elvidge, C.K.; Cooke, S.J. Niche separation, ontogeny, and heterospecific alarm responses in centrarchid sunfish. Behav. Ecol. 2018, 29, 862–868. [Google Scholar] [CrossRef]
- Zhang, N.; Elvidge, C.K.; Li, Q.; Fu, S.; Xia, J. Does mutualism provide additional indirect benefits? Behavioral indicators of chemical communication in a temporally dynamic fish-mussel mutualism. Behav. Ecol. Sociobiol. 2024, 78, 21. [Google Scholar] [CrossRef]
- Taga, M.E.; Bassler, B.L. Chemical communication among bacteria. Proc. Natl. Acad. Sci. USA 2003, 100, 14549–14554. [Google Scholar] [CrossRef]
- van Dam, N.M.; Bouwmeester, H.J. Metabolomics in the Rhizosphere: Tapping into belowground chemical communication. Trends Plant Sci. 2016, 21, 256–265. [Google Scholar] [CrossRef]
- Barriuso, J.; Hogan, D.A.; Keshavarz, T.; Martínez, M.J. Role of quorum sensing and chemical communication in fungal biotechnology and pathogenesis. FEMS Microbiol. Rev. 2018, 42, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Achtymichuk, G.H.; Crane, A.L.; Wrynn, T.E.; Ferrari, M.C.O. Exploring the potency and replenishment of woodfrog disturbance cues, a nonspecific communication system in aquatic species. Anim. Behav. 2025, 219, 123034. [Google Scholar] [CrossRef]
- Breithaupt, T.; Thiel, M. (Eds.) Chemical Communication in Crustaceans; Springer Science & Business Media: New York, NY, USA, 2011. [Google Scholar]
- Chaverri, G.; Ancillotto, L.; Russo, D. Social communication in bats. Biol. Rev. 2018, 93, 1938–1954. [Google Scholar] [CrossRef]
- Singletary, B.; Tecot, S. Multimodal pair-bond maintenance: A review of signaling across modalities in pair-bonded nonhuman primates. Am. J. Primatol. 2020, 82, e23105. [Google Scholar] [CrossRef]
- Xia, J.; Peng, M.; Huang, Y.; Elvidge, C.K. Acute warming in winter eliminates chemical alarm responses in threatened Qinling lenok Brachymystax lenok tsinlingensis. Sci. Total Environ. 2021, 764, 142807. [Google Scholar] [CrossRef]
- Brusseau, A.J.P.; Feyten, L.E.A.; Crane, A.L.; Ramnarine, I.W.; Ferrari, M.C.O.; Brown, G.E. Antipredator decisions of male Trinidadian guppies (Poecilia reticulata) depend on social cues from females. Curr. Zool. 2025, 71, 205–211. [Google Scholar] [CrossRef]
- Smith, R.J.F. Alarm signals in fishes. Rev. Fish Biol. Fish. 1992, 2, 33–63. [Google Scholar] [CrossRef]
- Ferrari, M.C.O.; Elvidge, C.K.; Jackson, C.D.; Chivers, D.P.; Brown, G.E. The responses of prey fish to temporal variation in predation risk: Sensory habituation or risk assessment? Behav. Ecol. 2010, 21, 532–536. [Google Scholar] [CrossRef]
- Xia, J.; Cheng, M.; Cai, R.; Fu, S.; Cooke, S.J.; Elvidge, C.K. Ontogenetic changes in chemical alarm cue recognition and fast-start performance in guppies (Poecilia reticulata). Ethology 2017, 123, 916–923. [Google Scholar] [CrossRef]
- Chung-Davidson, Y.W.; Huertas, M.; Li, W.M. A review of research in fish pheromones. In Chemical Communication in Crustaceans; Breithaupt, T., Thiel, M., Eds.; Springer: New York, NY, USA, 2010; pp. 467–482. [Google Scholar]
- Elvidge, C.K.; Brown, G.E. Size-based differences determine the contextual value of risky information in heterospecific information use. Anim. Behav. 2015, 102, 7–14. [Google Scholar] [CrossRef]
- Brönmark, C.; Hansson, L. Chemical communication in aquatic systems: An introduction. Oikos 2000, 88, 103–109. [Google Scholar] [CrossRef]
- Burks, R.L.; Lodge, D.M. Cued in: Advances and opportunities in freshwater chemical ecology. J. Chem. Ecol. 2002, 28, 1901–1917. [Google Scholar] [CrossRef] [PubMed]
- Scrimgeour, G.J.; Culp, J.M. Foraging and evading predators: The effect of predator species on a behavioural trade-off by a lotic mayfly. Oikos 1994, 69, 71. [Google Scholar] [CrossRef]
- Cowlishaw, G. Trade-offs between foraging and predation risk determine habitat use in a desert baboon population. Anim. Behav. 1997, 53, 667–686. [Google Scholar] [CrossRef]
- Lima, S.L.; Dill, L.M. Behavioral decisions made under the risk of predation: A review and prospectus. Can. J. Zool. 1990, 68, 619–640. [Google Scholar] [CrossRef]
