Beneficial Effects of Fermented Blueberry Pomace Supplementation on Carcass Traits, Meat Quality, and Antioxidant Capacity of Spent Hens
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of FBP
2.2. Animals and Feeding Management
2.3. Sample Collection
2.4. Carcass Traits and Organ Index Analysis
2.5. Meat Quality Analysis
2.6. Oxidative Stress Status Analysis
2.7. Gene Expression Analysis
2.8. Network Pharmacology Analysis
2.9. Molecular Docking
2.10. Statistical Analyses
3. Results
3.1. Effects of FBP on Carcass Traits and Organ Index of Spent Hens
3.2. Effects of FBP on Meat Quality of Spent Hens
3.3. Effects of FBP on the Antioxidant Capacity of Spent Hens
3.4. Effects of FBP on Oxidative-Status-Related Gene Expressions of Spent Hens
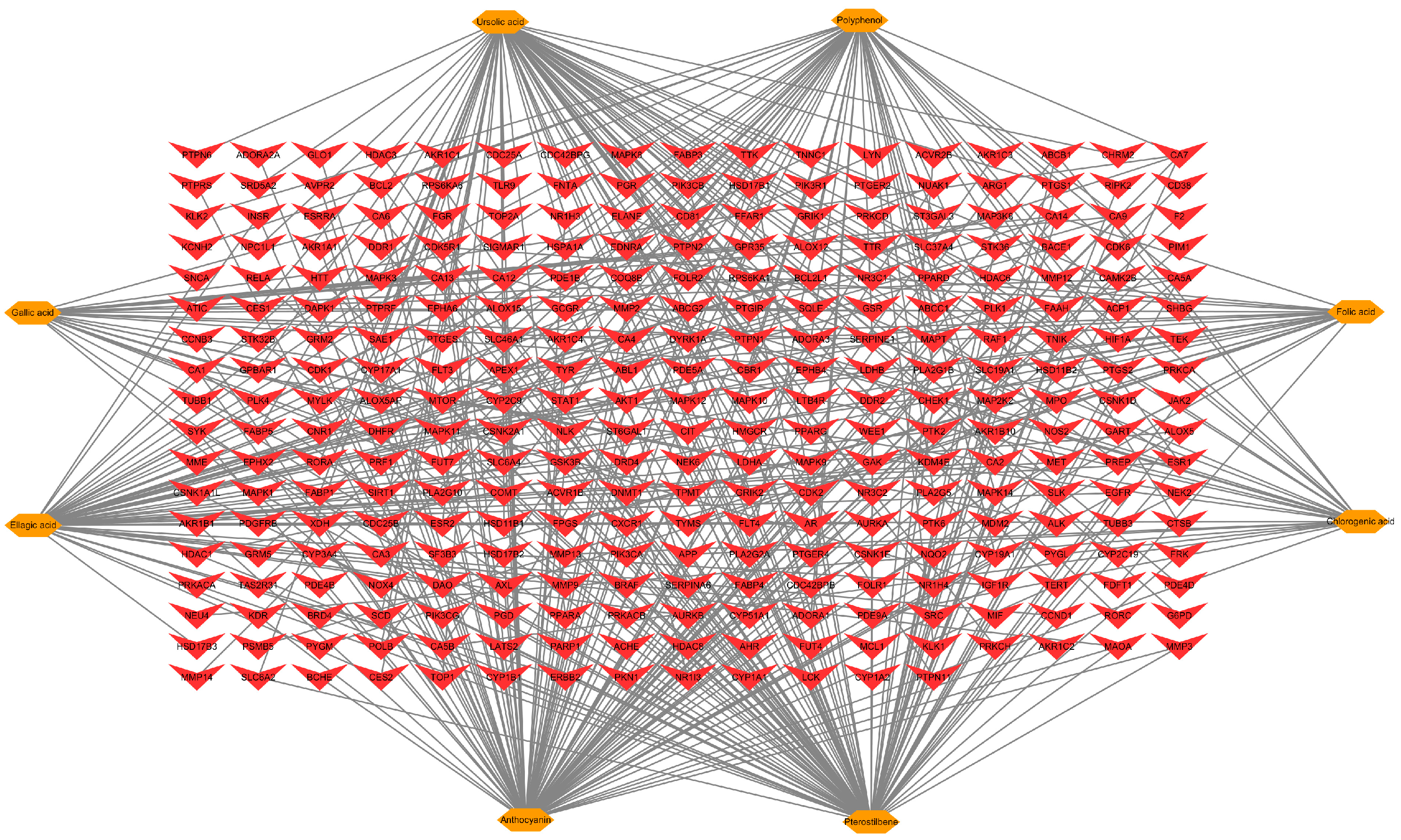
3.5. Identification of Bioactive Compound in FBP and Potential Targets Related to Meat Quality
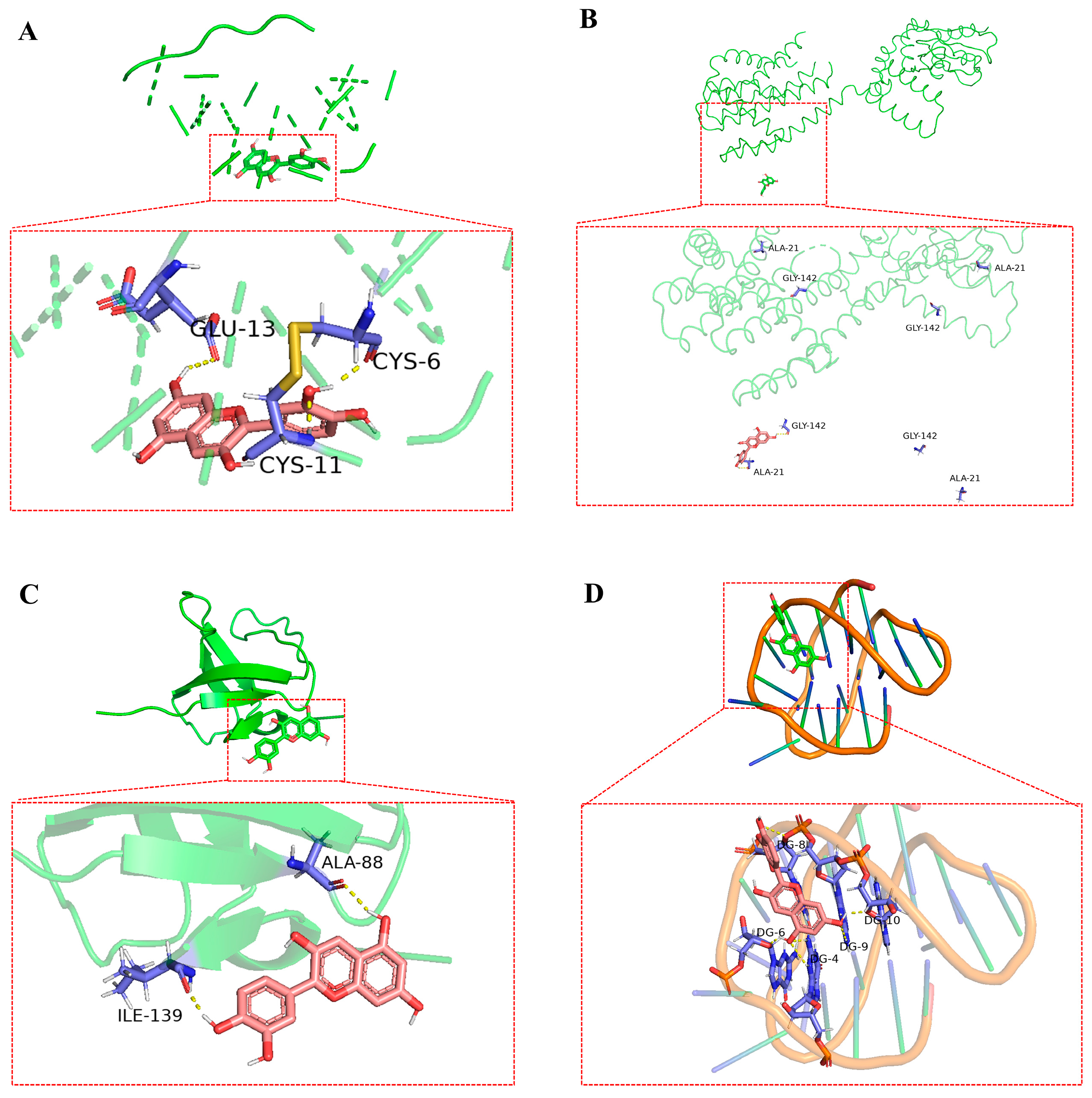
3.6. Molecular Docking of Bioactive Compounds
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hjellnes, V.; Šližyte, R.; Rustad, T.; Carvajal, A.K.; Greiff, K. Utilization of egg-laying hens (Gallus Gallus domesticus) for production of ingredients for human consumption and animal feed. BMC Biotechnol. 2020, 20, 22. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, H.Y. Comparison of quality and sensory characteristics of spent hen and broiler in South Korea. Animals 2021, 11, 2565. [Google Scholar] [CrossRef]
- Silva, F.A.; Estévez, M.; Ferreira, V.C.; Silva, S.A.; Lemos, L.T.; Ida, E.I.; Shimokomaki, M.; Madruga, M.S. Protein and lipid oxidations in Jerky chicken and consequences on sensory quality. LWT Food Sci. Technol. 2018, 97, 341–348. [Google Scholar] [CrossRef]
- Jiang, J.; Xiong, Y.L. Natural antioxidants as food and feed additives to promote health benefits and quality of meat products: A review. Meat Sci. 2016, 120, 107–117. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Pateiro, M.; Domínguez, R.; Barba, F.J.; Putnik, P.; Kovačević, D.B.; Shpigelman, A.; Granato, D.; Franco, D. Berries extracts as natural antioxidants in meat products: A review. Food Res. Int. 2018, 106, 1095–1104. [Google Scholar] [CrossRef]
- Liu, B.; Jiang, J.; Lin, G. Upregulation of antioxidant enzymes by organic mineral co-factors to improve oxidative stability and quality attributes of muscle from laying hens. Food Res. Int. 2019, 125, 108575. [Google Scholar] [CrossRef]
