Passive Immunity Establishment Through Colostral IgG Absorption in Neonatal Ruminants: Foundation for Efficient Ruminant Production
Simple Summary
Abstract
1. Introduction
2. Passive Immunity Transfer in Neonatal Animals
2.1. Passive Immunity Transfer and Systemic Immunity
2.1.1. Passive Immunity Transfer During the Pre-Natal Period
2.1.2. Passive Immunity Transfer in Both Pre- and Post-Natal Periods
2.1.3. Passive Immunity Transfer During the Postnatal Period
2.2. Passive Immunity Transfer and Local Mucosal Immunity
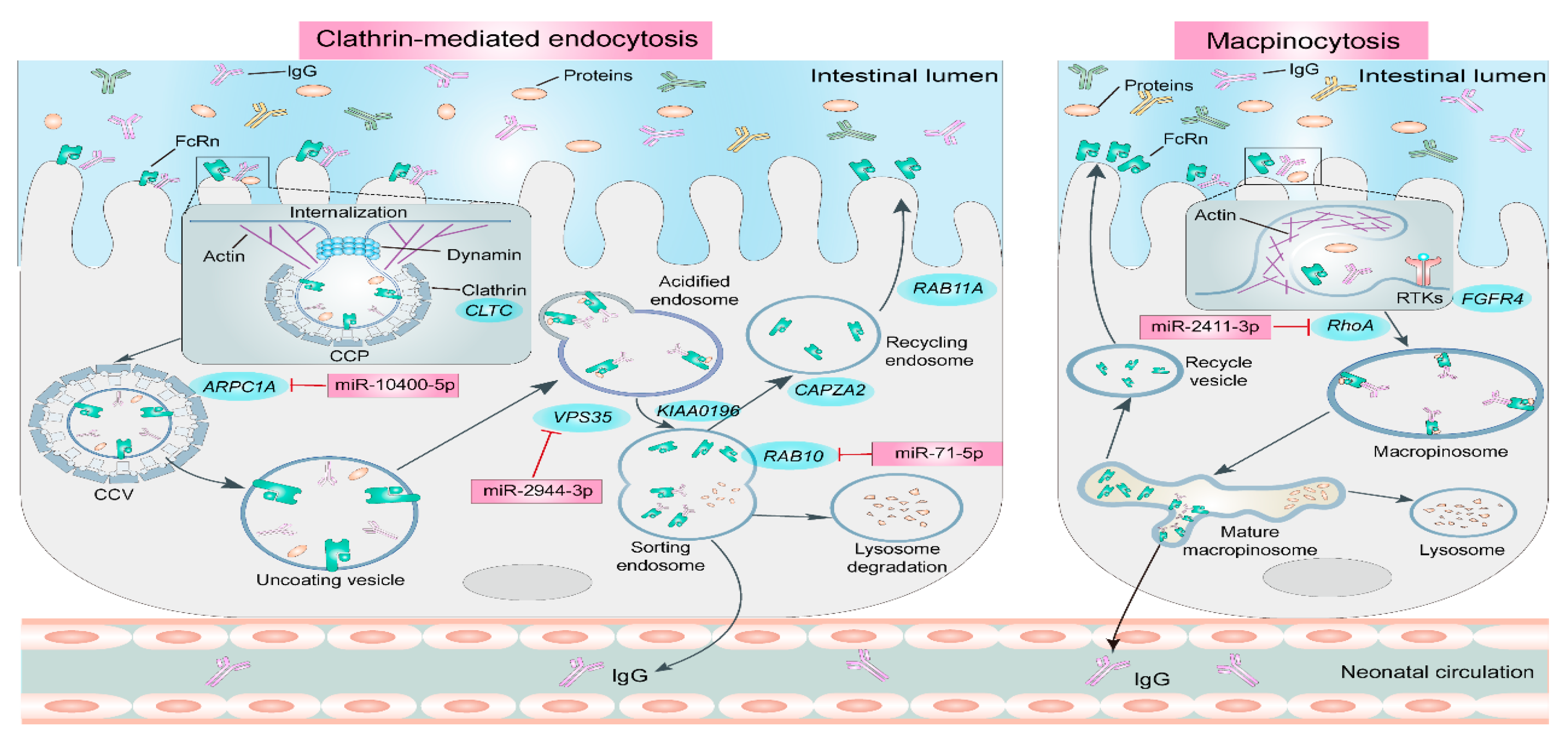
3. Pathways and Molecular Mechanisms of Passive Immunity Establishment in Neonatal Ruminants
4. Factors Influencing the Establishment of Passive Immunity in Neonatal Ruminants
4.1. Colostrum Quality
4.2. Colostrum Feeding Volume
4.3. Colostrum Feeding Time
4.4. Other Influencing Factors
5. Colostrum Feeding, Passive Immunity Establishment and Efficient Production of Ruminants
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vlaicu, P.A.; Gras, M.A.; Untea, A.E.; Lefter, N.A.; Rotar, M.C. Advancing livestock technology: Intelligent systemization for enhanced productivity, welfare, and sustainability. AgriEngineering 2024, 6, 1479–1496. [Google Scholar] [CrossRef]
- Compton, C.; Heuer, C.; Thomsen, P.T.; Carpenter, T.; Phyn, C.; McDougall, S. Invited review: A systematic literature review and meta-analysis of mortality and culling in dairy cattle. J. Dairy Sci. 2017, 100, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zamuner, F.; Carpenter, E.; Arcos-Gómez, G.; Parkinson, A.; Cameron, A.; Leury, B.; DiGiacomo, K. Evaluation of plasma immunoglobulin G and BW thresholds for predicting preweaning mortality in commercially raised dairy goat kids. Animal 2023, 17, 100989. [Google Scholar] [CrossRef]
- Holmøy, I.; Waage, S.; Granquist, E.; L’Abée-Lund, T.; Ersdal, C.; Hektoen, L.; Sørby, R. Early neonatal lamb mortality: Postmortem findings. Animal 2017, 11, 295–305. [Google Scholar] [CrossRef]
- Wang, D.; Liu, K.; Hashan, M. Analysis of factors influencing the mortality rates of xinjiang brown cattle and hostein cattle calves. China Anim. Husb. Vet. Med. 2017, 44, 1363–1368. [Google Scholar]
- Raboisson, D.; Trillat, P.; Cahuzac, C. Failure of passive immune transfer in calves: A meta-analysis on the consequences and assessment of the economic impact. PLoS ONE 2016, 11, e0150452. [Google Scholar] [CrossRef]
- Santos, R.; Cachapa, A.; Carvalho, G.P.; Silva, C.B.; Hernández, L.; Costa, L.; Pereira, L.S.; Minas, M.; Vala, H. Mortality and Morbidity of Beef Calves in Free-Range Farms in Alentejo, Portugal—A Preliminary Study. Vet. Med. Int. 2019, 2019, 3616284. [Google Scholar] [CrossRef] [PubMed]
- Dubrovsky, S.; Van Eenennaam, A.; Karle, B.; Rossitto, P.; Lehenbauer, T.; Aly, S. Epidemiology of bovine respiratory disease (BRD) in preweaned calves on California dairies: The BRD 10K study. J. Dairy Sci. 2019, 102, 7306–7319. [Google Scholar] [CrossRef] [PubMed]
- Godden, S.M.; Lombard, J.E.; Woolums, A.R. Colostrum management for dairy calves. Vet. Clin. N. Am. Food Anim. Pract. 2019, 35, 535. [Google Scholar] [CrossRef] [PubMed]
- Renegar, K.B. Passive immunization: Systemic and mucosal. Mucosal Immunol. 2007, 9, 841–851. [Google Scholar]
- Peter, A.T. Bovine placenta: A review on morphology, components, and defects from terminology and clinical perspectives. Theriogenology 2013, 80, 693–705. [Google Scholar] [CrossRef]
- Lombard, J.; Urie, N.; Garry, F.; Godden, S.; Quigley, J.; Earleywine, T.; McGuirk, S.; Moore, D.; Branan, M.; Chamorro, M. Consensus recommendations on calf-and herd-level passive immunity in dairy calves in the United States. J. Dairy Sci. 2020, 103, 7611–7624. [Google Scholar] [CrossRef]
