Simple Summary
The global demand for grain-based feed in the livestock sector has risen significantly in recent years. The utilization of food processing by-products as alternative feed ingredients presents a viable strategy to mitigate this issue. Citrus pomace, a residual by-product derived from juice and citrus product manufacturing, exhibits considerable promise as a dietary component for swine due to its high nutritional value and bioactive constituents. Findings from this study suggest that a 5% inclusion of citrus pomace in the diet of Tibetan pigs is sustainable over extended periods.
Abstract
This study aimed to evaluate the impacts of dietary inclusion of citrus pomace on growth performance, intestinal morphology, digestive enzyme activity, antioxidant status, and colonic microbiota in Tibetan pigs in a 90-day feeding trial. Eighty Tibetan pigs (75-day-age, 16.62 ± 1.50 kg) were divided into four dietary treatment groups, each containing four replicates with five pigs per replicate. The experimental diets comprised a control diet alongside three diets incorporating 5%, 10%, or 15% citrus pomace, respectively. The results showed that the feed conversion ratio of pigs was lower in the 5% citrus pomace group compared to the other three treatment groups. Compared to the control group, dietary inclusion of 5% citrus pomace increased villus height in the duodenum and jejunum of pigs. Additionally, it elevated β-amylase activity in the duodenum while increasing sucrase activity in the jejunum. Furthermore, dietary inclusion of 5% citrus pomace enhanced lipase activity in the duodenum, jejunum, and ileum of pigs compared to the control group. It also significantly increased catalase activity in the serum of pigs. However, dietary inclusion of 5% citrus pomace decreased the abundances of Lactobacillaceae and Lactobacillus while increasing the abundances of Streptococcaceae, Turicibacteraceae, Streptococcus, and Turicibacter in the colonic digesta of pigs. Collectively, long-term dietary inclusion with 5% citrus pomace reduced the feed conversion ratio, improved intestinal morphology, enhanced digestive enzyme activity in the small intestine, and elevated serum antioxidant status, while exerting complex effects on colonic microbiota in Tibetan pigs. Future research should prioritize in vitro fermentation to develop fermented citrus pomace as a feed ingredient for pigs.
1. Introduction
The global demand for grain production in the livestock industry has increased in recent years. Utilizing food by-products as feed ingredients has emerged as a potential solution to address this challenge [1]. Citrus pomace, an agricultural by-product from juice or citrus-based product processing, demonstrates significant potential as a pig feed ingredient due to its rich traditional nutrient content and bioactive compounds [2]. Citrus fruit processing generates substantial by-products, including peels, pulp, membranes, and seeds [3]. While these by-products pose economic and environmental challenges, they can also be utilized as valuable dietary components for ruminants and rabbits [4,5]. Researchers have prioritized by-product utilization to effectively reduce environmental impacts, alleviate competition for food and feed resources, and optimize land use [1].
According to the World Citrus Organization, China accounts for approximately 28% of global citrus production, establishing itself as a leader in both citrus orchard area and output worldwide [6]. Citrus fruits are rich in traditional nutrients, including carbohydrates, proteins, lipids, vitamins, and minerals, as well as bioactive compounds such as flavonoids, essential oils, carotenoids, limonoids, and coumarins [7]. Citrus pulp has demonstrated antioxidant and antimicrobial properties [8,9]. In poultry production, dietary inclusion of dried sweet orange (Citrus sinensis) pulp enhanced growth performance (feed intake and body weight gain) while reducing hepatic and abdominal fat deposition in broiler chickens [10]. Additionally, dietary supplementation with 2% Citrus junos probiotics enhanced immune function and reduced thiobarbituric acid reactive substances in broiler breast meat [11]. In growing rabbits, citrus pomace consumption showed no adverse effects on growth performance but instead improved immunity and hepatic antioxidant status [12]. Furthermore, the Tibetan pig, a native Chinese breed with herbivorous tendencies, has more diverse intestinal microbiota, characterized by a high abundance of fiber-degrading bacteria in the large intestine. These functional microorganisms are capable of decomposing cellulosic and other complex substances present in roughage, thereby facilitating the host’s nutrient digestion and absorption [13]. Growing pigs can adapt their gastrointestinal tract to efficiently utilize citrus pulp as a source of highly fermentable carbohydrates [14,15]. However, limited information is available regarding the impact of long-term dietary citrus pulp inclusion on the growth performance, antioxidant status, and intestinal health of Tibetan pigs.
Therefore, this study was conducted to investigate the effects of dietary inclusion of citrus pomace on growth performance, intestinal morphology, digestive enzyme activity, antioxidant status, and colonic microbiota of Tibetan pigs in a 90-day feeding trial.