- Sih, A. Prey uncertainty and the balancing of antipredator and feeding needs. Am. Nat. 1992, 139, 1052–1069. [Google Scholar] [CrossRef]
- Smith, R.J.F. Avoiding and deterring predators. In Behavioural Ecology of Teleost Fishes; JGodin, J.-G., Ed.; Oxford University Press: Oxford, UK, 1997; pp. 163–190. [Google Scholar] [CrossRef]
- Lima, S.L.; Bednekoff, P.A. Temporal variation in danger drives antipredator behavior: The predation risk allocation hypothesis. Am. Nat. 1999, 153, 649–659. [Google Scholar] [CrossRef]
- Walzer, A.; Schausberger, P. Phenotypic plasticity in anti-intraguild predator strategies: Mite larvae adjust their behaviours according to vulnerability and predation risk. Exp. Appl. Acarol. 2013, 60, 95–115. [Google Scholar] [CrossRef]
- Baras, E.; Arifin, O.Z.; Slembrouck, J.; Subagja, J.; Kristanto, A.H.; Legendre, M. Oil globule size in fish eggs: A matter of biome and reproductive strategy. Fish Fish. 2018, 19, 996–1002. [Google Scholar] [CrossRef]
- Teletchea, S.; Teletchea, F. STOREFISH 2.0: A database on the reproductive strategies of teleost fishes. Database 2020, 2020, baaa095. [Google Scholar] [CrossRef]
- Xia, J.; Deng, C.; Zheng, X.; Huang, Y.; Elvidge, C.K.; Fu, S. Differential effects of parental and developmental temperatures on larval thermal adaptation in oviparous and viviparous model fish species. J. Therm. Biol. 2023, 117, 103695. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.; Yu, H.; Zhong, J.; Qu, M.; Zhang, Y.; Shan, B.; Qin, G.; Zhang, H.; Huang, L.; et al. Mouthbrooding behavior and sexual immune dimorphism in Indian perch Jaydia lineata. Innov. Life 2024, 2, 100066-12. [Google Scholar] [CrossRef]
- La Mesa, M.; Llompart, F.; Riginella, E.; Eastman, J.T. Parental care and reproductive strategies in notothenioid fishes. Fish Fish. 2021, 22, 356–376. [Google Scholar] [CrossRef]
- Butler, J.M.; Herath, E.M.; Rimal, A.; Whitlow, S.M.; Maruska, K.P. Galanin neuron activation in feeding, parental care, and infanticide in a mouthbrooding African cichlid fish. Horm. Behav. 2020, 126, 104870. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Shine, R. The behavioural and physiological ecology of embryos: Responding to the challenges of life inside an egg. Biol. Rev. 2022, 97, 1272–1286. [Google Scholar] [CrossRef]
- Blaser, R.E.; Chadwick, L.; McGinnis, G.C. Behavioral measures of anxiety in zebrafish (Danio rerio). Behav. Brain Res. 2010, 208, 56–62. [Google Scholar] [CrossRef]
- Ulloa, P.E.; Iturra, P.; Neira, R.; Araneda, C. Zebrafish as a model organism for nutrition and growth: Towards comparative studies of nutritional genomics applied to aquacultured fishes. Rev. Fish Biol. Fish. 2011, 21, 649–666. [Google Scholar] [CrossRef]
- Brennan, C.H.; Parker, M.O. Zebrafish (Danio rerio) models of substance abuse: Harnessing the capabilities. Behaviour 2012, 149, 1037–1062. [Google Scholar] [CrossRef]
- Geletu, T.T.; Zhao, J. Genetic resources of Nile tilapia (Oreochromis niloticus Linnaeus, 1758) in its native range and aquaculture. Hydrobiologia 2023, 850, 2425–2445. [Google Scholar] [CrossRef]
- Podgorniak, T.; Brockmann, S.; Konstantinidis, I.; Fernandes, J.M.O. Differences in the fast muscle methylome provide insight into sex-specific epigenetic regulation of growth in Nile tilapia during early stages of domestication. Epigenetics 2019, 14, 818–836. [Google Scholar] [CrossRef]
- Longrie, N.; Poncin, P.; Denoël, M.; Gennotte, V.; Delcourt, J.; Parmentier, E. Behaviours associated with acoustic communication in Nile tilapia (Oreochromis niloticus). PLoS ONE 2013, 8, e61467. [Google Scholar] [CrossRef]
- Westerfield, M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio); University of Oregon Press: Eugene, OR, USA, 1995. [Google Scholar]
- Wood, S.N. Stable and efficient multiple smoothing parameter estimation for generalized additive models. Am. Stat. Assoc. 2004, 99, 673–686. [Google Scholar] [CrossRef]
- Pinheiro, J. nlme: Linear and Nonlinear Mixed Effects Models, R package Version 3; R Core Team: Vienna, Austria, 2011.