- Miller, K.; Feucht, W.; Schmid, M. Bioactive compounds of strawberry and blueberry and their potential health effects based on human intervention studies: A brief overview. Nutrients 2019, 11, 1510. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.F.; Yan, S.; Tian, M.L. Blueberry polyphenol extracts enhance the intestinal antioxidant capacity in weaned rats by modulating the Nrf2-Keap1 signal pathway. Front. Physiol. 2021, 12, 640737. [Google Scholar] [CrossRef]
- Liu, X.Y.; Zang, L.L.; Yu, J.B.; Yu, J.J.; Wang, S.Q.; Zhou, L.L.; Song, H.X.; Ma, Y.J.; Niu, X.F.; Li, W.F. Anti-inflammatory effect of proanthocyanidins from blueberry through NF-κβ/NLRP3 signaling pathway in vivo and in vitro. Immunopharmacol. Immunotoxicol. 2024, 46, 425–435. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, F.; Chai, Z.; Liu, M.; Battino, M.; Meng, X. Mixed fermentation of blueberry pomace with L. rhamnosus GG and L. plantarum-1: Enhance the active ingredient, antioxidant activity and health-promoting benefits. Food Chem. Toxicol. 2019, 131, 110541. [Google Scholar] [CrossRef]
- Cheng, Y.; Wu, T.; Tang, S.; Liang, F.; Fang, Y.; Cao, W.; Pan, S.; Xu, X. Fermented blueberry pomace ameliorates intestinal barrier function through the NF-κB-MLCK signaling pathway in high-fat diet mice. Food Funct. 2020, 11, 3167–3179. [Google Scholar] [CrossRef] [PubMed]
- Chai, Z.; Yan, Y.; Zan, S.; Meng, X.; Zhang, F. Probiotic-fermented blueberry pomace alleviates obesity and hyperlipidemia in high-fat diet C57BL/6J mice. Food Res. Int. 2022, 157, 111396. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.; Li, Z.; Azad, M.A.K.; Chen, T.; Cui, Y.; Lan, W.; Wang, H.; Kong, X. Fermented blueberry pomace supplementation improves egg quality, liver synthesis, and ovary antioxidant capacity of laying hens. Poult. Sci. 2024, 103, 104241. [Google Scholar] [CrossRef]
- Qin, B.; Li, Z.; Zhu, Q.; Chen, T.; Lan, W.; Cui, Y.; Azad, M.A.K.; Kong, X. Dietary fermented blueberry pomace supplementation improves small intestinal barrier function and modulates cecal microbiota in aged laying hens. Animals 2024, 14, 2786. