Effects of Ursolic Acid on Immune Function and Antioxidative Capacity in Weaned Rabbits
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design, Animals, and Management
2.2. Sample Collection
2.3. Intestinal Histological Analysis
2.4. Blood and Tissue Indicators
2.5. Cecum-Related Gene Expression
2.6. Data Statistics and Analysis
3. Results
3.1. Growth Performance and Diarrhea Rate
3.2. Organ Indices
3.3. Intestinal Morphology
3.4. Serum and Cecal Immune Capacity
3.5. Serum and Cecum Antioxidant Indicators
3.6. Serum and Cecal Inflammatory Cytokines
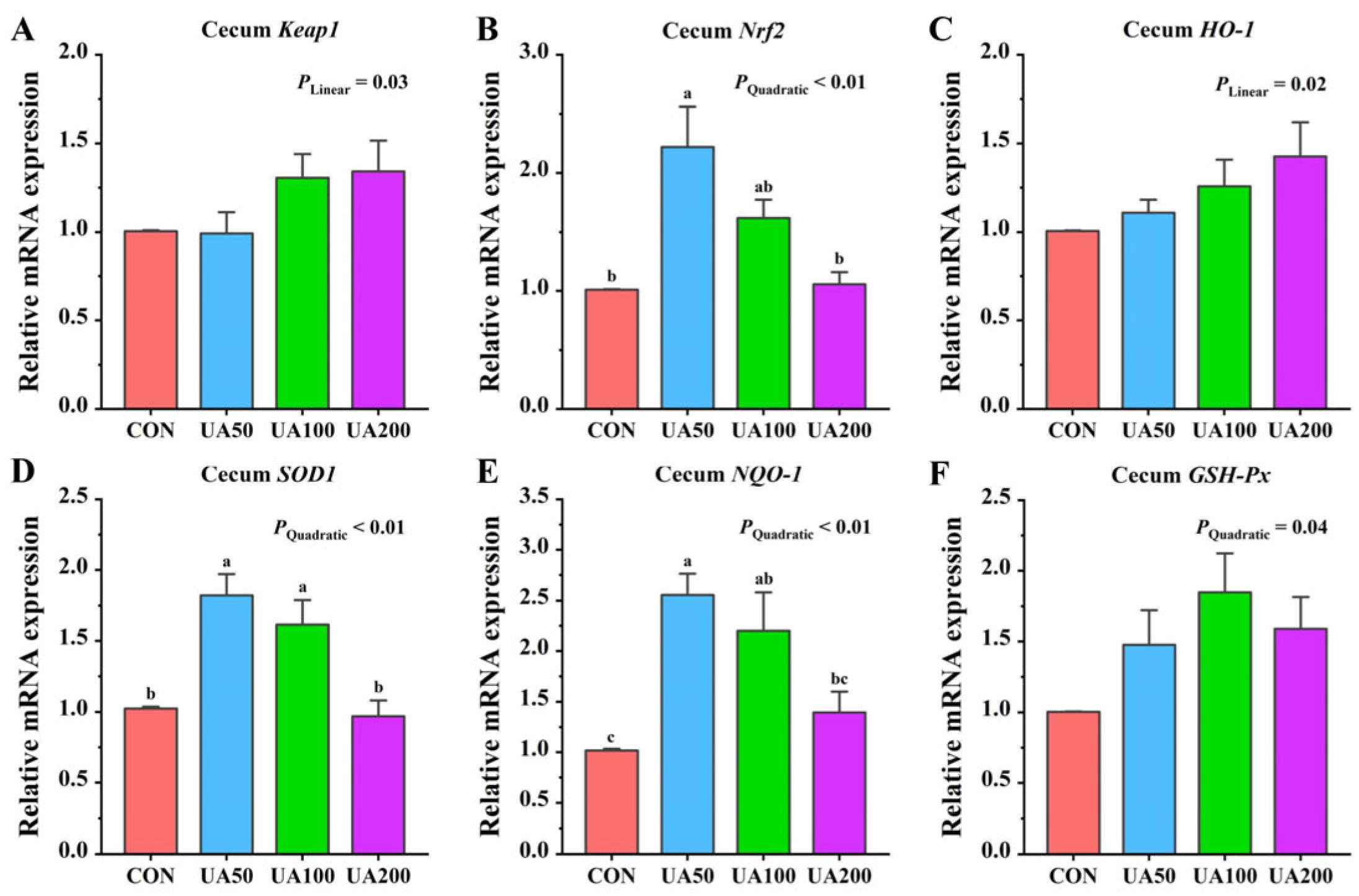
3.7. Cecal-Antioxidant-Related Gene Expression
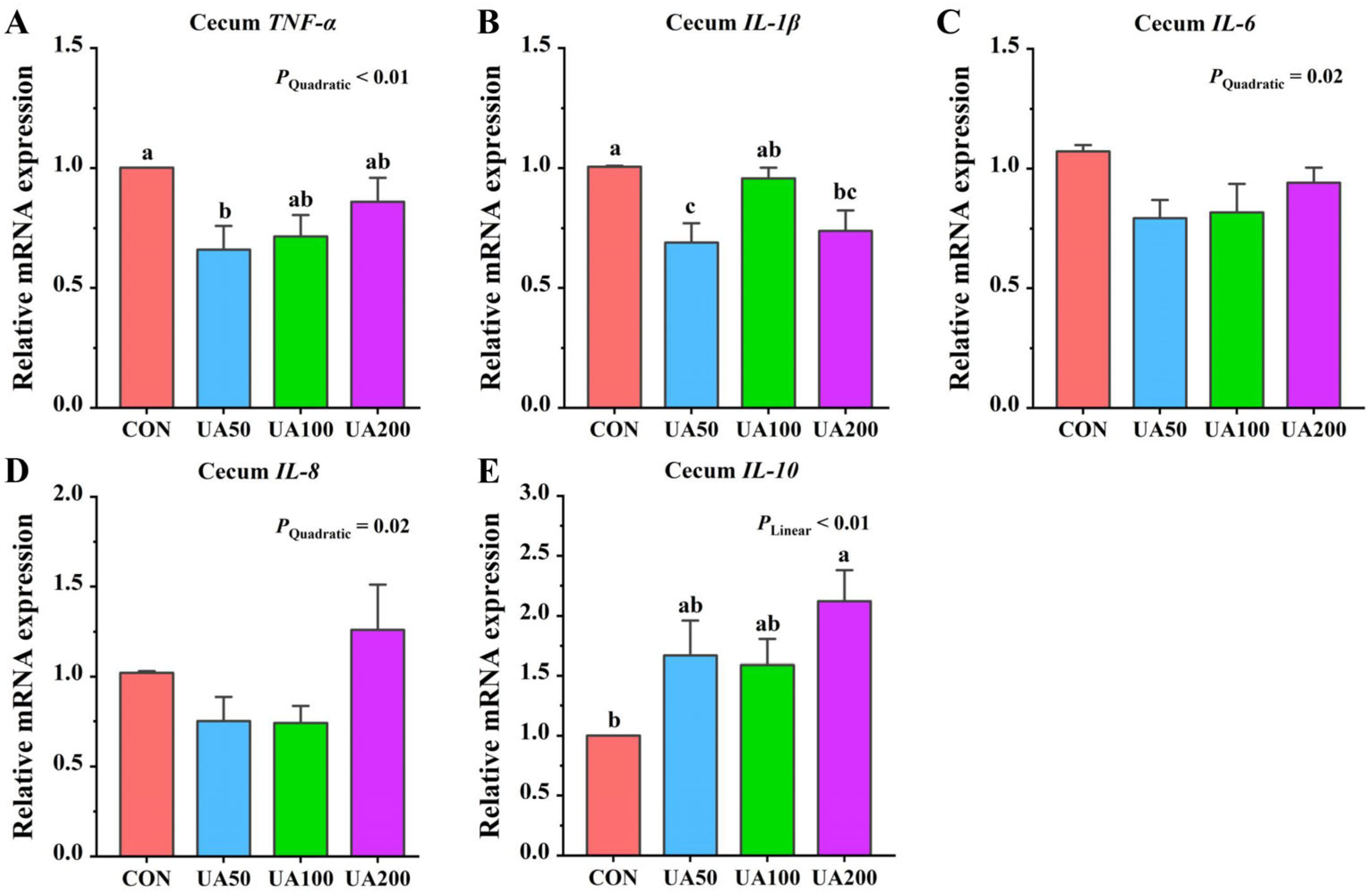
3.8. Cecal Inflammation-Related Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Cerqueira Magalhães, L.C.; Costa, R.B.; de Camargo, G.M.F. Consumption of Rabbit Meat in Brazil: Potential and Limitations. Meat Sci. 2022, 191, 108873. [Google Scholar] [CrossRef]
- Li, S.; Zeng, W.; Li, R.; Hoffman, L.C.; He, Z.; Sun, Q.; Li, H. Rabbit Meat Production and Processing in China. Meat Sci. 2018, 145, 320–328. [Google Scholar] [CrossRef]
- Honrado, A.; Aínsa, A.; Marquina, P.L.; Beltrán, J.A.; Calanche, J.B. Low-Fat Fresh Sausage from Rabbit Meat: An Alternative to Traditional Rabbit Consumption. Meat Sci. 2022, 194, 108973. [Google Scholar] [CrossRef]
- Xia, M.; LI, C.; Wu, D.; Wu, F.; Kong, L.; Jia, Z.; Han, W.; Chen, S.; Fang, W.; Liu, Y.; et al. Benefits of Heat-Killed Lactobacillus Acidophilus on Growth Performance, Nutrient Digestibility, Antioxidant Status, Immunity, and Cecal Microbiota of Rabbits. Front. Vet. Sci. 2024, 11, 1361908. [Google Scholar] [CrossRef]