- Crannell, P.; Abuelo, A. Comparison of calf morbidity, mortality, and future performance across categories of passive immunity: A retrospective cohort study in a dairy herd. J. Dairy Sci. 2023, 106, 2729–2738. [Google Scholar] [CrossRef] [PubMed]
- Godden, S. Colostrum Management for Dairy Calves. Vet. Clin. N. Am. Food Anim. Pract. 2008, 24, 19–39. [Google Scholar] [CrossRef] [PubMed]
- Mellado, M.; Del Angel, E.; Rebolloso, O.; García, E. Immunoglobulin G concentration and neonatal survival of goat kids delivered in a pen or on open range. Prev. Vet. Med. 1998, 37, 33–39. [Google Scholar] [CrossRef]
- O’brien, J.; Sherman, D. Serum immunoglobulin concentrations of newborn goat kids and subsequent kid survival through weaning. Small Rumin. Res. 1993, 11, 71–77. [Google Scholar] [CrossRef]
- Fischer, A.; Song, Y.; He, Z.; Haines, D.; Guan, L.; Steele, M. Effect of delaying colostrum feeding on passive transfer and intestinal bacterial colonization in neonatal male Holstein calves. J. Dairy Sci. 2018, 101, 3099–3109. [Google Scholar] [CrossRef]
- Fischer, A.J.; Malmuthuge, N.; Steele, M.A. The effect of heat treatment of bovine colostrum on the concentration of oligosaccharides in colostrum and in the intestine of neonatal male Holstein calves. J. Dairy Sci. 2018, 101, 401–407. [Google Scholar] [CrossRef]
- Flynn, A.; Leech, J.; McFadden, M.; McAloon, C.; Murphy, J.P.; Crispie, F.; Cotter, P.D.; McAloon, C.; Kennedy, E. The effects of offering adequate-quality or high-quality colostrum on the passive immunity, health, growth, and fecal microbiome development of dairy heifer calves. J. Dairy Sci. 2025, 108, 6254–6272. [Google Scholar] [CrossRef] [PubMed]
- Clem, A.S. Fundamentals of vaccine immunology. J. Glob. Infect. Dis. 2011, 3, 73–78. [Google Scholar] [CrossRef]
- Baxter, D. Active and passive immunity, vaccine types, excipients and licensing. Occup. Med. 2007, 57, 552–556. [Google Scholar] [CrossRef]
- Kalenik, B.; Sawicka, R.; Góra-Sochacka, A.; Sirko, A. Influenza prevention and treatment by passive immunization. Acta Biochim. Pol. 2014, 61, 573–587. [Google Scholar] [CrossRef]
- Chucri, T.M.; Monteiro, J.; Lima, A.; Salvadori, M.; Junior, J.K.; Miglino, M.A. A review of immune transfer by the placenta. J. Reprod. Immunol. 2010, 87, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Borghesi, J.; Mario, L.C.; Rodrigues, M.N.; Favaron, P.O.; Miglino, M.A. Immunoglobulin Transport during Gestation in Domestic Animals and Humans—A Review. Open J. Anim. Sci. 2014, 04, 323–336. [Google Scholar] [CrossRef]
- Borghi, S.; Bournazos, S.; Thulin, N.K.; Li, C.; Gajewski, A.; Sherwood, R.W.; Zhang, S.; Harris, E.; Jagannathan, P.; Wang, L.-X. FcRn, but not FcγRs, drives maternal-fetal transplacental transport of human IgG antibodies. Proc. Natl. Acad. Sci. USA 2020, 117, 12943–12951. [Google Scholar] [CrossRef]
- Pyzik, M.; Kozicky, L.K.; Gandhi, A.K.; Blumberg, R.S. The therapeutic age of the neonatal Fc receptor. Nat. Rev. Immunol. 2023, 23, 415–432. [Google Scholar] [CrossRef] [PubMed]
- Constantin, N.T.; Sipos, A. Passive transfer of immunoglobulins from ewe to lamb. Sci. Work. Ser. C Vet. Med. 2021, 67, 53–58. [Google Scholar]
- Ciobanu, A.M.; Dumitru, A.E.; Gica, N.; Botezatu, R.; Peltecu, G.; Panaitescu, A.M. Benefits and risks of IgG transplacental transfer. Diagnostics 2020, 10, 583. [Google Scholar] [CrossRef]
- Palmeira, P.; Quinello, C.; Silveira-Lessa, A.L.; Zago, C.A.; Carneiro-Sampaio, M. IgG placental transfer in healthy and pathological pregnancies. J. Immunol. Res. 2012, 2012, 985646. [Google Scholar] [CrossRef]