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.Y.; Zheng, J.H.; Li, S. TCM network pharmacology: A new trend towards combining computational, experimental and clinical approaches. Chin. J. Nat. Med. 2021, 19, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yuan, Y.; Wang, W.; He, Y.; Zhong, H.; Zhou, X.; Chen, Y.; Cai, X.J.; Liu, L.Q. Mechanisms underlying the therapeutic effects of Qingfeiyin in treating acute lung injury based on GEO datasets, network pharmacology and molecular docking. Comput. Biol. Med. 2022, 145, 105454. [Google Scholar] [CrossRef]
- NY/T 33-2004; China National Feeding Standard of Chicken. China Agriculture Press: Beijing, China, 2004.
- NY/T 823-2020; Performance Terminology and Measurements for Poultry. China Agriculture Press: Beijing, China, 2020.
- NY/T 1333-2007; Determination of Livestock and Poultry Meat Quality. China Agriculture Press: Beijing, China, 2007.
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Li, D.; Dai, Y.; Chen, W.; Liu, H. Research progress on nutritional value and health function of blueberry. Agric. Prod. Process 2018, 4, 69–70, 74. (In Chinese) [Google Scholar] [CrossRef]
- Kalt, W.; Cassidy, A.; Howard, L.R.; Krikorian, R.; Stull, A.J.; Tremblay, F.; Zamora-Ros, R. Recent research on the health benefits of blueberries and their anthocyanins. Adv. Nutr. 2020, 11, 224–236. [Google Scholar] [CrossRef]
- Zhao, M. Research and application progress of active ingredients in blueberry. Agric. Technol. Serv. 2021, 38, 54–56. (In Chinese) [Google Scholar]
- Zou, J.; Tu, Y.; Shu, J.; Zhang, M.; Xu, S.; Wu, J.; Shan, Y.; Ji, G.; Sheng, Z. Comparison on slaughter performance and meat quality of different crossing high-quality chilled chicken. Chin. J. Anim. Sci. 2017, 53, 49–52. (In Chinese) [Google Scholar] [CrossRef]
- Xiao, Z.; Teng, S.; Zhou, J.; Feng, X.; Huang, L.; Liang, J. Study on application of Siraitia grosvenorii residue added into diets in Guangxi Ma chicken. Chin. Feed. 2023, 13, 158–161. (In Chinese) [Google Scholar] [CrossRef]
- Hu, Y.; Tang, S.; Zhao, W.; Wang, S.; Sun, C.; Chen, B.; Zhu, Y. Effects of dried blueberry pomace and pineapple pomace on growth performance and meat quality of broiler chickens. Animals 2023, 13, 2198. [Google Scholar] [CrossRef]