- Li, H.; Leng, C.; Chen, N.; Ding, Q.; Yuan, Y.; Zheng, Y.; Zhu, G.; Chen, C.; Xu, L.; Shuai, J.; et al. Lactic Acid Bacteria Reduce Bacterial Diarrhea in Rabbits via Enhancing Immune Function and Restoring Intestinal Microbiota Homeostasis. BMC Vet. Res. 2024, 20, 151. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.; Zhang, Z.; Zhu, Y.; Xu, Z.; Liu, W.; Liang, K.; Li, F. Assessment of the Impact of Dietary Supplementation with Epigallocatechin Gallate (EGCG) on Antioxidant Status, Immune Response, and Intestinal Microbiota in Post-Weaning Rabbits. Animals 2024, 14, 3011. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Liu, L.; Li, F.; Chen, J. Grape Seed Proanthocyanidin Extract Improves Growth Performance and Protects against Hydrogen Peroxide-Induced Oxidative Stress to the Liver and Intestine in Weaned Hyla Rabbits. Animals 2025, 15, 327. [Google Scholar] [CrossRef] [PubMed]
- Imbabi, T.; Sabeq, I.; Osman, A.; Mahmoud, K.; Amer, S.A.; Hassan, A.M.; Kostomakhin, N.; Habashy, W.; Easa, A.A. Impact of Fennel Essential Oil as an Antibiotic Alternative in Rabbit Diet on Antioxidant Enzymes Levels, Growth Performance, and Meat Quality. Antioxidants 2021, 10, 1797. [Google Scholar] [CrossRef]
- Cargnin, S.T.; Gnoatto, S.B. Ursolic Acid from Apple Pomace and Traditional Plants: A Valuable Triterpenoid with Functional Properties. Food Chem. 2017, 220, 477–489. [Google Scholar] [CrossRef]