- da Silva Marques, R.; Vulcano, M.; Cazerta, S.M.; Miglino, M.A.; de Assis Neto, A.C.; Pereira, F.T.V. Caracterização morfológica da região intercaruncular uterina de vacas e búfalas gestantes. Biotemas 2007, 20, 103–114. [Google Scholar]
- Trundley, A.; Moffett, A. Human uterine leukocytes and pregnancy. Tissue Antigens 2004, 63, 1–12. [Google Scholar] [CrossRef]
- Kane, S.V.; Acquah, L.A. Placental transport of immunoglobulins: A clinical review for gastroenterologists who prescribe therapeutic monoclonal antibodies to women during conception and pregnancy. Off. J. Am. Coll. Gastroenterol. ACG 2009, 104, 228–233. [Google Scholar] [CrossRef]
- Sand, K.M.K.; Gruber, M.M.; Sandlie, I.; Mathiesen, L.; Andersen, J.T.; Wadsack, C. Contribution of the ex vivo placental perfusion model in understanding transplacental immunoglobulin G transfer. Placenta 2022, 127, 77–87. [Google Scholar] [CrossRef]
- Schmidt, A.; Schmidt, A.; Markert, U.R. The road (not) taken–placental transfer and interspecies differences. Placenta 2021, 115, 70–77. [Google Scholar] [CrossRef]
- Miglino, M.A.; Ambrósio, C.E.; dos Santos Martins, D.; Wenceslau, C.V.; Pfarrer, C.; Leiser, R. The carnivore pregnancy: The development of the embryo and fetal membranes. Theriogenology 2006, 66, 1699–1702. [Google Scholar] [CrossRef]
- Furukawa, S.; Kuroda, Y.; Sugiyama, A. A comparison of the histological structure of the placenta in experimental animals. J. Toxicol. Pathol. 2014, 27, 11–18. [Google Scholar] [CrossRef]
- Kowalewski, M.P.; Kazemian, A.; Klisch, K.; Gysin, T.; Tavares Pereira, M.; Gram, A. Canine endotheliochorial placenta: Morpho-functional aspects. Adv. Anat. Embryol. Cell Biol. 2021, 234, 155–179. [Google Scholar]
- Ursell, E. Maternal Diet and Visceral Yolk Sac Function During Mouse Development. Ph.D. Thesis, University of Southampton, Southampton, UK, 2004. [Google Scholar]
- Weström, B.; Arévalo Sureda, E.; Pierzynowska, K.; Pierzynowski, S.G.; Pérez-Cano, F.-J. The immature gut barrier and its importance in establishing immunity in newborn mammals. Front. Immunol. 2020, 11, 1153. [Google Scholar] [CrossRef]
- Wooding, F. The ruminant placental trophoblast binucleate cell: An evolutionary breakthrough. Biol. Reprod. 2022, 107, 705–716. [Google Scholar] [CrossRef]
- Johnson, G.A.; Bazer, F.W.; Seo, H.; Burghardt, R.C.; Wu, G.; Pohler, K.G.; Cain, J.W. Understanding placentation in ruminants: A review focusing on cows and sheep. Reprod. Fertil. Dev. 2023, 36, 93–111. [Google Scholar] [CrossRef]
- Dudley, J.S.; Murphy, C.R.; Thompson, M.B.; McAllan, B.M. Uterine cellular changes during mammalian pregnancy and the evolution of placentation. Biol. Reprod. 2021, 105, 1381–1400. [Google Scholar] [CrossRef]
- dos Santos Baptista, V.; de Mattos Guttmann, P.; Rusca, A.C.; da Silva, K.M.; de Barros Macieira, D.; de Alencar, N.X.; Lessa, D.A.B. Evaluation of acquired passive immunity in mule foals up to 60 days of age. J. Equine Sci. 2020, 31, 1–4. [Google Scholar] [CrossRef]
- Bigler, N.A.; Bruckmaier, R.M.; Gross, J.J. Implications of placentation type on species-specific colostrum properties in mammals. J. Anim. Sci. 2022, 100, skac287. [Google Scholar] [CrossRef]
- Ghafoori, E.M.; Narmuratova, M.; Mohammadi, M.H.; Narmuratova, Z. A Comprehensive Review of General Characteristics of Peptides of Serum Immunoglobulins and Their Health Benefits. Eur. J. Theor. Appl. Sci. 2024, 2, 659–671. [Google Scholar] [CrossRef]
- Niewiesk, S. Maternal antibodies: Clinical significance, mechanism of interference with immune responses, and possible vaccination strategies. Front. Immunol. 2014, 5, 446. [Google Scholar] [CrossRef]
- Stott, G.; Menefee, B. Selective Absorption of Immunoglobulin lgM in the New Born Calf. J. Dairy Sci. 1978, 61, 461–466. [Google Scholar] [CrossRef]