2. Materials and Methods
2.1. Experimental Design
The animal protocols were approved by the Animal Care and Use Committee of Jiangxi Agricultural University (JAXULL-0132). Eighty 75-day-old Tibetan pigs, with an average body weight of 16.62 ± 1.50 kg, were assigned to four dietary treatments, each with four replicate pens, and each pen housing five pigs. The study included four experimental diets: a control diet and three diets supplemented with 5%, 10%, or 15% citrus pomace. The citrus pomace used in this study was sourced from the by-product of Newhall Navel oranges produced in Jiangxi Province, the primary production area for Newhall Navel oranges in China. The ingredient composition and nutrient levels of the experimental diets are presented in Table 1. The feeding trial lasted 90 days, during which pigs had ad libitum access to feed and fresh water.

Table 1.
Ingredient composition and nutrient level of the experimental diets (as-fed basis).
2.2. Data and Sample Collection
2.2.1. Growth Performance
At the beginning and end of the feeding trial, pigs were weighed to determine the average daily gain (ADG). Feed intake per pen was recorded to calculate the average daily feed intake (ADFI). The feed conversion ratio (FCR) was determined accordingly.
2.2.2. Sample Collection
At the end of the trial, one pig with a body weight similar to the average body weight in each pen was selected for sampling in the control group and the 5% citrus pomace group (the 5% citrus pomace group was selected based on growth performance). Firstly, following a 12 h fasting period, one pig per pen, as described above, was sampled for blood collection via the anterior vena cava. The blood samples were centrifuged at 4 °C and 3000 rpm for 15 min to harvest serum, which was promptly frozen in liquid nitrogen and stored at –80 °C until subsequent analysis of antioxidant parameters. Subsequently, the pigs were euthanized via intravenous injection of pentobarbital sodium. The intestinal tract was promptly excised and segmented into the duodenum, jejunum, and ileum. Samples of 2 g intestinal contents from each segment were collected and stored at –80 °C for subsequent analysis of digestive enzyme activity. Segments approximately 2 cm from the mid-portions of the duodenum, jejunum, and ileum were sampled and fixed in 4% paraformaldehyde for morphological analysis. Finally, the colonic digesta was collected and preserved in liquid nitrogen for subsequent microbiota analysis.
2.3. Laboratory Analysis
2.3.1. Intestinal Morphology
The intestinal morphology was determined as described in our previous study [16]. Briefly, the intestinal segments were fixed, dehydrated, embedded in paraffin, and sectioned. The sections were then deparaffinized, rehydrated, and stained with hematoxylin and eosin (H&E). Digital images of the H&E-stained sections were acquired using an EVOS microscope (Advanced Microscopy Group, Bothell, WA, USA). The villus height and crypt depth were measured using Image-Pro Plus 6.0 software, and the villus height-to-crypt depth ratio was subsequently calculated using the formula: villus height divided by crypt depth.
2.3.2. Digestive Enzyme Activity in the Small Intestine
Approximately 1 g of frozen duodenum, jejunum, or ileum digesta samples was weighed and homogenized with nine volumes (w/v) of precooled physiological saline. The mixture was centrifuged at 4000× g for 10 min at 4 °C. The supernatant protein concentration was determined using a protein quantification kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Subsequently, the activities of α-amylase, β-amylase, sucrase, lipase, and neutral protease in the supernatant were measured using commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) following the manufacturer’s instructions.
2.3.3. Serum Antioxidant Parameters
The serum antioxidant parameters, including glutathione peroxidase (GSH-Px) activity, superoxide dismutase (SOD) activity, catalase (CAT) activity, and malondialdehyde (MDA) content, were measured using commercial assay kits (Sino Best Biological Technology, Shanghai, China) following the manufacturer’s specifications.
2.3.4. Colonic Microbiota Analysis
Genomic DNA was extracted from colonic digesta using the OMEGA Soil DNA Kit (Omega Bio-Tek, Norcross, GA, USA). The V3–V4 region of bacterial 16S rRNA genes was amplified via PCR with primers 338F (5′-ACTCCTACGGGAGGCAGCA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′). Sample-specific 7 bp barcodes were integrated into the primers for multiplex sequencing. The amplicons were pooled in equimolar amounts, and paired-end sequencing was performed on the Illumina NovaSeq platform using the NovaSeq 6000 SP Reagent Kit (500 cycles). Data processing and bioinformatics analyses were carried out by Shanghai Personal Biotechnology Co., Ltd. (Shanghai, China).
2.4. Statistical Analysis
Data were analyzed using SPSS 26.0 software (SPSS, Chicago, IL, USA). For the growth performance data, each pen was considered the experimental unit, and one-way analysis of variance was performed, followed by Duncan’s multiple range test for multiple comparisons among treatments. All other variables, excluding growth performance, were analyzed using a t-test, with each sampled pig per pen serving as the experimental unit. A significance level of p < 0.05 was considered statistically significant.