- Liu, G.; Qin, P.; Cheng, X.; Wu, L.; Wang, R.; Gao, W. Ursolic Acid: Biological Functions and Application in Animal Husbandry. Front. Vet. Sci. 2023, 10, 1251248. [Google Scholar] [CrossRef]
- Zhao, M.; Cui, Y.; Wang, F.; Wu, F.; Li, C.; Liu, S. Ursolic Acid Regulates Immune Balance, Modulates Gut Microbial Metabolism, and Improves Liver Health in Mice. Int. J. Mol. Sci. 2024, 25, 10623. [Google Scholar] [CrossRef]
- Zhang, X.W.; Li, X.; Yin, Y.; Wang, M.; Wang, Y.F.; Chen, J.Y.; Zhao, Y.R. Effects of Ursolic Acid on Growth Performance, Serum Biochemistry, Antioxidant Capacity, and Intestinal Health of Broilers. Animal 2025, 19, 101385. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Y.; Li, X.; Yin, Y.; Zhang, X.; Wu, S.; Wang, H.; Zhao, Y. Effects of Dietary Ursolic Acid on Growth Performance and Intestinal Health of Largemouth Bass (Micropterus salmoides). Animals 2024, 14, 2492. [Google Scholar] [CrossRef]
- Sheng, Q.; Li, F.; Chen, G.; Li, J.; Li, J.; Wang, Y.; Lu, Y.; Li, Q.; Li, M.; Chai, K. Ursolic Acid Regulates Intestinal Microbiota and Inflammatory Cell Infiltration to Prevent Ulcerative Colitis. J. Immunol. Res. 2021, 2021, 6679316. [Google Scholar] [CrossRef]
- Chun, J.; Lee, C.; Hwang, S.W.; Im, J.P.; Kim, J.S. Ursolic Acid Inhibits Nuclear Factor-ΚB Signaling in Intestinal Epithelial Cells and Macrophages, and Attenuates Experimental Colitis in Mice. Life Sci. 2014, 110, 23–34. [Google Scholar] [CrossRef] [PubMed]
- NY/T 4049-2021; Nutritional Requirements of Meat Rabbits. Ministry of Agriculture and Rural Affairs: Beijing, China, 2021.