- Jeni, R.E.; Dittoe, D.K.; Olson, E.G.; Lourenco, J.; Seidel, D.S.; Ricke, S.C.; Callaway, T.R. An overview of health challenges in alternative poultry production systems. Poult. Sci. 2021, 100, 101173. [Google Scholar] [CrossRef]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive compounds and antioxidant activity in different types of berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef]
- Ruedt, C.; Gibis, M.; Weiss, J. Meat color and iridescence: Origin, analysis, and approaches to modulation. Compr. Rev. Food Sci. Food Saf. 2023, 22, 3366–3394. [Google Scholar] [CrossRef]
- Chen, R.; Wen, C.; Gu, Y.; Wang, C.; Chen, Y.; Zhuang, S.; Zhou, Y. Dietary betaine supplementation improves meat quality of transported broilers through altering muscle anaerobic glycolysis and antioxidant capacity. J. Sci. Food Agric. 2020, 100, 2656–2663. [Google Scholar] [CrossRef]
- Watanabe, A.; Daly, C.C.; Devine, C.E. The effects of the ultimate pH of meat on tenderness changes during ageing. Meat Sci. 1996, 42, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Sosnówka-Czajka, E.; Skomorucha, I.; Obremski, K.; Wojtacha, P. Performance and meat quality of broiler chickens fed with the addition of dried fruit pomace. Poult. Sci. 2023, 102, 102631. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Chen, Z.; Ke, H.; Zhu, C.; Fang, X.; Zhao, W.; Wang, L. Effects of fermented soybean meal on growth performance, carcass traits, meat quality and intramuscular amino acid content of fattening black pigs. Swine Prod. 2020, 4, 5–8. (In Chinese) [Google Scholar] [CrossRef]
- Quan, T.H.; Benjakul, S.; Sae-leaw, T.; Balange, A.K.; Maqsood, S. Protein–polyphenol conjugates: Antioxidant property, functionalities and their applications. Trends Food Sci. Technol. 2019, 91, 507–517. [Google Scholar] [CrossRef]
- Khani, N.; Shakeri, A.H.; Ashkezary, M.R.; Aghapour, B.; Soleimani, R.A.; Hosseinzadeh, N.; Rezaei-Savadkouhid, N.; Khorrami, R.; Shkouhian, S.M.J.; Homayouni-Rad, A. Potential of postbiotics in the biodegradation of antinutrients in foods. Probiotics Antimicrob. Prot. 2025; ahead of print. [Google Scholar] [CrossRef]
- Zaboli, G.; Huang, X.; Feng, X.; Ahn, D.U. How can heat stress affect chicken meat quality?—A review. Poult. Sci. 2019, 98, 1551–1556. [Google Scholar] [CrossRef]