3. Results
3.1. Growth Performance
As shown in Table 2, no statistical differences were observed in the final body weight and average daily gain of pigs among the four dietary treatment groups (p > 0.05). However, compared with the control group without citrus pomace inclusion, pigs in the 10% citrus pomace group exhibited increased average daily feed intake, while those in the 5% citrus pomace group showed a reduced feed conversion ratio (p < 0.05). Therefore, pigs from the control group and the 5% citrus pomace inclusion group were selected for subsequent analysis of intestinal morphology, digestive enzyme activity in the small intestine, serum antioxidant status, and colonic microbiota.

Table 2.
Effects of dietary inclusion with citrus pomace on growth performance of Tibetan pigs (n=4, mean ± SEM).
3.2. Intestinal Morphology
The effects of dietary inclusion with 5% citrus pomace on intestinal morphology in Tibetan pigs are shown in Figure 1. No statistical differences were found in crypt depth and the villus height-to-crypt depth ratio of the duodenum, jejunum, and ileum in Tibetan pigs (p > 0.05). However, compared to the control group without citrus pomace inclusion, dietary inclusion of 5% citrus pomace increased villus height in the duodenum and jejunum of Tibetan pigs (p < 0.05).
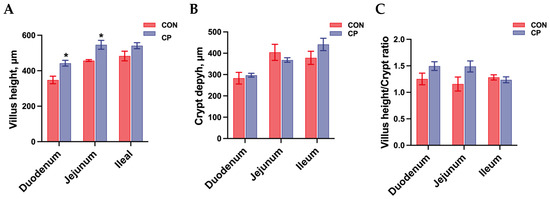
Figure 1.
Effects of dietary inclusion with 5% citrus pomace on intestinal morphology of Tibetan pigs (n = 4, mean ± SEM): (A). Villus height. (B). Crypt depth. (C). Villus height/crypt depth ratio. * p < 0.05. Abbreviations: CON, control group; CP, 5% citrus pomace group.
3.3. Digestive Enzyme Activity in Small Intestine
As illustrated in Figure 2, no statistical differences were observed in α-amylase activity, and neutral protease activity in the small intestine of Tibetan pigs between treatment groups (p > 0.05). However, compared with the control group, dietary inclusion of 5% citrus pomace increased β-amylase activity and decreased sucrase activity in the duodenum, while increasing sucrase activity in the jejunum of Tibetan pigs (p < 0.05). Moreover, dietary inclusion of 5% citrus pomace elevated lipase activity in the duodenum, jejunum, and ileum of Tibetan pigs compared to the control group (p < 0.05).
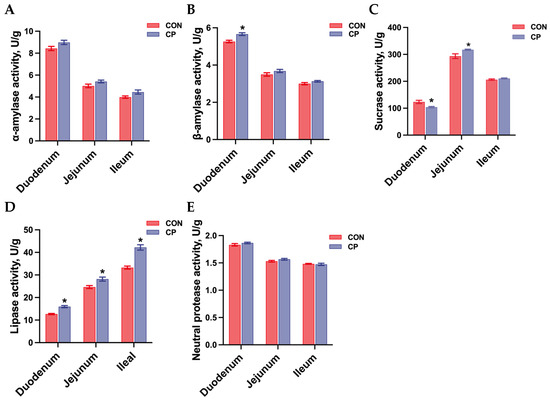
Figure 2.
Effects of dietary inclusion with 5% citrus pomace on digestive enzyme activity in the small intestine of Tibetan pigs (n = 4, mean ± SEM): (A). α-amylase activity. (B). β-amylase activity. (C). Sucrase activity. (D). Lipase activity. (E). Neutral protease activity. * p < 0.05. Abbreviations: CON, control group; CP, 5% citrus pomace group.
3.4. Serum Antioxidant Status
The effects of dietary inclusion of 5% citrus pomace on the antioxidant status in the serum of Tibetan pigs are shown in Figure 3. No significant differences were observed in GSH-Px activity, SOD activity, and MDA content in the serum of Tibetan pigs (p > 0.05). However, compared to the control group, dietary inclusion of 5% citrus pomace significantly increased CAT activity in the serum of Tibetan pigs (p < 0.05).
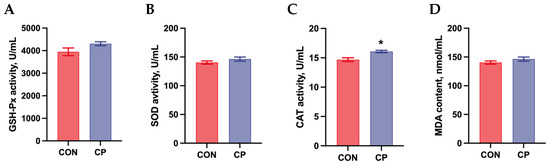
Figure 3.
Effects of dietary inclusion with 5% citrus pomace on antioxidant status in the serum of Tibetan pigs (n = 4, mean ± SEM): (A). Glutathione peroxidase (GSH-Px) activity. (B). Superoxide dismutase (SOD) activity. (C). Catalase (CAT) activity. (D). Malondialdehyde (MDA) content. * p < 0.05. Abbreviations: CON, control group; CP, 5% citrus pomace group.