- AOAC. Official Methods of Analysis of AOAC International, 18th ed.; Hortwitz, W., Latimer, G.W., Jr., Eds.; AOAC Int.: Gaithersburg, MD, USA, 2007. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Javidul, M.; Bhuiyan, H.; Jun, H.; Hae, J.; Hien, M.; Lee, H.; Kim, N.; Lee, D.; Yeon, K.; et al. Ursolic Acid Is a PPAR-α Agonist That Regulates Hepatic Lipid Metabolism. Bioorg. Med. Chem. Lett. 2011, 21, 5876–5880. [Google Scholar] [CrossRef]
- He, Y.; Li, Y.; Zhao, T.; Wang, Y.; Sun, C. Ursolic Acid Inhibits Adipogenesis in 3T3-L1 Adipocytes through LKB1/AMPK Pathway. PLoS ONE 2013, 8, e70135. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Zhang, H.; He, X.; Song, Z. Effects of Ursolic Acid on Intestinal Health and Gut Bacteria Antibiotic Resistance in Mice. Front. Physiol. 2021, 12, 650190. [Google Scholar] [CrossRef]
- Salomón, R.; Firmino, J.P.; Reyes-López, F.E.; Andree, K.B.; González-Silvera, D.; Esteban, M.A.; Tort, L.; Quintela, J.C.; Pinilla-Rosas, J.M.; Vallejos-Vidal, E.; et al. The Growth Promoting and Immunomodulatory Effects of a Medicinal Plant Leaf Extract Obtained from Salvia Officinalis and Lippia Citriodora in Gilthead Seabream (Sparus aurata). Aquaculture 2020, 524, 735291. [Google Scholar] [CrossRef]
- Bivolarski, B.L.; Vachkova, E.G. Morphological and Functional Events Associated to Weaning in Rabbits. J. Anim. Physiol. Anim. Nutr. 2014, 98, 9–18. [Google Scholar] [CrossRef]
- He, J.; Su, X.; Guo, S.; Shi, H.; Guo, C.; Li, J.; Lv, J.; Yu, M.; Huang, M. Effects of Compound Essential Oil and Oregano Oil on Production Performance, Immunity and Antioxidant Capacity of Meat Rabbits. Ital. J. Anim. Sci. 2023, 22, 934–941. [Google Scholar] [CrossRef]
- Kong, F.; Wu, F.; Liu, Y.; Lai, N.; Wang, G.; Shen, S.; Han, S.; Li, B.; Zhi, Y.; Chen, S.; et al. Effects of Enzymolytic Soybean Meal on the Growth Performance, Digestive Enzyme Activity, Some Serum Indexes, Carcase Performance and Meat Quality of Rex Rabbits. Ital. J. Anim. Sci. 2022, 21, 1307–1314. [Google Scholar] [CrossRef]
- Molnar, D.S.; Granger, D.A.; Shisler, S.; Eiden, R.D. Prenatal and Postnatal Cigarette and Cannabis Exposure: Effects on Secretory Immunoglobulin A in Early Childhood. Neurotoxicol. Teratol. 2018, 67, 31–36. [Google Scholar] [CrossRef] [PubMed]
- León, E.D.; Francino, M.P. Roles of Secretory Immunoglobulin A in Host-Microbiota Interactions in the Gut Ecosystem. Front. Microbiol. 2022, 13, 880484. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, D.K.; Heilmann, R.M.; Paital, B.; Patel, A.; Yadav, V.K.; Wong, D.; Jergens, A.E. Oxidative Stress, Hormones, and Effects of Natural Antioxidants on Intestinal Inflammation in Inflammatory Bowel Disease. Front. Endocrinol. 2023, 14, 1217165. [Google Scholar] [CrossRef] [PubMed]