- Park, M.W.; Cha, H.W.; Kim, J.; Kim, J.H.; Yang, H.; Yoon, S.; Boonpraman, N.; Yi, S.S.; Yoo, I.D.; Moon, J.S. NOX4 promotes ferroptosis of astrocytes by oxidative stress-induced lipid peroxidation via the impairment of mitochondrial metabolism in Alzheimer’s diseases. Redox Biol. 2021, 41, 101947. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.S.; Ahsan, H.; Zia, M.K.; Siddiqui, T.; Khan, F.H. Understanding oxidants and antioxidants: Classical team with new players. J. Food Biochem. 2020, 44, e13145. [Google Scholar] [CrossRef] [PubMed]
- Borges, G.; Degeneve, A.; Mullen, W.; Crozier, A. Identification of flavonoid and phenolic antioxidants in black currants, blueberries, raspberries, red currants, and cranberries. J. Agric. Food Chem. 2010, 58, 3901–3909. [Google Scholar] [CrossRef]
- Simunkova, M.; Barbierikova, Z.; Jomova, K.; Hudecova, L.; Lauro, P.; Alwasel, S.H.; Alhazza, I.; Rhodes, C.J.; Valko, M. Antioxidant vs. prooxidant properties of the flavonoid, kaempferol, in the presence of Cu(II) ions: A ROS-scavenging activity, fenton reaction and DNA damage study. Int. J. Mol. Sci. 2021, 22, 1619. [Google Scholar] [CrossRef]
- Salzano, A.; Damiano, S.; D’Angelo, L.; Ballistreri, G.; Claps, S.; Rufrano, D.; Maggiolino, A.; Neglia, G.; De Palo, P.; Ciarcia, R. Productive performance and meat characteristics of kids fed a red orange and lemon extract. Animals 2021, 11, 809. [Google Scholar] [CrossRef]
- Quadros, D.G.; Whitney, T.R.; Kerth, C.R.; Miller, R.; Tolleson, D.R.; Redden, R.R.; Xu, W. Intake, growth performance, carcass traits, and meat quality of feedlot lambs fed novel anthocyanin-rich corn cobs. Transl. Anim. Sci. 2023, 7, txac171. [Google Scholar] [CrossRef]
- Jiang, L.; Cheng, J. Effects of tea polyphenols on growth performance and meat quality of broilers. J. Xichang Coll. 2021, 35, 39–42+48. (In Chinese) [Google Scholar] [CrossRef]
- Li, H.Q.; Wang, B.; Li, Z.; Luo, H.L.; Zhang, C.; Jian, L.Y.; Gao, Y.F.; Lu, W.; Zhao, X.G. Effects of maternal and post-weaned rumen-protected folic acid supplementation on slaughter performance and meat quality in offspring lambs. Br. J. Nutr. 2021, 126, 1140–1148. [Google Scholar] [CrossRef]
- Yao, P.P.; Sheng, M.J.; Weng, W.H.; Long, Y.; Liu, H.; Chen, L.; Lu, J.J.; Rong, A.; Li, B. High glucose causes apoptosis of rabbit corneal epithelial cells involving activation of PERK-eIF2α-CHOP-caspase-12 signaling pathway. Int. J. Ophthalmol. 2019, 12, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