- Corthésy, B. Role of secretory immunoglobulin A and secretory component in the protection of mucosal surfaces. Future Microbiol. 2010, 5, 817–829. [Google Scholar] [CrossRef]
- Lamm, M.E. Interaction of antigens and antibodies at mucosal surfaces. Annu. Rev. Microbiol. 1997, 51, 311–340. [Google Scholar] [CrossRef]
- Saif, L.J.; Smith, K.L. Enteric viral infections of calves and passive immunity. J. Dairy Sci. 1985, 68, 206–228. [Google Scholar] [CrossRef]
- El-Loly, M.M. Colostrum ingredients, its nutritional and health benefits-an overview. Clin. Nutr. Open Sci. 2022, 44, 126–143. [Google Scholar] [CrossRef]
- Jermsutjarit, P.; Venkateswaran, D.; Indrawattana, N.; Na Plord, J.; Tantituvanont, A.; Nilubol, D. The development of a lateral flow immunochromatographic test strip for measurement of specific IgA and IgG antibodies level against porcine epidemic diarrhea virus in pig milk. Vet. Q. 2024, 44, 1–15. [Google Scholar] [CrossRef]
- Campo, J.J.; Seppo, A.E.; Randall, A.Z.; Pablo, J.; Hung, C.; Teng, A.; Shandling, A.D.; Truong, J.; Oberai, A.; Miller, J. Human milk antibodies to global pathogens reveal geographic and interindividual variations in IgA and IgG. J. Clin. Investig. 2024, 134, e168789. [Google Scholar] [CrossRef]
- Hockenberry, A.; Slack, E.; Stadtmueller, B.M. License to Clump: Secretory IgA structure–function relationships across scales. Annu. Rev. Microbiol. 2023, 77, 645–668. [Google Scholar] [CrossRef]
- Matsukawa, S.; Ueno, K.; Sugino, T.; Yoshimura, Y.; Isobe, N. Effects of colostrum whey on immune function in the digestive tract of goats. Anim. Sci. 2018, 89, 1152–1160. [Google Scholar] [CrossRef]
- Ahmann, J.; Steinhoff-Wagner, J.; Büscher, W. Determining immunoglobulin content of bovine colostrum and factors affecting the outcome: A review. Animals 2021, 11, 3587. [Google Scholar] [CrossRef]
- Klobasa, F.; Werhahn, E. Variations in the concentrations of the immunoglobulins IgG1, IgG2, IgM and IgA in sheep. 1. Changes in the blood serum and in the milk of sheep of different breeds and cross-breeds during lactation. Berl. Munch. Tierarztl. Wochenschr. 1989, 102, 123–129. [Google Scholar]
- Hurley, W.L.; Theil, P.K. Perspectives on immunoglobulins in colostrum and milk. Nutrients 2011, 3, 442–474. [Google Scholar] [CrossRef]
- Hăbeanu, M.; Lefter, N.; Gheorghe, A. Alterations in essential fatty acids, immunoglobulins (IgA, IgG, and IgM), and enteric methane emission in primiparous sows fed hemp seed oil and their offspring response. Vet. Sci. 2022, 9, 352. [Google Scholar] [CrossRef]
- Akhter, H.; Aziz, F.; Ullah, F.R.; Ahsan, M.; Islam, S.N. Immunoglobulins content in colostrum, transitional and mature milk of Bangladeshi mothers: Influence of parity and sociodemographic characteristics. J. Mother Child 2021, 24, 8. [Google Scholar]
- Ceniti, C.; Costanzo, N.; Morittu, V.M.; Tilocca, B.; Roncada, P.; Britti, D. Colostrum as an emerging food: Nutraceutical properties and food supplement. Food Rev. Int. 2023, 39, 4636–4664. [Google Scholar] [CrossRef]
- Cervenak, J.; Kacskovics, I. The neonatal Fc receptor plays a crucial role in the metabolism of IgG in livestock animals. Vet. Immunol. Immunopathol. 2009, 128, 171–177. [Google Scholar] [CrossRef]
- Ortiz-Alegría, L.B.; Xicoténcatl-García, L.; Cañedo-Solares, I.; Rico-Torres, C.P.; Gómez-Chávez, F. The FcRn from gene to protein and function: Comparison between species. Front. Immunol. 2025, 16, 1608426. [Google Scholar] [CrossRef]
- Roopenian, D.C.; Akilesh, S. FcRn: The neonatal Fc receptor comes of age. Nat. Rev. Immunol. 2007, 7, 715–725. [Google Scholar] [CrossRef]
- Pyzik, M.; Rath, T.; Lencer, W.I.; Baker, K.; Blumberg, R.S. FcRn: The architect behind the immune and nonimmune functions of IgG and albumin. J. Immunol. 2015, 194, 4595–4603. [Google Scholar] [CrossRef] [PubMed]