- Silvestrini, A.; Meucci, E.; Ricerca, B.M.; Mancini, A. Total Antioxidant Capacity: Biochemical Aspects and Clinical Significance. Int. J. Mol. Sci. 2023, 24, 10978. [Google Scholar] [CrossRef]
- Zhao, M.; Wu, F.; Tang, Z.; Yang, X.; Liu, Y.; Wang, F.; Chen, B. Anti-Inflammatory and Antioxidant Activity of Ursolic Acid: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2023, 14, 1256946. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, T.; He, S.; Zhang, Y.; Tan, Y.; Bai, Y.; Shi, J.; Deng, W.; Qiu, J.; Wang, Z.; et al. Ursolic Acid Reduces Oxidative Stress Injury to Ameliorate Experimental Autoimmune Myocarditis by Activating Nrf2/HO-1 Signaling Pathway. Front. Pharmacol. 2023, 14, 1189372. [Google Scholar] [CrossRef]
- Jia, Z.; Li, W.; Bian, P.; Yang, L.; Liu, H.; Pan, D. Ursolic Acid Treats Renal Tubular Epithelial Cell Damage Induced by Calcium Oxalate Monohydrate via Inhibiting Oxidative Stress and Inflammation. Bioengineered 2021, 12, 5450–5461. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, Z.; Liu, Y.; Kang, X.; Zhang, H.; Meng, M. Suppression of Oxidative Stress and Improvement of Liver Functions in Mice by Ursolic Acid via LKB1-AMP-Activated Protein Kinase Signaling. J. Gastroenterol. Hepatol. 2015, 30, 609–618. [Google Scholar] [CrossRef]
- Zhang, W.; Gan, D.; Jian, J.; Huang, C.; Luo, F.; Wan, S. Protective Effect of Ursolic Acid on the Intestinal Mucosal Barrier in a Rat Model of Liver Fibrosis. Front. Physiol. 2019, 10, 956. [Google Scholar] [CrossRef]
- Pei, J.; Wu, M.; Cai, S.; Peng, J.; Zhan, X.; Wang, D.; Wang, W.; An, N. The Protective Effect of Ursolic Acid on Unilateral Ureteral Obstruction in Rats by Activating the Nrf2/HO-1 Antioxidant Signaling Pathway. Comput. Intell. Neurosci. 2022, 2022, 13. [Google Scholar] [CrossRef]
- Liu, S.; Pi, J.; Zhang, Q. Redox Biology Signal Amplification in the KEAP1-NRF2-ARE Antioxidant Response Pathway. Redox Biol. 2022, 54, 102389. [Google Scholar] [CrossRef]
- Li, L.; Zhang, X.; Cui, L.; Wang, L.; Liu, H.; Ji, H.; Du, Y. Ursolic Acid Promotes the Neuroprotection by Activating Nrf2 Pathway after Cerebral Ischemia in Mice. Brain Res. 2013, 1497, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Ding, J.; Zhang, L.; Liu, C. Protective Effects of Ursolic Acid in an Experimental Model of Liver Fibrosis through Nrf2/ARE Pathway. Clin. Res. Hepatol. Gastroenterol. 2015, 39, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Wang, H.; Zhu, L.; Wei, W. Ursolic Acid Ameliorates Early Brain Injury after Xeperimental Traumatic Brain Injury in Mice by Activating the Nrf2 Pathway. Neurochem. Res. 2017, 42, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Ramirez, C.N.; Su, Z.; Kong, A.T. Epigenetic Modifications of Triterpenoid Ursolic Acid in Activating Nrf2 and Blocking Cellular Transformation of Mouse Epidermal Cells. J. Nutr. Biochem. 2016, 33, 54–62. [Google Scholar] [CrossRef]