3.5. Diversity of Colonic Microbiota
As shown in Figure 4, there were no statistical differences in the Chao1 index, Shannon index, and Simpson index between treatment groups (p > 0.05). However, a significant difference was found in β-diversity between treatment groups (p < 0.05).
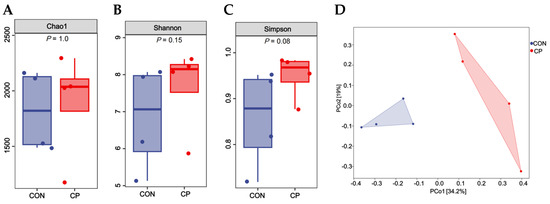
Figure 4.
Effects of dietary inclusion with 5% citrus pomace on the diversity of colonic microbiota in Tibetan pigs (n = 4): (A). Chao1 index. (B). Shannon index. (C). Simpson index. (D). β-diversity. Abbreviations: CON, control group; CP, 5% citrus pomace group.
3.6. Composition of Colonic Microbiota
The colonic microbiota composition of Tibetan pigs fed the two experimental diets is presented in Figure 5. Compared to the control group, dietary inclusion of 5% citrus pomace reduced the abundances of Lactobacillaceae and Lactobacillus, while increasing the abundances of Streptococcaceae, Turicibacteraceae, Streptococcus, and Turicibacter in the colonic digesta of Tibetan pigs (p < 0.05). The LEfSe analysis was further conducted to assess alterations in specific bacterial taxa across taxonomic levels, including phylum (p), class (c), order (o), family (f), genus (g), and species (s), within the colonic digesta of Tibetan pigs fed the 5% citrus pomace-included diet vs. the control diet (Figure 6). The results show that, compared to the control group, Tibetan pigs fed diets containing 5% citrus pomace exhibited reduced abundances of Lactobacillus (g), Lactobacillaceae (f), Dehalobacterium (g), and Dehalobacteriaceae (f), while showing increased abundances of Streptococcaceae (f), Streptococcus (g), Turicibacteraceae (f), Turicibacter (g), and Turicibacterales (o) in the colonic digesta (p < 0.05).
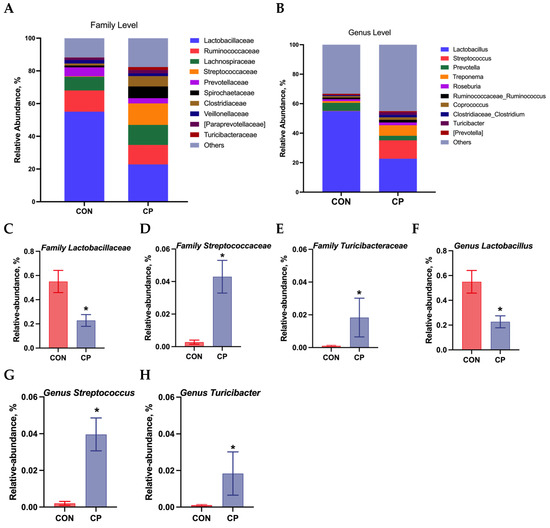
Figure 5.
Colonic microbiota composition in Tibetan pigs fed the two experimental diets: (A,B). Relative abundance at family and genus levels (n = 4). (C–H). Relative abundance of Lactobacillaceae, Streptococcaceae, Turicibacteraceae, Lactobacillus, Streptococcus, and Turicibacter (n = 4, mean ± SEM). * p < 0.05. Abbreviations: CON, control group; CP, 5% citrus pomace group.
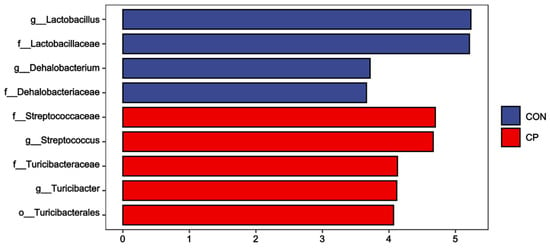
Figure 6.
LEfSe analysis of colonic microbiota in Tibetan pigs (LDA scores > 3.0; n = 4). Abbreviations: CON, control group; CP, 5% citrus pomace group.
4. Discussion
The primary objective of this study was to assess the impact of long-term dietary citrus pomace inclusion on growth performance, intestinal morphology, digestive enzyme activity, antioxidant status, and colonic microbiota in Tibetan pigs, thereby addressing the existing gap in research concerning the utilization of food by-products as feed ingredients. In this study, dietary inclusion of citrus pomace at 5%, 10%, and 15% did not adversely affect the ADG, ADFI, or FCR of Tibetan pigs during the 90-day feeding period. Importantly, dietary 5% citrus pomace inclusion decreased FCR of Tibetan pigs. The results align with previous research on weanling pigs, which demonstrated that supplementation with 7.5% citrus pulp did not compromise growth performance [17]. Additionally, studies on finishing pigs demonstrated no adverse effects from a 15% dried citrus pulp supplementation, while research on growing pigs indicated similar outcomes with a 10% inclusion rate [14]. Interestingly, previous studies also found that dietary inclusion of 2.5% and 5% citrus pomace did not adversely affect growth performance in growing pigs. However, increasing the inclusion rate to 10% resulted in compromised growth performance [18]. This phenomenon can be explained by the fact that higher concentrations of citrus pomace may increase dietary soluble non-starch polysaccharide levels, potentially impairing growth performance [19]. Therefore, based on growth performance, pigs from the control group and the 5% citrus pomace group were chosen for further evaluation of intestinal morphology, digestive enzyme activity in the small intestine, serum antioxidant capacity, and colonic microbiota.