- Mayer, B.; Zolnai, A.; Frenyó, L.V.; Jancsik, V.; Szentirmay, Z.; Hammarström, L.; Kacskovics, I. Redistribution of the sheep neonatal Fc receptor in the mammary gland around the time of parturition in ewes and its localization in the small intestine of neonatal lambs. Immunology 2002, 107, 288–296. [Google Scholar] [CrossRef]
- Ober, R.J.; Martinez, C.; Lai, X.; Zhou, J.; Ward, E.S. Exocytosis of IgG as mediated by the receptor, FcRn: An analysis at the single-molecule level. Proc. Natl. Acad. Sci. USA 2004, 101, 11076–11081. [Google Scholar] [CrossRef]
- Yang, C.; Cheng, Y.; Zhang, T.; Gebeyew, K.; Fischer-Tlustos, A.; Guan, L.; Steele, M.; Tan, Z.; He, Z. Multi-omics analysis provides new insights into the molecular mechanisms underlying colostral immunoglobulin G absorption in the gut of neonatal goat kids. Anim. Nutr. 2025, 21, 166–178. [Google Scholar] [CrossRef]
- Sato, K.; Nagai, J.; Mitsui, N.; Yumoto, R.; Takano, M. Effects of endocytosis inhibitors on internalization of human IgG by Caco-2 human intestinal epithelial cells. Life Sci. 2009, 85, 800–807. [Google Scholar] [CrossRef]
- Laegreid, W.W.; Heaton, M.P.; Keen, J.E.; Grosse, W.M.; Chitko-McKown, C.G.; Smith, T.P.; Keele, J.W.; Bennett, G.L.; Besser, T.E. Association of bovine neonatal Fc receptor a-chain gene (FCGRT) haplotypes with serum IgG concentration in newborn calves. Mamm. Genome 2002, 13, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Geiger, A.J. Colostrum: Back to basics with immunoglobulins. J. Anim. Sci. 2020, 98, S126–S132. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.; Echeverry-Munera, J.; McCarthy, H.; Welboren, A.; Pineda, A.; Nagorske, M.; Renaud, D.; Steele, M. Effects of enriching IgG concentration in low-and medium-quality colostrum with colostrum replacer on IgG absorption in newborn Holstein calves. J. Dairy Sci. 2023, 106, 3680–3691. [Google Scholar] [CrossRef]
- Swan, H.; Godden, S.; Bey, R.; Wells, S.; Fetrow, J.; Chester-Jones, H. Passive transfer of immunoglobulin G and preweaning health in Holstein calves fed a commercial colostrum replacer. J. Dairy Sci. 2007, 90, 3857–3866. [Google Scholar] [CrossRef] [PubMed]
- Avendaño-Reyes, L.; Macías-Cruz, U.; Sánchez-Castro, M.; Anzures-Olvera, F.; Vicente-Pérez, R.; Mellado, M.; Zamorano-Algándar, R.; Robinson, P.; Castañeda-Bustos, V.; López-Baca, A. Effects of parity, seasonal heat stress, and colostrum collection time postpartum on colostrum quality of Holstein cattle in an arid region. Int. J. Biometeorol. 2024, 68, 427–434. [Google Scholar] [CrossRef]
- Yang, M.; Zou, Y.; Wu, Z.; Li, S.; Cao, Z. Colostrum quality affects immune system establishment and intestinal development of neonatal calves. J. Dairy Sci. 2015, 98, 7153–7163. [Google Scholar] [CrossRef] [PubMed]
- Jaster, E. Evaluation of quality, quantity, and timing of colostrum feeding on immunoglobulin G1 absorption in Jersey calves. J. Dairy Sci. 2005, 88, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Hue, D.T.; Skirving, R.; Chen, T.; Williams, J.L.; Bottema, C.D.; Petrovski, K. Colostrum source and passive immunity transfer in dairy bull calves. J. Dairy Sci. 2021, 104, 8164–8176. [Google Scholar] [CrossRef]
- Hernández-Castellano, L.E.; Suárez-Trujillo, A.; Martell-Jaizme, D.; Cugno, G.; Argüello, A.; Castro, N. The effect of colostrum period management on BW and immune system in lambs: From birth to weaning. Animal 2015, 9, 1672–1679. [Google Scholar] [CrossRef]
- Moretti, D.; Kindlein, L.; Pauletti, P.; Machado-Neto, R. IgG absorption by Santa Ines lambs fed Holstein bovine colostrum or Santa Ines ovine colostrum. Animal 2010, 4, 933–937. [Google Scholar] [CrossRef]