- Chong, S.J.F.; Marchi, S.; Petroni, G.; Kroemer, G.; Galluzzi, L.; Pervaiz, S. Noncanonical cell fate regulation by Bcl-2 proteins. Trends Cell Biol. 2020, 30, 537–555. [Google Scholar] [CrossRef] [PubMed]
- Gellhaus, B.; Böker, K.O.; Gsaenger, M.; Rodenwaldt, E.; Hüser, M.A.; Schilling, A.F.; Saul, D. Foxo3 knockdown mediates decline of Myod1 and Myog reducing myoblast conversion to myotubes. Cells 2023, 12, 2167. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Xiao, W.; Chen, B.; Zou, Z.; Zhu, J.; Li, D.; Yu, J.; Yang, H. Single nucleotide polymorphisms in the MRFs gene family associated with growth in Nile tilapia. Mol. Biol. Rep. 2024, 51, 128. [Google Scholar] [CrossRef]
- Zanders, L.; Kny, M.; Hahn, A.; Schmidt, S.; Wundersitz, S.; Todiras, M.; Lahmann, I.; Banyopadhyay, A.; Wollersheim, T.; Kaderali, L.; et al. Sepsis induces interleukin 6, gp130/JAK2/STAT3, and muscle wasting. J. Cachexia Sarcopenia Muscle 2022, 13, 713–727. [Google Scholar] [CrossRef]







| Composition | Content | Nutrient | Level 2 |
|---|---|---|---|
| Corn | 64.20 | Crude protein | 15.56 |
| Soybean meal | 21.60 | Crude fat | 5.10 |
| Soybean oil | 1.20 | Metabolizable energy, MJ/kg | 11.38 |
| Limestone | 8.00 | Lysine | 0.95 |
| Premix 1 | 5.00 | Methionine | 0.33 |
| Total | 100.00 | Calcium | 3.52 |
| Total phosphorus | 0.42 |
| Gene Names | Accession No. | Primer Sequence (5′–3′) | Product Size (bp) |
|---|---|---|---|
| CAT | NM_001031215.2 | F: AGATGGCGTATGACCCTAGC R: CCTCTGATAATTGGCCACGC | 173 |
| GPX1 | NM_001277853.2 | F: ATGTTCGAGAAGTGCGAGGT R: AGTTCCAGGAGACGTCGTTG | 160 |
| HO-1 | NM_205344.1 | F: ATGCCTACACCCGCTATTTG R: ATCTCAAGGGCATTCATTCG | 178 |
| Keap1 | MN416132.1 | F: CATCAACTGGGTGCAGTACG R: AGGGTGAGGTCCTGGAAGAT | 183 |
| NQO1 | NM_001277619.1 | F: AAGAAGATTGAAGCGGCTGA R: GCATGGCTTTCTTCTTCTGG | 166 |
| Nrf2 | NM_205117.1 | F: CCACCCTAAAGCTCCATTCA R: ATTCTTGCCTCTCCTGCGTA | 217 |
| SOD1 | NM_205064.1 | F: ATTACCGGCTTGTCTGATGG R: CCTCCCTTTGCAGTCACATT | 173 |
| SOD2 | NM_204211.2 | F: CCTTCGCAAACTTCAAGGAG R: CCAGCAATGGAATGAGACCT | 162 |
| β-actin | NM_205518.1 | F: ATGAAGCCCAGAGCAAAAGA R: GGGGTGTTGAAGGTCTCAAA | 223 |
| Items | Treatments | SEM | p-Values | |||||
|---|---|---|---|---|---|---|---|---|
| Control | 0.25% FBP | 0.5% FBP | 1.0% FBP | FBP | Linear | Quadratic | ||