- Chen, X.; Wan, Y.; Zhou, T.; Li, J.; Wei, Y. Ursolic Acid Attenuates Lipopolysaccharide-Induced Acute Lung Injury in a Mouse Model. Immunotherapy 2013, 5, 39–47. [Google Scholar] [CrossRef]
- Xu, T.; Wang, X.; Zhong, B.; Roza, I.; Ding, S.; Dong, C. Ursolic Acid Suppresses Interleukin-17 (IL-17) Production by Selectively Antagonizing the Function of RORγt Protein. J. Biol. Chem. 2011, 286, 22707–22710. [Google Scholar] [CrossRef]
- Kobayashi, E.H.; Suzuki, T.; Funayama, R.; Nagashima, T.; Hayashi, M.; Sekine, H.; Tanaka, N.; Moriguchi, T.; Motohashi, H.; Nakayama, K.; et al. Nrf2 Suppresses Macrophage Inflammatory Response by Blocking Proinflammatory Cytokine Transcription. Nat. Commun. 2016, 7, 11624. [Google Scholar] [CrossRef]
- Lei, P.; Li, Z.; Hua, Q.; Song, P.; Gao, L.; Zhou, L.; Cai, Q. Ursolic Acid Alleviates Neuroinflammation after Intracerebral Hemorrhage by Mediating Microglial Pyroptosis via the NF-ΚB/NLRP3/GSDMD Pathway. Int. J. Mol. Sci. 2023, 24, 14771. [Google Scholar] [CrossRef] [PubMed]
- Checker, R.; Sandur, S.K.; Sharma, D.; Patwardhan, R.S.; Jayakumar, S.; Kohli, V.; Sethi, G.; Aggarwal, B.B.; Sainis, K.B. Potent Anti-Inflammatory Activity of Ursolic Acid, a Triterpenoid Antioxidant, Is Mediated through Suppression of NF-ΚB, AP-1 and NF-AT. PLoS ONE 2012, 7, e31318. [Google Scholar] [CrossRef]
| Ingredient | Content | Nutritional Level (2) | Content |
|---|---|---|---|
| Corn | 15.00 | Digestible energy/(MJ/kg) | 10.06 |
| Wheat bran | 15.50 | Dry matter | 89.71 |
| Dried whey | 3.00 | Crude protein | 15.62 |
| Soybean meal | 15.00 | Crude fiber | 19.98 |
| Peanut vine | 8.00 | Ether extract | 2.91 |
| Peanut shell | 20.00 | Neutral detergent fiber | 35.70 |
| Corn germ meal | 3.00 | Acid detergent fiber | 17.98 |
| Soybean oil | 1.00 | Calcium | 1.26 |
| Rice husk | 4.00 | Total phosphorus | 0.62 |
| Chili stalk powder | 12.00 | ||
| Limestone | 1.00 | ||
| CaHPO4 | 0.50 | ||
| NaCl | 0.50 | ||
| L-Lys | 0.35 | ||
| DL-Met | 0.15 | ||
| Premix (1) | 1.00 | ||
| Total | 100.00 |
| Target Gene | Accession No. | Primer Sequence (5′→3′) | Product Size (bp) |
|---|---|---|---|
| TNF-α | NM_001082263.1 | F: GACGGGCTGTACCTCATCTACTC R: ACGGCGAAGCGGCTGAC | 95 |
| IL-1β | NM_001082201.1 | F: TGTCCAGACGAGGGCATCCAG R: GAGCCACAACGACTGACAAGACC | 85 |
| IL-6 | NM_001082064.2 | F: GAGGCACTGGCGGAAGTCAATC R: TCAGCAGGCAGGTCTCATTATTCAC | 94 |
| IL-8 | NM_001082293.1 | F: GCTGTGGCTCTCTTGGCAACC R: ATTTGGGATGGAAAGGTGTGGAGTG | 127 |
| IL-10 | NC_067389.1 | F: AAACAAGAGCAAGGCAGTGG R: GGATGGAGTTCTCCTGGCTT | 170 |
