Development and Validation of an Owner-Assessed Feline Acute Pain Scale: Validation and Agreement with Veterinary Scales
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Study Design
2.2.1. Development of the Owner-Assessed Feline Acute Pain Scale
2.2.2. Study Protocol
2.2.3. Statistical Analysis
3. Results
3.1. Study Population
3.2. Pain Scores Across Clinical Groups
3.3. Correlation, Agreement, and Internal Consistency
3.4. Owner-Related Factors Associated with Pain Scores
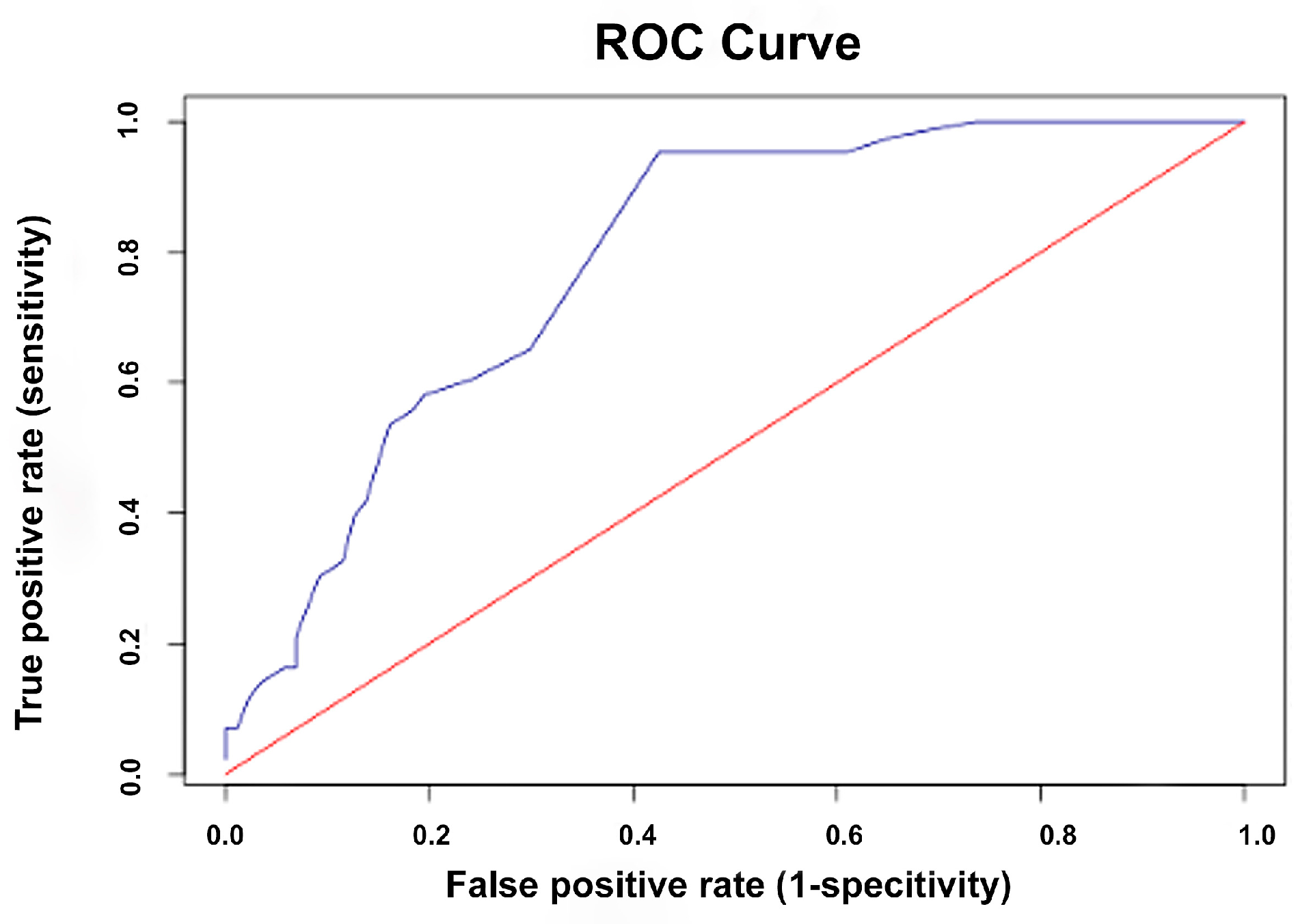
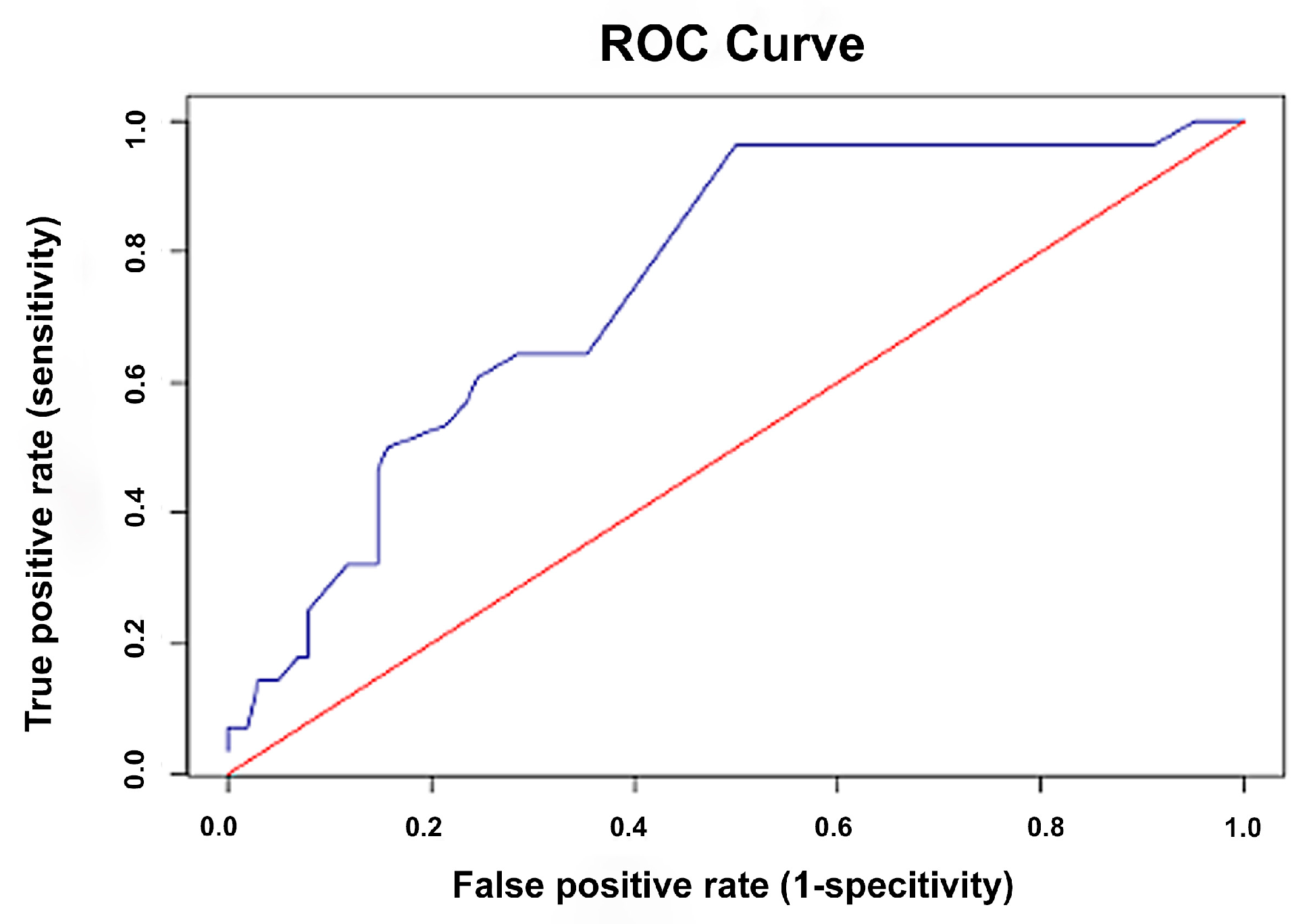
3.5. ROC Curve Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CMPS-Feline | Glasgow Composite Measure Pain Scale-Feline |
| FGS | Feline Grimace Scale |
| CSU-FPS | Colorado State University Feline Acute Pain Scale |
| MCP | Multidimensional Composite Pain Scale |
| TECA | Total Ear Canal Ablation |
| CVI | Content Validity Index |
| VAS | Visual Analog Scale |
| ROC | Receiver Operating Characteristic |
| IQR | Interquartile Range |
| CI | Confidence Interval |
| AUC | Area Under the Curve |
| OA | Osteoarthritis |
References
- Steagall, P.V.; Robertson, S.; Simon, B.; Warne, L.N.; Shilo-Benjamini, Y.; Taylor, S. 2022 ISFM Consensus guidelines on the management of acute pain in cats. J. Feline Med. Surg. 2021, 24, 4–30. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.; St Denis, K.; Collins, S.; Dowgray, N.; Ellis, S.L.; Heath, S.; Rodan, I.; Ryan, L. 2022 ISFM/AAFP Cat friendly veterinary environment guidelines. J. Feline Med. Surg. 2022, 24, 1133–1163. [Google Scholar] [CrossRef]
- Monteiro, B.P.; Lascelles, B.D.X.; Murrell, J.; Robertson, S.; Steagall, P.V.M.; Wright, B. 2023 WSAVA guidelines for the recognition, assessment and treatment of pain. J. Small Anim. Pract. 2023, 64, 177–254. [Google Scholar] [CrossRef]
- Epstein, M.E.; Rodan, I.; Griffenhagen, G.; Kadrlik, J.; Petty, M.C.; Robertson, S.A.; Simpson, W.; American Animal Hospital Association (AAHA); American Association of Feline Practitioners (AAFP). 2015 AAHA/AAFP pain management guidelines for dogs and cats. J. Feline Med. Surg. 2015, 17, 251–272. [Google Scholar] [CrossRef]