The intestine is the primary site for nutrient digestion and absorption in pigs. A reduction in villus height accompanied by an increase in crypt depth often indicates intestinal stress, reflecting a suboptimal intestinal environment. In the current study, compared to the control group, dietary inclusion of 5% citrus pomace increased villus height in the duodenum and jejunum of Tibetan pigs. Similarly, morphological assessments revealed that citrus pulp supplementation increased villus height in the duodenum and jejunum in weaned piglets, as reported by Uerlings et al. (2021) [20]. Consistently, Zeng et al. (2022) observed increased ileal villus height and villus height-to-crypt depth ratio in weaned piglets fed diets supplemented with pomelo peel powder [21]. The improved intestinal morphology, which suggests an increased absorptive surface, further supports the lower FCR result in our study.
To further understand the changes in the digestive capacity of Tibetan pigs, we analyzed the activities of digestive enzymes, including α-amylase, β-amylase, sucrase, lipase, and neutral protease. The results showed that, compared to the control group, dietary inclusion of 5% citrus pomace significantly increased β-amylase activity in the duodenum and sucrase activity in the jejunum of Tibetan pigs. Additionally, lipase activity was elevated in the duodenum, jejunum, and ileum of Tibetan pigs fed the 5% citrus pomace diet relative to the control group.
The primary role of exocrine pancreatic secretion is to provide the enzymes necessary for the luminal digestion of carbohydrates, fats, and proteins. The pig’s pancreas secretes significant classes of digestive enzymes, including proteases and lipases, whose activities are crucial markers for assessing digestive capacity [22]. Furthermore, the intensity of sucrase activity, a pancreatic enzyme, serves as an indicator for evaluating intestinal development [23,24,25,26]. Overall, these results suggest that dietary inclusion of 5% citrus pomace positively influences the digestive capacity of the intestine by increasing the activities of key digestive enzymes such as β-amylase, sucrase, and lipase.
Citrus is abundant in bioactive compounds, including carotenoids, flavonoids, terpenes, and limonoids, which exhibit antioxidant properties [27]. In our study, compared to the control group, dietary inclusion of 5% citrus pomace significantly increased CAT activity in the serum of Tibetan pigs. Consistent with our findings, Zeng et al. (2022) demonstrated that dietary supplementation with 8 g/kg pomelo peel powder significantly increased serum CAT activity (11.00 vs. 7.25 U/mL) in weaned piglets compared to the control group [21]. Liu et al. (2024) also reported that dietary supplementation with 10% fermented or unfermented citrus pomace increased CAT activity in both serum and breast muscle of yellow-feathered broilers [28]. The findings further support our results that citrus pomace inclusion enhanced serum antioxidant status in Tibetan pigs.
The gut microbiome plays a pivotal role in maintaining gastrointestinal homeostasis, with diet being a significant factor influencing the gut microbiota [29]. In this study, a significant difference was found in β-diversity between treatment groups. Compared to the control group, dietary inclusion of 5% citrus pomace reduced the abundances of Lactobacillaceae and Lactobacillus, while increasing the abundances of Streptococcaceae, Streptococcus, Turicibacteraceae, and Turicibacter in the colonic digesta of Tibetan pigs. The LEfSe analysis further confirmed these findings. The enrichment of Streptococcaceae may be linked to the high fructose content in the citrus pomace-supplemented diet, a finding consistent with observations reported by Jones et al. (2019) [30]. Streptococcaceae and Streptococcus are documented to be associated with metabolic processes and gastrointestinal inflammatory responses [31,32]. It is proposed that the abundances of Turicibacteraceae and Turicibacter correlated with the production of short-chain fatty acids [33]. Lactobacillaceae and Lactobacillus are commonly recognized as probiotics [34,35], though some studies have documented their potential as pathogenic microorganisms [36]. Our findings indicate that long-term dietary supplementation may adversely impact the gut microbiota of pigs, as evidenced by reduced abundances of Lactobacillaceae and Lactobacillus. Therefore, the colonic microbiota of pigs responds to dietary citrus pomace inclusion in a complex manner.
This highlights that in vitro fermentation could be a viable strategy to address this issue [28,37]. Future research should focus on in vitro fermentation to provide fermented citrus pomace for pig nutrition.