- Glover, R.L. The Use of Bovine Colostrum as a Source of Immunoglobulin (Ig) for Lambs. Master’s Thesis, University of Ghana, Accra, Ghana, 2006. [Google Scholar]
- Van Hese, I.; Goossens, K.; Ampe, B.; Haegeman, A.; Opsomer, G. Exploring the microbial composition of Holstein Friesian and Belgian Blue colostrum in relation to the transfer of passive immunity. J. Dairy Sci. 2022, 105, 7623–7641. [Google Scholar] [CrossRef]
- Elizondo-Salazar, J.A.; Heinrichs, A.J. Feeding heat-treated colostrum to neonatal dairy heifers: Effects on growth characteristics and blood parameters. J. Dairy Sci. 2009, 92, 3265–3273. [Google Scholar] [CrossRef]
- Mann, S.; Curone, G.; Chandler, T.; Sipka, A.; Cha, J.; Bhawal, R.; Zhang, S. Heat treatment of bovine colostrum: II. Effects on calf serum immunoglobulin, insulin, and IGF-I concentrations, and the serum proteome. J. Dairy Sci. 2020, 103, 9384–9406. [Google Scholar] [CrossRef]
- Malik, M.I.; Rashid, M.A.; Raboisson, D. Heat treatment of colostrum at 60 °C decreases colostrum immunoglobulins but increases serum immunoglobulins and serum total protein: A meta-analysis. J. Dairy Sci. 2022, 105, 3453–3467. [Google Scholar] [CrossRef]
- Lora, I.; Barberio, A.; Contiero, B.; Paparella, P.; Bonfanti, L.; Brscic, M.; Stefani, A.; Gottardo, F. Factors associated with passive immunity transfer in dairy calves: Combined effect of delivery time, amount and quality of the first colostrum meal. Animal 2018, 12, 1041–1049. [Google Scholar] [CrossRef]
- Ullah, S.; Rizwana, H.; Kabir, A.; Behan, A.; Naeem, M.; Barham, G.; Bukero, A.; Rahman, A.; Ullah, N.; Shah, S. The effect of colostrum feeding quantities on calf immunity and health. Vet. Sci. Res. Rev. 2023, 9, 74–81. [Google Scholar] [CrossRef]
- Gamsjäger, L.; Haines, D.; Pajor, E.; Lévy, M.; Windeyer, M. Impact of volume, immunoglobulin G concentration, and feeding method of colostrum product on neonatal nursing behavior and transfer of passive immunity in beef calves. Animal 2021, 15, 100345. [Google Scholar] [CrossRef] [PubMed]
- Chigerwe, M.; Tyler, J.W.; Summers, M.K.; Middleton, J.R.; Schultz, L.G.; Nagy, D.W. Evaluation of factors affecting serum IgG concentrations in bottle-fed calves. J. Am. Vet. Med. Assoc. 2009, 234, 785–789. [Google Scholar] [CrossRef]
- Conneely, M.; Berry, D.; Murphy, J.P.; Lorenz, I.; Doherty, M.L.; Kennedy, E. Effect of feeding colostrum at different volumes and subsequent number of transition milk feeds on the serum immunoglobulin G concentration and health status of dairy calves. J. Dairy Sci. 2014, 97, 6991–7000. [Google Scholar] [CrossRef]
- Stott, G.; Fellah, A. Colostral immunoglobulin absorption linearly related to concentration for calves. J. Dairy Sci. 1983, 66, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Ramos, J.; Loste, A.; Ferrer, L.; Fernández, A.; Castro, N.; Ortín, A.; Verde, M.; Argüello, A.; Figueras, L. Effect of addition of soybean trypsin inhibitor to colostrum on immunological status in goat kids. J. Anim. Physiol. Anim. Nutr. 2010, 94, 93–98. [Google Scholar] [CrossRef]
- Lorenz, I.; Fagan, J.; More, S.J. Calf health from birth to weaning. II. Management of diarrhoea in pre-weaned calves. Ir. Vet. J. 2011, 64, 9. [Google Scholar] [CrossRef] [PubMed]
- Zamuner, F.; Cameron, A.; Carpenter, E.; Arcos-Gómez, G.; Kirkham, J.; Leury, B.; DiGiacomo, K. Assessing the impact of colostrum feeding delay on serum immunoglobulin G and total protein in dairy goat kids. Animal 2024, 18, 101246. [Google Scholar] [CrossRef]