- Mathews, K.; Kronen, P.W.; Lascelles, D.; Nolan, A.; Robertson, S.; Steagall, P.V.; Wright, B.; Yamashita, K. Guidelines for recognition, assessment and treatment of pain: WSAVA Global Pain Council members and co-authors of this document. J. Small Anim. Pract. 2014, 55, E10–E68. [Google Scholar] [CrossRef]
- Steagall, P.V.; Monteiro, B.P. Acute pain in cats: Recent advances in clinical assessment. J. Feline Med. Surg. 2019, 21, 25–34. [Google Scholar] [CrossRef]
- Merola, I.; Mills, D.S. Systematic review of the behavioural assessment of pain in cats. J. Feline Med. Surg. 2016, 18, 60–76. [Google Scholar] [CrossRef]
- Lamont, L.A. Feline perioperative pain management. Vet. Clin. N. Am. Small Anim. Pract. 2002, 32, 747–763. [Google Scholar] [CrossRef]
- Calvo, G.; Holden, E.; Reid, J.; Scott, E.M.; Firth, A.; Bell, A.; Robertson, S.; Nolan, A.M. Development of a behaviour-based measurement tool with defined intervention level for assessing acute pain in cats. J. Small Anim. Pract. 2014, 55, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Reid, J.; Scott, E.M.; Calvo, G.; Nolan, A.M. Definitive Glasgow acute pain scale for cats: Validation and intervention level. Vet. Rec. 2017, 180, 449. [Google Scholar] [CrossRef] [PubMed]
- Brondani, J.T.; Luna, S.P.; Padovani, C.R. Refinement and initial validation of a multidimensional composite scale for use in assessing acute postoperative pain in cats. Am. J. Vet. Res. 2011, 72, 174–183. [Google Scholar] [CrossRef]
- Holden, E.; Calvo, G.; Collins, M.; Bell, A.; Reid, J.; Scott, E.M.; Nolan, A.M. Evaluation of facial expression in acute pain in cats. J. Small Anim. Pract. 2014, 55, 615–621. [Google Scholar] [CrossRef]
- Marangoni, S.; Beatty, J.; Steagall, P.V. An ethogram of acute pain behaviors in cats based on expert consensus. PLoS ONE 2023, 18, e0292224. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, M.C.; Watanabe, R.; Leung, V.S.Y.; Monteiro, B.P.; O’Toole, E.; Pang, D.S.J.; Steagall, P.V. Facial expressions of pain in cats: The development and validation of a Feline Grimace Scale. Sci. Rep. 2019, 9, 19128. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, M.C.; Steagall, P.V. Agreement and reliability of the Feline Grimace Scale among cat owners, veterinarians, veterinary students and nurses. Sci. Rep. 2021, 11, 5262. [Google Scholar] [CrossRef]