| Keap1 | XM_008251550.3 | F: TCCTCAACCGCCTGCTCTATGC R: TCATCCGCCACTCGTTCCTCTC | 99 |
| Nrf2 | MK645905.1 | F: AAGCAACTCAGCACCTTGTATCTGG R: GAATACATTGCCGTCCCTCGTCTG | 114 |
| HO-1 | XM_002711415.3 | F: CCACCAAGTTCAAGCAGCTCTACC R: TTAGCCTCTTCCACCACCCTCTG | 88 |
| NQO-1 | XM_002711667.3 | F: CAGGAAGGACATCACAGGCAAGC R: CAGAATGGCAGGGACTCCAAACC | 184 |
| SOD1 | NM_001082627.2 | F: AAGGCTGTGTGCGTGCTGAAG R: GTCAGTCCTGTTATGCGTCCCTTG | 107 |
| GSH-Px | NM_001085444.1 | F: CAGGAGAACGCCAAGAATGAGGAG R: GTTCACCTCGCACTTCTGGAAGAG | 105 |
| GAPDH | NM_001082253.1 | F: CCACTTTGTGAAGCTCATTTCCT R: TCTCGTCCTCCTCTGGTGCT | 142 |
| Item | CON | UA Level, mg/kg | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| 50 | 100 | 200 | Treatment | Linear | Quadratic | |||
| IBW, g | 1023.18 | 1008.00 | 1033.25 | 1001.88 | 16.26 | 0.11 | 0.30 | 0.36 |
| FBW, g | 2178.32 | 2220.56 | 2198.66 | 2158.95 | 16.59 | 0.06 | 0.13 | 0.03 |
| ADG, g | 41.27 b | 43.31 a | 41.62 b | 41.32 b | 0.25 | <0.01 | 0.32 | 0.06 |
| ADFI, g | 130.33 b | 135.14 a | 130.21 b | 130.74 ab | 0.66 | 0.02 | 0.38 | 0.21 |
| F/G | 3.16 | 3.12 | 3.13 | 3.16 | 0.02 | 0.76 | 0.77 | 0.38 |
| Diarrhea rate, % | 0.73 | 0.55 | 0.56 | 0.47 | - | 0.86 | - | - |
| Mortality rate, % | 2.50 | 2.50 | 5.00 | 5.00 | - | 0.88 | - | - |
| Item | CON | UA Level, mg/kg | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| 50 | 100 | 200 | Treatment | Linear | Quadratic | |||
| Liver index | 2.89 | 2.89 | 2.86 | 2.99 | 0.05 | 0.86 | 0.52 | 0.60 |
| Kidney index | 0.62 | 0.62 | 0.63 | 0.62 | 0.01 | 0.90 | 0.58 | 0.77 |
| Spleen index | 0.05 | 0.06 | 0.07 | 0.06 | 0.01 | 0.23 | 0.44 | 0.06 |
| Item | CON | UA Level, mg/kg | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| 50 | 100 | 200 | Treatment | Linear | Quadratic | |||
| Villus height, μm | 626.00 b | 788.73 a | 766.35 a | 775.36 a | 20.31 | <0.01 | <0.01 | <0.01 |
| Crypt depth, μm | 128.44 a | 106.77 b | 113.79 ab | 112.54 ab | 2.95 | 0.03 | 0.09 | 0.04 |
| Villus height/crypt depth | 4.88 b | 7.40 a | 6.77 a | 6.89 a | 0.30 | <0.01 | <0.01 | <0.01 |
| Item | CON | UA Level, mg/kg | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| 50 | 100 | 200 | Treatment | Linear | Quadratic | |||
| Serum, μg/mL | ||||||||
| IgM | 1161.91 | 1170.38 | 1185.16 | 1184.36 | 18.91 | 0.97 | 0.68 | 0.83 |
| IgG | 3303.49 | 3421.67 | 3618.64 | 3539.85 | 64.54 | 0.35 | 0.18 | 0.27 |
| IgA | 5100.36 | 5117.62 | 5215.24 | 5321.79 | 67.77 | 0.67 | 0.23 | 0.95 |
| C3 | 232.26 | 240.70 | 231.05 | 237.58 | 3.26 | 0.72 | 0.79 | 0.98 |
| C4 | 227.40 | 245.60 | 246.62 | 240.79 | 2.51 | 0.05 | 0.08 | 0.03 |
| Cecum, pg/mL | ||||||||
| sIgA | 471.16 b | 561.33 a | 517.64 ab | 482.73 b | 10.11 | <0.01 | 0.51 | <0.01 |