| Live weight, kg | 1.72 | 1.68 | 1.72 | 1.74 | 0.028 | 0.877 | 0.620 | 0.668 |
| Dressed weight, kg | 1.59 | 1.55 | 1.59 | 1.60 | 0.026 | 0.931 | 0.729 | 0.813 |
| Dressing percentage, % | 92.24 | 92.61 | 92.67 | 91.81 | 0.278 | 0.693 | 0.495 | 0.316 |
| Percentage of half-eviscerated yield, % | 79.43 | 78.52 | 79.14 | 77.85 | 0.442 | 0.610 | 0.256 | 0.895 |
| Percentage of eviscerated yield, % | 64.57 | 63.66 | 62.81 | 63.74 | 0.496 | 0.665 | 0.589 | 0.295 |
| Percentage of breast muscle, % | 11.96 | 10.56 | 12.37 | 12.04 | 0.265 | 0.067 | 0.409 | 0.605 |
| Percentage of thigh muscle, % | 13.39 a | 11.16 b | 12.60 ab | 12.81 ab | 0.274 | 0.017 | 0.924 | 0.059 |
| Liver index, g/kg | 17.38 | 17.94 | 20.62 | 19.83 | 0.676 | 0.077 | 0.095 | 0.426 |
| Spleen index, g/kg | 1.08 | 1.05 | 1.18 | 1.03 | 0.029 | 0.594 | 0.688 | 0.202 |
| Abdominal fat index, g/kg | 42.89 | 42.06 | 34.14 | 34.88 | 2.398 | 0.720 | 0.167 | 0.609 |
| Items | Treatments | SEM | p-Values | |||||
|---|---|---|---|---|---|---|---|---|
| Control | 0.25% FBP | 0.5% FBP | 1.0% FBP | FBP | Linear | Quadratic | ||
| Breast muscle | ||||||||
| L* | 53.40 | 52.85 | 53.91 | 54.11 | 0.308 | 0.322 | 0.241 | 0.790 |
| a* | 11.27 | 11.25 | 11.34 | 11.30 | 0.240 | 0.999 | 0.940 | 0.958 |
| b* | 12.49 | 12.92 | 12.75 | 12.95 | 0.250 | 0.919 | 0.606 | 0.800 |
| pH45min | 6.12 | 6.10 | 5.95 | 5.98 | 0.074 | 0.832 | 0.455 | 0.715 |
| pH48h | 5.94 a | 5.65 b | 5.73 b | 5.72 b | 0.028 | 0.001 | 0.050 | 0.006 |
| 24 h drip loss, % | 6.75 a | 6.48 a | 2.85 b | 3.91 ab | 0.565 | 0.017 | 0.028 | 0.130 |
| 48 h drip loss, % | 8.43 a | 5.13 b | 4.54 b | 5.47 ab | 0.475 | 0.007 | 0.044 | 0.006 |
| Cooking loss, % | 14.58 b | 17.79 a | 17.42 a | 15.47 ab | 0.437 | 0.009 | 0.914 | 0.003 |
| Shear force, N | 26.12 | 25.17 | 27.40 | 29.78 | 1.169 | 0.921 | 0.186 | 0.652 |
| Thigh muscle | ||||||||
| L* | 40.61 | 41.92 | 42.32 | 40.70 | 2.605 | 0.494 | 0.895 | 0.123 |
| a* | 19.37 | 17.30 | 18.14 | 17.82 | 1.689 | 0.079 | 0.209 | 0.146 |
| b* | 7.86 | 8.12 | 8.19 | 8.55 | 1.342 | 0.804 | 0.317 | 0.971 |
| pH45min | 6.63 | 6.52 | 6.51 | 6.48 | 0.153 | 0.223 | 0.081 | 0.308 |
| pH48h | 5.93 | 6.00 | 5.92 | 5.96 | 0.118 | 0.529 | 0.946 | 0.862 |
| 24 h drip loss, % | 4.46 | 5.97 | 5.38 | 4.87 | 2.610 | 0.773 | 0.947 | 0.355 |