- Shipley, H.; Guedes, A.; Graham, L.; Goudie-DeAngelis, E.; Wendt-Hornickle, E. Preliminary appraisal of the reliability and validity of the Colorado State University Feline Acute Pain Scale. J. Feline Med. Surg. 2019, 21, 335–339. [Google Scholar] [CrossRef]
- Väisänen, M.A.; Tuomikoski, S.K.; Vainio, O.M. Behavioral alterations and severity of pain in cats recovering at home following elective ovariohysterectomy or castration. J. Am. Vet. Med. Assoc. 2007, 231, 236–242. [Google Scholar] [CrossRef]
- Lascelles, B.D.; Hansen, B.D.; Roe, S.; DePuy, V.; Thomson, A.; Pierce, C.C.; Smith, E.S.; Rowinski, E. Evaluation of client-specific outcome measures and activity monitoring to measure pain relief in cats with osteoarthritis. J. Vet. Intern. Med. 2007, 21, 410–416. [Google Scholar] [CrossRef]
- Klinck, M.P.; Gruen, M.E.; del Castillo, J.R.E.; Guillot, M.; Thomson, A.E.; Heit, M.; Lascelles, B.D.X. Development and preliminary validity and reliability of the Montreal Instrument for Cat Arthritis Testing, for Use by Caretaker/Owner, MI-CAT(C), via a randomised clinical trial. Appl. Anim. Behav. Sci. 2018, 200, 96–105. [Google Scholar] [CrossRef]
- Monteiro, B.P.; Lee, N.H.; Steagall, P.V. Can cat caregivers reliably assess acute pain in cats using the Feline Grimace Scale? A large bilingual global survey. J. Feline Med. Surg. 2023, 25, 1098612X221145499. [Google Scholar] [CrossRef]
- Quimby, J.M.; Smith, M.L.; Lunn, K.F. Evaluation of the effects of hospital visit stress on physiologic parameters in the cat. J. Feline Med. Surg. 2011, 13, 733–737. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical Power Analyses Using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef]
- Cat Carer Guide: Acute Pain. Available online: https://icatcare.org/resources/cat-carer-guide-acute-pain.pdf (accessed on 18 March 2025).
- Feline Grimace Scale. Available online: https://www.felinegrimacescale.com/ (accessed on 18 March 2025).
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]
- Ponterotto, J.G.; Ruckdeschel, D.E. An overview of coefficient alpha and a reliability matrix for estimating adequacy of internal consistency coefficients with psychological research measures. Percept. Mot. Skills. 2007, 105, 997–1014. [Google Scholar] [CrossRef] [PubMed]
- Hosmer, D.W.; Lemeshow, S. Applied Logistic Regression, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2000; pp. 160–164. [Google Scholar]