- Hernández-Castellano, L.E.; Morales-delaNuez, A.; Sánchez-Macías, D.; Moreno-Indias, I.; Torres, A.; Capote, J.; Argüello, A.; Castro, N. The effect of colostrum source (goat vs. sheep) and timing of the first colostrum feeding (2 h vs. 14 h after birth) on body weight and immune status of artificially reared newborn lambs. J. Dairy Sci. 2015, 98, 204–210. [Google Scholar] [CrossRef]
- Al-Saiady, M. Effect of probiotic bacteria on immunoglobulin G concentration and other blood components of Newborn calves. J. Anim. Vet. Adv. 2010, 9, 604–609. [Google Scholar] [CrossRef]
- Brady, M.; Godden, S.; Haines, D. Supplementing fresh bovine colostrum with gut-active carbohydrates reduces passive transfer of immunoglobulin G in Holstein dairy calves. J. Dairy Sci. 2015, 98, 6415–6422. [Google Scholar] [CrossRef]
- Htun, A.; Sato, T.; Hanada, M. Effect of difructose anhydride III supplementation on passive immunoglobulin G transfer and serum immunoglobulin G concentration in newborn Holstein calves fed pooled colostrum. J. Dairy Sci. 2016, 99, 5701–5706. [Google Scholar] [CrossRef]
- Rodas, E.R.; Ayala, L.E.; Dután, J.B.; Gañan, G.E.; Pesántez, J.L.; González-Martín, J.V. Influence of Capsaicin Supplementation on the Enhancement of Passive Immunity Transfer Through Modulation of Immunoglobulin Absorption in Neonatal Calves. Animals 2025, 15, 1676. [Google Scholar] [CrossRef]
- Reis, M.; Cantor, M.; Bittar, C.M.M.; Costa, J.H. Association of a green tea extract with serum immunoglobulin G status and neonatal vitality in newborn dairy calves. J. Dairy Sci. 2022, 105, 9961–9970. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, T.; Tian, Q.; Cheng, Y.; Gebeyew, K.; Liu, G.; Tan, Z.; He, Z. Supplementing mannan oligosaccharide reduces the passive transfer of immunoglobulin g and improves antioxidative capacity, immunity, and intestinal microbiota in neonatal goats. Front. Microbiol. 2022, 12, 795081. [Google Scholar] [CrossRef]
- Soberon, F.; Raffrenato, E.; Everett, R.; Van Amburgh, M. Preweaning milk replacer intake and effects on long-term productivity of dairy calves. J. Dairy Sci. 2012, 95, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Faber, S.; Faber, N.; McCauley, T.; Ax, R. Case study: Effects of colostrum ingestion on lactational performance 1. Prof. Anim. Sci. 2005, 21, 420–425. [Google Scholar] [CrossRef]
- Kenéz, Á.; Koch, C.; Korst, M.; Kesser, J.; Eder, K.; Sauerwein, H.; Huber, K. Different milk feeding intensities during the first 4 weeks of rearing dairy calves: Part 3: Plasma metabolomics analysis reveals long-term metabolic imprinting in Holstein heifers. J. Dairy Sci. 2018, 101, 8446–8460. [Google Scholar] [CrossRef]
- Armengol, R.; Fraile, L. Feeding calves with pasteurized colostrum and milk has a positive long-term effect on their productive performance. Animals 2020, 10, 1494. [Google Scholar] [CrossRef] [PubMed]
- Blum, J. Nutritional physiology of neonatal calves. J. Anim. Physiol. Anim. Nutr. 2006, 90, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Roffler, B.; Fäh, A.; Sauter, S.; Hammon, H.; Gallmann, P.; Brem, G.; Blum, J. Intestinal morphology, epithelial cell proliferation, and absorptive capacity in neonatal calves fed milk-born insulin-like growth factor-I or a colostrum extract. J. Dairy Sci. 2003, 86, 1797–1806. [Google Scholar] [CrossRef] [PubMed]
- Hammon, H.; Liermann, W.; Frieten, D.; Koch, C. Importance of colostrum supply and milk feeding intensity on gastrointestinal and systemic development in calves. Animal 2020, 14, s133–s143. [Google Scholar] [CrossRef]
| Species | Percentage of Immunoglobulin (%) | References | ||
|---|---|---|---|---|
| IgA | IgG | IgM | ||
| Cow | 5.0 | 88.0 | 7.0 | Ahmann et al., 2021 [56] |
| Sheep | 2.0 | 86.0 | 12.0 | Klobasa and Werhahn, 1989 [57] |
| Horse | 1.2 | 89.2 | 9.6 | Hurley and Theil, 2011 [58] |
| Pig | 14.9 | 77.7 | 7.4 | Hăbeanu et al., 2022 [59] |