- Griffiths, S.W.; Magurran, A.E. Schooling preferences for familiar fish vary with group size in a wild guppy population. Proc. R. Soc. B 1997, 264, 547–551. [Google Scholar] [CrossRef]
- Hesse, S.; Bakker, T.C.M.; Baldauf, S.A.; Thünken, T. Kin recognition by phenotype matching is family- rather than self-referential in juvenile cichlid fish. Anim. Behav. 2012, 84, 451–457. [Google Scholar] [CrossRef]
- Wells, M.W.; Wright, P.A. Do not eat your kids: Embryonic kin recognition in an amphibious fish. Behav. Ecol. Sociobiol. 2017, 71, 140. [Google Scholar] [CrossRef]
- Ray, E.J.; Maruska, K.P. Sensory mechanisms of parent-offspring recognition in fishes, amphibians, and reptiles. Integr. Comp. Biol. 2023, 63, 1168–1181. [Google Scholar] [CrossRef]
- Atherton, J.A.; McCormick, M.I. Active in the sac: Damselfish embryos use innate recognition of odours to learn predation risk before hatching. Anim. Behav. 2015, 103, 1–6. [Google Scholar] [CrossRef]
- Mourabit, S.; Rundle, S.D.; Spicer, J.I.; Sloman, K.A. Alarm substance from adult zebrafish alters early embryonic development in offspring. Biol. Lett. 2010, 6, 525–528. [Google Scholar] [CrossRef]
- Wisenden, B.D.; Paulson, D.C.; Orr, M. Zebrafish embryos hatch early in response to chemical and mechanical indicators of predation risk, resulting in underdeveloped swimming ability of hatchling larvae. Biol. Open 2022, 11, bio059229. [Google Scholar] [CrossRef]
- Ward, A.J.W.; Hart, P.J.B. The effects of kin and familiarity on interactions between fish. Fish Fish. 2003, 4, 348–358. [Google Scholar] [CrossRef]
- Auld, H.L.; Noakes, D.L.G.; Banks, M.A. Advancing mate choice studies in salmonids. Rev. Fish Biol. Fish. 2019, 29, 249–276. [Google Scholar] [CrossRef]
- Meldrum, D.R.; Casper, R.F.; Diez-Juan, A.; Simon, C.; Domar, A.D.; Frydman, R. Aging and the environment affect gamete and embryo potential: Can we intervene? Fertil. Steril. 2016, 105, 548–559. [Google Scholar] [CrossRef] [PubMed]
- Burford, B.P.; Wild, L.A.; Schwarz, R.; Chenoweth, E.M.; Sreenivasan, A.; Elahi, R.; Carey, N.; Hoving, H.-J.T.; Straley, J.M.; Denny, M.W. Rapid range expansion of a marine ectotherm reveals the demographic and ecological consequences of short-term variability in seawater temperature and dissolved oxygen. Am. Nat. 2022, 199, 523–550. [Google Scholar] [CrossRef]
- Candolin, U.; Goncalves, S.; Pant, P. Delayed early life effects in the threespine stickleback. Proc. R. Soc. B 2022, 289, 20220554. [Google Scholar] [CrossRef] [PubMed]
- Keller-Costa, T.; Canário, A.V.M.; Hubbard, P.C. Chemical communication in cichlids: A mini-review. Gen. Comp. Endocrinol. 2015, 221, 64–74. [Google Scholar] [CrossRef]
- Maruska, K.P.; Butler, J.M. Reproductive-and social-state plasticity of multiple sensory systems in a cichlid fish. Integr. Comp. Biol. 2021, 61, 249–268. [Google Scholar] [CrossRef] [PubMed]
- Roff, D.A. The evolution of threshold traits in animals. Q. Rev. Biol. 1996, 71, 3–35. Available online: https://www.jstor.org/stable/3037828 (accessed on 5 February 2025). [CrossRef]