- Väisänen, M.A.; Tuomikoski-Alin, S.K.; Brodbelt, D.C.; Vainio, O.M. Opinions of Finnish small animal owners about surgery and pain management in small animals. J. Small Anim. Pract. 2008, 49, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Steagall, P.V.; Monteiro, B.P.; Ruel, H.L.M.; Beauchamp, G.; Luca, G.; Berry, J.; Little, S.; Stiles, E.; Hamilton, S.; Pang, D. Perceptions and opinions of Canadian pet owners about anaesthesia, pain and surgery in small animals. J. Small Anim. Pract. 2017, 58, 380–388. [Google Scholar] [CrossRef]
- Robinson, A.R.; Steagall, P.V. Effects of training on Feline Grimace Scale scoring for acute pain assessment in cats. J. Feline Med. Surg. 2024, 26, 1098612X241275284. [Google Scholar] [CrossRef]
- Adami, C.; Filipas, M.; John, C.; Skews, K.; Dobson, E. Inter-observer reliability of three feline pain scales used in clinical practice. J. Feline Med. Surg. 2023, 25, 1098612X231194423. [Google Scholar] [CrossRef]
- Barletta, M.; Young, C.N.; Quandt, J.E.; Hofmeister, E.H. Agreement between veterinary students and anesthesiologists regarding postoperative pain assessment in dogs. Vet. Anaesth. Analg. 2016, 43, 91–98. [Google Scholar] [CrossRef]
- Mich, P.M.; Hellyer, P.W.; Kogan, L.; Schoenfeld-Tacher, R. Effects of a pilot training program on veterinary students’ pain knowledge, attitude, and assessment skills. J. Vet. Med. Educ. 2010, 37, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Doodnaught, G.M.; Benito, J.; Monteiro, B.P.; Beauchamp, G.; Grasso, S.C.; Steagall, P.V. Agreement among undergraduate and graduate veterinary students and veterinary anesthesiologists on pain assessment in cats and dogs: A preliminary study. Can. Vet. J. 2017, 58, 805–808. [Google Scholar] [PubMed]
- Streiner, D.L.; Norman, G.R.; Cairney, J. Health Measurement Scales: A Practical Guide to Their Development and Use, 5th ed.; Oxford University Press: New York, NY, USA, 2015; pp. 7–18. [Google Scholar]
- DeVon, H.A.; Block, M.E.; Moyle-Wright, P.; Ernst, D.M.; Hayden, S.J.; Lazzara, D.J.; Savoy, S.M.; Kostas-Polston, E. A psychometric toolbox for testing validity and reliability. J. Nurs. Scholarsh. 2007, 39, 155–164. [Google Scholar] [CrossRef]