| 48 h drip loss, % | 6.25 | 8.65 | 6.78 | 5.39 | 3.009 | 0.245 | 0.307 | 0.204 |
| Cooking loss, % | 32.57 | 32.10 | 33.36 | 32.67 | 1.654 | 0.552 | 0.671 | 0.658 |
| Items | Treatments | SEM | p-Values | |||||
|---|---|---|---|---|---|---|---|---|
| Control | 0.25% FBP | 0.5% FBP | 1.0% FBP | FBP | Linear | Quadratic | ||
| GSH, U/mgprot | 0.80 | 0.88 | 0.93 | 0.84 | 0.035 | 0.834 | 0.769 | 0.179 |
| GSH-PX, U/mgprot | 4.14 | 4.57 | 6.91 | 6.15 | 0.540 | 0.118 | 0.128 | 0.299 |
| MDA, nmol/mgprot | 0.27 b | 0.29 ab | 0.48 a | 0.27 b | 0.029 | 0.018 | 0.889 | 0.015 |
| SOD, U/mL | 7.86 b | 7.22 b | 13.92 a | 10.27 ab | 0.852 | 0.009 | 0.144 | 0.103 |
| T-AOC, mmol/mg | 0.07 ab | 0.05 b | 0.11 a | 0.09 ab | 0.006 | 0.005 | 0.104 | 0.352 |
| Ingredient | Targets | Binding Energy, kJ/mol |
|---|---|---|
| Anthocyanin | INS | −4.32 |
| PRKACB | −2.27 | |
| SRC | −2.82 | |
| BCL2 | −5.10 | |
| Ursolic acid | INS | −6.28 |
| PRKACB | −4.57 | |
| SRC | −4.89 | |
| BCL2 | −6.39 | |
| Pterostilbene | INS | −5.29 |
| PRKACB | −2.69 | |
| SRC | −2.92 | |
| BCL2 | −3.98 | |
| Ellagic acid | INS | −5.08 |
| PRKACB | −2.76 | |
| SRC | −3.61 | |
| BCL2 | −5.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, B.; Chen, T.; Li, Z.; Lan, W.; Cui, Y.; Azad, M.A.K.; Kong, X. Beneficial Effects of Fermented Blueberry Pomace Supplementation on Carcass Traits, Meat Quality, and Antioxidant Capacity of Spent Hens. Animals 2025, 15, 2799. https://doi.org/10.3390/ani15192799
Qin B, Chen T, Li Z, Lan W, Cui Y, Azad MAK, Kong X. Beneficial Effects of Fermented Blueberry Pomace Supplementation on Carcass Traits, Meat Quality, and Antioxidant Capacity of Spent Hens. Animals. 2025; 15(19):2799. https://doi.org/10.3390/ani15192799
Chicago/Turabian StyleQin, Binghua, Ting Chen, Zhihua Li, Wei Lan, Yadong Cui, Md. Abul Kalam Azad, and Xiangfeng Kong. 2025. "Beneficial Effects of Fermented Blueberry Pomace Supplementation on Carcass Traits, Meat Quality, and Antioxidant Capacity of Spent Hens" Animals 15, no. 19: 2799. https://doi.org/10.3390/ani15192799
APA StyleQin, B., Chen, T., Li, Z., Lan, W., Cui, Y., Azad, M. A. K., & Kong, X. (2025). Beneficial Effects of Fermented Blueberry Pomace Supplementation on Carcass Traits, Meat Quality, and Antioxidant Capacity of Spent Hens. Animals, 15(19), 2799. https://doi.org/10.3390/ani15192799