- Fabre, N.; García-Galea, E.; Vinyoles, D. Parents’ presence affects embryos’ development in Salaria fluviatilis (Asso, 1801), a fish with parental care. Anim. Biol. 2014, 64, 295–309. [Google Scholar] [CrossRef]
- Wu, S.; Zeng, W.; Deng, W.; Li, J.; Li, M.; Tan, L.; Cai, H.; Li, X.; Li, Y.; Zhou, Z. Parental sex and not kinship determines egg cannibalism in Arma custos Fallou (Hemiptera: Pentatomidae: Asopinae). Front. Ecol. Evol. 2022, 10, 758587. [Google Scholar] [CrossRef]
- Thomas, L.K.; Manica, A. Filial cannibalism in an assassin bug. Anim. Behav. 2003, 66, 205–210. [Google Scholar] [CrossRef]
- FitzGerald, G.J. Filial cannibalism in fishes: Why do parents eat their offspring? Trends Ecol. Evol. 1992, 7, 7–10. [Google Scholar] [CrossRef]
- Manica, A. Filial cannibalism in teleost fish. Biol. Rev. 2002, 77, 261–277. [Google Scholar] [CrossRef]
- Mrowka, W. Filial cannibalism and reproductive success in the maternal mouthbrooding cichlid fish Pseudocrenilabrus multicolor. Behav. Ecol. Sociobiol. 1987, 21, 257–265. [Google Scholar] [CrossRef]







| Embryonic Heart Rate | Hatching Performance | |||||||
|---|---|---|---|---|---|---|---|---|
| Species | Difference in Heart Rate | Hatching Rate | Deformation Rate | Mortality | Initial Hatching Time | Final Hatching Time | Membrane Rupture Duration | Hatching Duration |
| Zebrafish | H4 = 53.621 p < 0.0001 | H4 = 1.420 p = 0.841 | H4 = 4.000 p = 0.406 | H4 = 2.232 p = 0.693 | H4 = 16.953 p = 0.002 | H4 = 30.426 p < 0.0001 | F4,65 = 0.335 p = 0.853 | H4 = 26.865 p < 0.0001 |
| Nile tilapia | H4 = 24.063 p < 0.0001 | H4 = 26.171 p < 0.0001 | H4 = 3.070 p = 0.546 | H4 = 25.954 p < 0.0001 | H4 = 24.916 p < 0.0001 | H4 = 22.225 p < 0.001 | F4,44 = 23.472 p < 0.001 | H4 = 25.935 p < 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, N.; Li, Q.; Li, M.; Elvidge, C.K.; Deng, C.; Wang, D.; Fu, S.; Xia, J. Divergent Embryo Responses to Chemical Cues in Two Freshwater Fishes with Different Parental Care Strategies. Animals 2025, 15, 3511. https://doi.org/10.3390/ani15243511
Zhang N, Li Q, Li M, Elvidge CK, Deng C, Wang D, Fu S, Xia J. Divergent Embryo Responses to Chemical Cues in Two Freshwater Fishes with Different Parental Care Strategies. Animals. 2025; 15(24):3511. https://doi.org/10.3390/ani15243511
Chicago/Turabian StyleZhang, Ning, Qinlei Li, Minghui Li, Chris K. Elvidge, Chuke Deng, Deshou Wang, Shijian Fu, and Jigang Xia. 2025. "Divergent Embryo Responses to Chemical Cues in Two Freshwater Fishes with Different Parental Care Strategies" Animals 15, no. 24: 3511. https://doi.org/10.3390/ani15243511
APA StyleZhang, N., Li, Q., Li, M., Elvidge, C. K., Deng, C., Wang, D., Fu, S., & Xia, J. (2025). Divergent Embryo Responses to Chemical Cues in Two Freshwater Fishes with Different Parental Care Strategies. Animals, 15(24), 3511. https://doi.org/10.3390/ani15243511