- Benito, J.; Depuy, V.; Hardie, E.; Zamprogno, H.; Thomson, A.; Simpson, W.; Roe, S.; Hansen, B.; Lascelles, B.D. Reliability and discriminatory testing of a client-based metrology instrument, feline musculoskeletal pain index (FMPI) for the evaluation of degenerative joint disease-associated pain in cats. Vet. J. 2013, 196, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Ellingsen, K.; Zanella, A.J.; Bjerkas, E.; Indrebo, A. The Relationship between empathy, perception of pain and attitudes toward pets among Norwegian dog owners. Anthrozoös 2010, 23, 231–243. [Google Scholar] [CrossRef]
- Beswick, A.; Dewey, C.; Johnson, R.; Dowsett-Coope, J.; Niel, L. Survey of Ontario veterinarians’ knowledge and attitudes on pain in dogs and cats in 2012. Can. Vet. J. 2016, 57, 1274–1280. [Google Scholar]
- Tomsic, K.; Rakinic, K.; Sokolov, C.; Seliskar, A. A survey study on the recognition and treatment of pain in dogs and cats by Slovenian veterinarians. Vet. Anaesth. Analg. 2021, 48, 334–343. [Google Scholar] [CrossRef]
- Hernandez-Avalos, I.; Mota-Rojas, D.; Mora-Medina, P.; Martínez-Burnes, J.; Casas Alvarado, A.; Verduzco-Mendoza, A.; Lezama-García, K.; Olmos-Hernandez, A. Review of different methods used for clinical recognition and assessment of pain in dogs and cats. Int. J. Vet. Sci. Med. 2019, 7, 43–54. [Google Scholar] [CrossRef] [PubMed]


| Characteristic | Orthopedic (n = 48) | Soft Tissue (n = 36) | Postoperative (n = 46) | Total (n = 130) |
|---|---|---|---|---|
| Cat age (years), median (IQR) | 1.0 (0.3, 2.8) | 4.0 (2.3, 7.8) | 4.0 (1.3, 6.1) | 2.3 (0.8, 5.8) |
| Sex, n (%) | ||||
| Male | 24 (50.0%) | 23 (63.9%) | 24 (52.2%) | 71 (54.6%) |
| Female | 24 (50.0%) | 13 (36.1%) | 22 (47.8%) | 59 (45.4%) |
| Breed, n (%) | ||||
| Domestic Shorthair | 40 (83.3%) | 27 (75.0%) | 34 (73.9%) | 101 (77.7%) |
| Persian | 2 (4.2%) | 3 (8.3%) | 5 (10.9%) | 10 (7.7%) |
| Scottish Fold | 4 (8.3%) | 3 (8.3%) | 3 (6.5%) | 10 (7.7%) |
| American Shorthair | 1 (2.1%) | 1 (2.8%) | 1 (2.2%) | 3 (2.3%) |
| Devon Rex | 0 (0.0%) | 1 (2.8%) | 0 (0.0%) | 1 (0.8%) |
| British Longhair | 1 (2.1%) | 1 (2.8%) | 2 (4.3%) | 4 (3.1%) |
| Maine Coon | 0 (0.0%) | 0 (0.0%) | 1 (2.2%) | 1 (0.8%) |
| Group | Owner-Assessed Scale Median (IQR) | CMPS-Feline Median (IQR) | FGS Median (IQR) | CSU-FPS Median (IQR) |
|---|---|---|---|---|