Lastly, regarding the cost-effectiveness of citrus pomace in pig production, as an agricultural byproduct of citrus processing, moist citrus pulp (primarily from Navel oranges) has a relatively low market price. Although drying and storage incur additional costs, citrus pomace still offers a significant cost advantage compared to traditional feed ingredients. Thus, utilizing citrus pomace as swine feed presents considerable potential.
5. Conclusions
Dietary supplementation with 5% citrus pomace decreased feed conversion ratio, improved intestinal morphology, elevated digestive enzyme activity in the small intestine and serum antioxidant status, while having complex effects on colonic microbiota in Tibetan pigs. Our findings also highlight the potential application of citrus pomace in other commercial pig breeds. Future research should focus on in vitro fermentation to provide fermented citrus pomace as a feed ingredient for pigs.
Author Contributions
Conceptualization, J.Y. and J.C.; methodology, X.G. and H.Z.; software, X.G. and J.C.; validation, J.C. and J.Y.; formal analysis, X.G. and X.L.; investigation, X.G. and G.Z.; resources, J.Y.; data curation, X.G. and J.L.; writing—original draft preparation, X.G.; writing—review and editing, J.C.; visualization, J.C. and Y.H.; supervision, J.Y.; project administration, J.Y.; funding acquisition, J.Y. and X.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Jiangxi Provincial Cultivation Program for Academic and Technical Leaders of Major Subjects (No. 20213BCJ22005), the Key Research and Development Program of Jiangxi Province (No. 20223BBF61018), the Science and Technology Plan Project of Jiangxi Provincial Department of Education (No. GJJ210406), and the Research and Demonstration of Regional Feed Resources Utilizing in Fragrance pig (JXAFRP-20-09), China.
Institutional Review Board Statement
The animal protocols were approved by the Animal Care and Use Committee of Jiangxi Agricultural University (JAXULL-0132) (1 August 2023).
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw sequencing data of colonic microbiota were deposited in the National Center for Biotechnology Information (NCBI) with the accession number PRJNA1300782.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yang, K.; Qing, Y.; Yu, Q.; Tang, X.; Chen, G.; Fang, R.; Liu, H. By-product feeds: Current understanding and future perspectives. Agriculture 2021, 11, 207. [Google Scholar] [CrossRef]
- Vastolo, A.; Calabró, S.; Liotta, L.; Musco, N.; Di Rosa, A.R.; Cutrignelli, M.I.; Chiofalo, B. In vitro fermentation and chemical characteristics of Mediterranean by-products for swine nutrition. Animals 2019, 9, 556. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Panesar, P.S.; Chopra, H.K. Citrus processing by-products: An overlooked repository of bioactive compounds. Crit. Rev. Food Sci. Nutr. 2023, 63, 67–86. [Google Scholar] [CrossRef]
- Mahmoud Abdel Gawad, A.R.; Ahamed Hanafy, M.; Mohamed Mahmoud, A.E.; Hassan Al-Slibi, Y. Effect of tomato pomace, citrus and beet pulp on productive performance and milk quality of Egyptian buffaloes. Pak. J. Med. Sci. 2020, 23, 1210–1219. [Google Scholar]
- Tayengwa, T.; Chikwanha, O.C.; Raffrenato, E.; Dugan, M.E.R.; Mutsvangwa, T.; Mapiye, C. Comparative effects of feeding citrus pulp and grape pomace on nutrient digestibility and utilization in steers. Animal 2021, 15, 100020. [Google Scholar] [CrossRef]
- Zhao, H.; Dong, Z.; Liu, B.; Xiong, H.; Guo, C.; Lakshmanan, P.; Wang, X.; Chen, X.; Shi, X.; Zhang, F. Can citrus production in China become carbon-neutral? A historical retrospect and prospect. Agric. Ecosyst. Environ. 2023, 348, 108412. [Google Scholar] [CrossRef]
- Lu, X.; Zhao, C.; Shi, H.; Liao, Y.; Xu, F.; Du, H.; Xiao, H.; Zheng, J. Nutrients and bioactives in citrus fruits: Different citrus varieties, fruit parts, and growth stages. Rev. Food Sci. Nutr. 2023, 63, 2018–2041. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lee, W.W.; Yang, H.W.; Ryu, B.M.; Cui, Y.R.; Lee, S.C.; Lee, T.G.; Jeon, Y.J. Protective effect of water extract of citrus pomace against AAPH-induced oxidative stress in vitro in Vero cells and in vivo in zebrafish. Prev. Nutr. Food Sci. 2018, 23, 301–308. [Google Scholar] [CrossRef]