| Human | 91.6 | 1.5 | 6.8 | Akhter et al., 2022 [60] |
| Species | Additives and Dosage | Colostrum Quantity and Quality | The Impact on IgG Absorption | References |
|---|---|---|---|---|
| Bovine | Lactobacillus strains, 1.85 × 107 CFU | 8% body weight (BW) | A significant increase in serum Ig G concentration was observed in the Lactobacillus strains supplemented groups (Treatment: 273.49 ± 6.36 ng/mL, CON:159.51 ± 5.10 ng/mL) | Saiady, 2010 [95] |
| Mannan-oligosaccharide, 30 g | 3.8 L, IgG concentration of 105.6 g/L | Supplementation of Mannan-oligosaccharide in colostrum decreased serum IgG level (Treatment: 24.02 ± 1.05 g/L, CON: 30.75 ± 1.04 g/L) and apparent efficiency of absorption of IgG (Treatment: 23.9 ± 0.97%, CON: 30.4 ± 0.96%) | Brady et al., 2015 [96] | |
| Green tea extract, 15 mL | 3 L, IgG concentration of 50 g/L | Green tea extract of colostrum did not affect serum IgG concentration and apparent efficiency of absorption of IgG | Reis et al., 2022 [99] | |
| Capsaicin, 40 mg per kg of BW | 10% BW in 1 h, 2 L on 12 h and 2 L on 20 h after birth, IgG concentration of 50.75 g/L | Supplementation of capsaicin in colostrum significantly increased serum IgG concentrations (Treatment: 21.6 ± 0.43 g/L, CON: 15.5 ± 0.78 g/L) than those only received equal volume of colostrum | Rodas et al., 2025 [98] | |
| Difructose anhydride (DFA) III, 18 g | 2 L at 1, 10, and 24 h after birth, IgG concentration of 42.9 g/L | Supplementation of DFA III in colostrum increased serum IgG concentration on 10 h (Treatment: 13.8 ± 0.8 g/L, CON: 11.5 ± 0.9 g/L), 24 h (Treatment: 21.3 ± 1.5 g/L, CON: 17.5 ± 1.5 g/L), 36 h (Treatment: 22.3 ± 1.4 g/L, CON: 17.7 ± 1.4 g/L), day 4 (Treatment: 19.9 ± 1.2 g/L, CON: 15.7 ± 1.4 g/L) and day 7 (Treatment: 18.6 ± 1.1 g/L, CON: 14.5 ± 1.3 g/L) after birth, as well as apparent efficiency of absorption of IgG (Treatment: 36.1 ± 2.7%, CON: 29.5 ± 2.7%) at 36 h | Htun et al., 2016 [97] | |
| Goat | Soybean trypsin inhibitor, 1 g/L colostrum | 160 mL/kg BW, IgG concentration of 26.35 g/L | Supplemented soybean trypsin inhibitor in colostrum did not affect serum IgG levels at 24 h (Treatment: 13.7 ± 3.7 g/L, CON: 14.2 ± 3.7 g/L) and 48 h (Treatment: 12.6 ± 3.3 g/L, CON: 12.7 ± 3.6 g/L) and the apparent efficiency of absorption of IgG (Treatment: 25.2%, CON: 24.5%) in goat kids | Ramos et al., 2010 [91] |
| Mannan oligosaccharides, 0.06% of birth BW | 5% of BW colostrum, IgG concentration of 28.61 mg/mL | Supplemented mannan oligosaccharides in colostrum significantly decreased serum IgG concentration on 3 h and 6 h after birth | Yang et al., 2022 [100] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.; Du, M.; Ahmad, A.A.; Cheng, Y.; Gebeyew, K. Passive Immunity Establishment Through Colostral IgG Absorption in Neonatal Ruminants: Foundation for Efficient Ruminant Production. Animals 2025, 15, 3093. https://doi.org/10.3390/ani15213093
Yang C, Du M, Ahmad AA, Cheng Y, Gebeyew K. Passive Immunity Establishment Through Colostral IgG Absorption in Neonatal Ruminants: Foundation for Efficient Ruminant Production. Animals. 2025; 15(21):3093. https://doi.org/10.3390/ani15213093
Chicago/Turabian StyleYang, Chao, Mei Du, Anum Ali Ahmad, Yan Cheng, and Kefyalew Gebeyew. 2025. "Passive Immunity Establishment Through Colostral IgG Absorption in Neonatal Ruminants: Foundation for Efficient Ruminant Production" Animals 15, no. 21: 3093. https://doi.org/10.3390/ani15213093
APA StyleYang, C., Du, M., Ahmad, A. A., Cheng, Y., & Gebeyew, K. (2025). Passive Immunity Establishment Through Colostral IgG Absorption in Neonatal Ruminants: Foundation for Efficient Ruminant Production. Animals, 15(21), 3093. https://doi.org/10.3390/ani15213093