| Orthopedic | 9.0 (8.0, 16.0) a | 7.0 (3.0, 9.0) a | 3.0 (1.0, 5.0) a | 1.75 (0.75, 2.00) a |
| Soft tissue | 10.0 (5.0, 17.8) a,b | 5.0 (1.0, 7.0) b | 2.0 (0.0, 4.0) a,b | 0.75 (0.00, 1.69) b |
| Postoperative | 8.0 (3.0, 10.3) b | 3.0 (2.0, 6.0) b | 1.0 (0.0, 3.0) b | 0.50 (0.25, 1.00) b |
| p-value | 0.015 | <0.001 | <0.001 | <0.001 |
| Pain Scaling System | CMPS-FELINE Rho (95% CI) | FGS Rho (95% CI) | CSU-FPS Rho (95% CI) |
|---|---|---|---|
| Owner-assessed pain scale | 0.66 (0.50–0.72) | 0.53 (0.38–0.66) | 0.57 (0.44–0.68) |
| CMPS-Feline | - | 0.79 (0.70–0.85) | 0.83 (0.76–0.89) |
| FGS | - | - | 0.63 (0.51–0.73) |
| Pain Scaling System | CMPS-Feline Kappa (95% CI) | FGS Kappa (95% CI) | CSU-FPS Kappa (95% CI) |
|---|---|---|---|
| Owner-assessed pain scale | 0.74 (0.57–0.91) | 0.44 (0.23–0.59) | 0.28 (0.16–0.41) |
| CMPS-Feline | - | 0.57 (0.43–0.71) | 0.38 (0.22–0.54) |
| FGS | - | - | 0.37 (0.19–0.56) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojsiripornchai, S.; Niyom, S.; Koatsang, N.; Kathatip, S.; Thunpattranon, T.; Bunjerdsuwan, W.; Jaroensong, T.; Kasemsuwan, S. Development and Validation of an Owner-Assessed Feline Acute Pain Scale: Validation and Agreement with Veterinary Scales. Animals 2025, 15, 2801. https://doi.org/10.3390/ani15192801
Rojsiripornchai S, Niyom S, Koatsang N, Kathatip S, Thunpattranon T, Bunjerdsuwan W, Jaroensong T, Kasemsuwan S. Development and Validation of an Owner-Assessed Feline Acute Pain Scale: Validation and Agreement with Veterinary Scales. Animals. 2025; 15(19):2801. https://doi.org/10.3390/ani15192801
Chicago/Turabian StyleRojsiripornchai, Samolwan, Sirirat Niyom, Nattika Koatsang, Sakunrat Kathatip, Teerapat Thunpattranon, Wutti Bunjerdsuwan, Tassanee Jaroensong, and Suwicha Kasemsuwan. 2025. "Development and Validation of an Owner-Assessed Feline Acute Pain Scale: Validation and Agreement with Veterinary Scales" Animals 15, no. 19: 2801. https://doi.org/10.3390/ani15192801
APA StyleRojsiripornchai, S., Niyom, S., Koatsang, N., Kathatip, S., Thunpattranon, T., Bunjerdsuwan, W., Jaroensong, T., & Kasemsuwan, S. (2025). Development and Validation of an Owner-Assessed Feline Acute Pain Scale: Validation and Agreement with Veterinary Scales. Animals, 15(19), 2801. https://doi.org/10.3390/ani15192801