- Caputo, L.; Quintieri, L.; Cavalluzzi, M.M.; Lentini, G.; Habtemariam, S. Antimicrobial and antibiofilm activities of citrus water-extracts obtained by microwave-assisted and conventional methods. Biomedicines 2018, 6, 70. [Google Scholar] [CrossRef]
- Abbasi, H.; Seidavi, A.; Liu, W.; Asadpour, L. Investigation on the effect of different levels of dried sweet orange (Citrus sinensis) pulp on performance, carcass characteristics and physiological and biochemical parameters in broiler chicken. Saudi J. Biol. Sci. 2015, 22, 139–146. [Google Scholar] [CrossRef]
- Ahmed, S.T.; Mun, H.S.; Islam, M.M.; Kim, S.S.; Hwang, J.A.; Kim, Y.J.; Yang, C.J. Effects of citrus junos by-products fermented with multistrain probiotics on growth performance, immunity, caecal microbiology and meat oxidative stability in broilers. Br. Poult. Sci. 2014, 55, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Long, X.; He, Z.; Shen, Y.; Yang, Y.; Pan, Y.; Zhang, J.; Li, H. Effect of dietary inclusion of dried citrus pulp on growth performance, carcass characteristics, blood metabolites and hepatic antioxidant status of rabbits. J. Appl. Anim. Ethics. Res. 2017, 46, 529–533. [Google Scholar] [CrossRef]
- Gan, M.; Shen, L.; Fan, Y.; Guo, Z.; Liu, B.; Chen, L.; Tang, G.; Jiang, Y.; Li, X.; Zhang, S.; et al. High altitude adaptability and meat quality in Tibetan pigs: A reference for local pork processing and genetic improvement. Animals 2019, 9, 1080. [Google Scholar] [CrossRef] [PubMed]
- Crosswhite, J.; Myers, N.; Adesogan, A.; Brendemuhl, J.; Johnson, D.; Carr, C. The effect of dietary citrus pulp on the growth, feed efficiency, carcass merit, and lean quality of finishing pigs. Prof. Anim. Sci. 2013, 29, 345–358. [Google Scholar] [CrossRef]
- Cerisuelo, A.; Castelló, L.; Moset, V.; Martínez, M.; Hernández, P.; Piquer, O.; Gómez, E.; Gasa, J.; Lainez, M. The inclusion of ensiled citrus pulp in diets for growing pigs: Effects on voluntary intake, growth performance, gut microbiology and meat quality. Livest. Sci. 2010, 134, 180–182. [Google Scholar] [CrossRef]
- Chen, J.; Jia, X.; Hu, Y.; Zhao, X.; Cheng, Y.; Lu, L.; Zhong, S.; You, J.; Zou, T. Benzoic acid as a dietary supplement mitigates inflammation and intestinal injury in acute enterotoxigenic Escherichia coli-infected mice without adverse effects in healthy mice. Food Funct. 2025, 16, 3195–3210. [Google Scholar] [CrossRef]
- Almeida, V.V.; Nuñez, A.J.C.; Schinckel, A.P.; Alvarenga, P.V.A.; Castelini, F.R.; Silva-Guillen, Y.V.; Thomaz, M.C. Interactive effect of dietary protein and dried citrus pulp levels on growth performance, small intestinal morphology, and hindgut fermentation of weanling pigs. J. Anim. Sci. 2017, 95, 257–269. [Google Scholar]
- O’sullivan, T.; Lynch, P.; Morrissey, P.; O’Grady, J. Evaluation of citrus pulp in diets for sows and growing pigs. Irish J. Agric. Food Res. 2003, 42, 243–253. [Google Scholar]
- Choct, M. Feed non-starch polysaccharides for monogastric animals: Classification and function. Anim. Prod. Sci. 2015, 55, 1360–1366. [Google Scholar] [CrossRef]
- Uerlings, J.; Arevalo Sureda, E.; Schroyen, M.; Kroeske, K.; Tanghe, S.; De Vos, M.; Bruggeman, G.; Wavreille, J.; Bindelle, J.; Purcaro, G.; et al. Impact of citrus pulp or inulin on intestinal microbiota and metabolites, barrier, and immune function of weaned piglets. Front. Nutr. 2021, 8, 650211. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Dai, X.; Chen, Q.; Liu, Y.; Gifty, Z.B.; Sun, W.; Tang, Z. Effect of dietary pomelo peel powder on growth performance, diarrhea, immune function, antioxidant function, ileum morphology, and colonic microflora of weaned piglets. Animals 2022, 12, 3216. [Google Scholar] [CrossRef] [PubMed]
- Aranda-Aguirre, E.; Robles-Jimenez, L.E.; Osorio-Avalos, J.; Vargas-Bello-Pérez, E.; Gonzalez-Ronquillo, M. A systematic-review on the role of exogenous enzymes on the productive performance at weaning, growing and finishing in pigs. Vet. Anim. Sci. 2021, 14, 100195. [Google Scholar] [CrossRef]
- Gao, X.; Yu, B.; Yu, J.; Mao, X.; Huang, Z.; Luo, Y.; Luo, J.; Zheng, P.; Yan, H.; He, J.; et al. Developmental profiling of dietary carbohydrate digestion in piglets. Front. Microbiol. 2022, 13, 896660. [Google Scholar] [CrossRef]
- Pieper, R.; Scharek-Tedin, L.; Zetzsche, A.; Röhe, I.; Kröger, S.; Vahjen, W.; Zentek, J. Bovine milk–based formula leads to early maturation-like morphological, immunological, and functional changes in the jejunum of neonatal piglets. J. Anim. Sci. 2016, 94, 989–999. [Google Scholar] [CrossRef]
- Song, M.; Zhang, F.; Chen, L.; Yang, Q.; Su, H.; Yang, X.; He, H.; Ling, M.; Zheng, J.; Duan, C. Dietary chenodeoxycholic acid improves growth performance and intestinal health by altering serum metabolic profiles and gut bacteria in weaned piglets. Anim. Nutr. 2021, 7, 365–375. [Google Scholar] [CrossRef]
- Park, S.; Lee, J.J.; Yang, B.M.; Cho, J.H.; Kim, S.; Kang, J.; Oh, S.; Park, D.-J.; Perez-Maldonado, R.; Cho, J.-Y. Dietary protease improves growth performance, nutrient digestibility, and intestinal morphology of weaned pigs. J. Anim. Sci. Technol. 2020, 62, 21. [Google Scholar] [CrossRef]
- Saini, R.K.; Ranjit, A.; Sharma, K.; Prasad, P.; Shang, X.; Gowda, K.G.M.; Keum, Y.-S. Bioactive compounds of citrus fruits: A review of composition and health benefits of carotenoids, flavonoids, limonoids, and terpenes. Antioxidants 2022, 11, 239. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, Y.; Mei, H.; Liu, Z.; Li, Z.; Ma, X.; Luo, Z.; Huang, W.; Li, Y.; Yu, M. Feeding citrus pomace fermented with combined probiotics improves growth performance, meat quality, fatty acid profile, and antioxidant capacity in yellow-feathered broilers. Front. Vet. Sci. 2024, 11, 1469947. [Google Scholar] [CrossRef]
- Armet, A.M.; Deehan, E.C.; O’Sullivan, A.F.; Mota, J.F.; Field, C.J.; Prado, C.M.; Lucey, A.J.; Walter, J. Rethinking healthy eating in light of the gut microbiome. Cell Host Microbe 2022, 30, 764–785. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.B.; Alderete, T.L.; Kim, J.S.; Millstein, J.; Gilliland, F.D.; Goran, M.I. High intake of dietary fructose in overweight/obese teenagers associated with depletion of Eubacterium and Streptococcus in gut microbiome. Gut Microbes 2019, 10, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Ishaq, S.L.; Zhao, F.-Q.; Wright, A.-D.G. Colonic inflammation accompanies an increase of β-catenin signaling and Lachnospiraceae/Streptococcaceae bacteria in the hind gut of high-fat diet-fed mice. J. Nutr. Biochem. 2016, 35, 30–36. [Google Scholar] [CrossRef]
- Heidarian, F.; Noormohammadi, Z.; Aghdaei, H.A.; Alebouyeh, M. Relative abundance of Streptococcus spp. and its association with disease activity in inflammatory bowel disease patients compared with controls. Arch. Clin. Infect. Dis. 2017, 12, 10–5812. [Google Scholar] [CrossRef]
- Wang, X.; Chen, H.; Yang, M.; Huang, M.; Zhang, D.; Li, M.; Wang, H.; Zhou, Q.; Lu, L.; Li, Y.; et al. Influence of gut microbiota and immune markers in different stages of colorectal adenomas. Front. Microbiol. 2025, 16, 1556056. [Google Scholar] [CrossRef]
- Zhang, Z.; Lv, J.; Pan, L.; Zhang, Y. Roles and applications of probiotic Lactobacillus strains. Appl. Microbiol. Biotechnol. 2018, 102, 8135–8143. [Google Scholar] [CrossRef]
- Guo, H.; Yu, L.; Tian, F.; Chen, W.; Zhai, Q. The potential therapeutic role of Lactobacillaceae rhamnosus for treatment of inflammatory bowel disease. Foods 2023, 12, 692. [Google Scholar] [CrossRef]
- Parente, E.; Zotta, T.; Giavalisco, M.; Ricciardi, A. Metataxonomic insights in the distribution of Lactobacillaceae in foods and food environments. Int. J. Food Microbiol. 2023, 391–393, 110124. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Li, L.; Zhao, H.; Tu, Y.; Liu, M.; Jiang, L.; Zhao, Y. Characterization of the dynamic changes of ruminal microbiota colonizing citrus pomace waste during rumen incubation for volatile fatty acid production. Microbiol. Spectr. 2023, 11, e0351722. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).