| UA Level, mg/kg | SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|
| Item | CON | 50 | 100 | 200 | Treatment | Linear | Quadratic | |
| Serum | ||||||||
| T-AOC, U/mL | 39.56 | 41.75 | 39.31 | 39.35 | 0.61 | 0.44 | 0.55 | 0.61 |
| GSH-PX, U/L | 674.06 | 699.71 | 671.59 | 719.13 | 7.81 | 0.09 | 0.07 | 0.43 |
| SOD, U/mL | 333.78 | 347.37 | 353.14 | 353.59 | 3.57 | 0.17 | 0.06 | 0.20 |
| CAT, U/mL | 105.08 b | 110.02 ab | 110.75 ab | 114.23 a | 1.22 | 0.05 | 0.01 | 0.48 |
| MDA, nmol/L | 14.18 | 13.77 | 14.58 | 14.53 | 0.19 | 0.40 | 0.29 | 0.97 |
| Cecum | ||||||||
| T-AOC, U/mg | 4.06 | 4.55 | 3.99 | 3.75 | 0.13 | 0.21 | 0.17 | 0.37 |
| GSH-PX, U/g | 58.76 | 72.73 | 75.81 | 65.13 | 3.67 | 0.37 | 0.71 | 0.09 |
| SOD, U/mg | 27.37 | 33.90 | 24.63 | 28.21 | 1.50 | 0.11 | 0.22 | 0.67 |
| CAT, U/mg | 10.32 b | 14.69 a | 11.62 ab | 11.92 ab | 0.55 | 0.03 | 0.81 | 0.11 |
| MDA, nmol/g | 1.40 | 1.12 | 1.28 | 1.09 | 0.06 | 0.19 | 0.13 | 0.68 |
| UA Level, mg/kg | SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|
| Item | CON | 50 | 100 | 200 | Treatment | Linear | Quadratic | |
| Serum, pg/mL | ||||||||
| TNF-α | 348.49 a | 267.60 b | 267.38 b | 275.88 b | 7.99 | <0.01 | <0.01 | <0.01 |
| IL-1β | 85.61 | 84.35 | 89.19 | 91.65 | 1.65 | 0.40 | 0.43 | 0.38 |
| IL-6 | 48.91 | 48.15 | 47.00 | 47.69 | 0.53 | 0.67 | 0.13 | 0.87 |
| IL-8 | 171.04 a | 147.00 b | 146.62 b | 130.32 b | 3.65 | <0.01 | <0.01 | 0.12 |
| Cecum, pg/mg | ||||||||
| TNF-α | 65.60 | 64.97 | 63.52 | 61.13 | 0.88 | 0.30 | 0.06 | 0.88 |
| IL-1β | 17.40 a | 17.10 a | 15.90 ab | 15.18 b | 0.27 | <0.01 | <0.01 | 0.69 |
| IL-6 | 28.49 | 28.02 | 28.43 | 27.11 | 0.30 | 0.36 | 0.13 | 0.55 |
| IL-8 | 29.33 | 28.95 | 28.89 | 30.20 | 0.47 | 0.72 | 0.42 | 0.42 |
| IL-10 | 13.33 b | 15.42 a | 15.21 ab | 14.69 ab | 0.29 | 0.03 | 0.20 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Chen, S.; Wu, F.; Chen, B.; Li, C.; Yang, X.; Zhang, G.; Hu, M. Effects of Ursolic Acid on Immune Function and Antioxidative Capacity in Weaned Rabbits. Animals 2025, 15, 2159. https://doi.org/10.3390/ani15152159
Liu Y, Chen S, Wu F, Chen B, Li C, Yang X, Zhang G, Hu M. Effects of Ursolic Acid on Immune Function and Antioxidative Capacity in Weaned Rabbits. Animals. 2025; 15(15):2159. https://doi.org/10.3390/ani15152159
Chicago/Turabian StyleLiu, Yanhua, Saijuan Chen, Fengyang Wu, Baojiang Chen, Chong Li, Xinyu Yang, Gang Zhang, and Man Hu. 2025. "Effects of Ursolic Acid on Immune Function and Antioxidative Capacity in Weaned Rabbits" Animals 15, no. 15: 2159. https://doi.org/10.3390/ani15152159
APA StyleLiu, Y., Chen, S., Wu, F., Chen, B., Li, C., Yang, X., Zhang, G., & Hu, M. (2025). Effects of Ursolic Acid on Immune Function and Antioxidative Capacity in Weaned Rabbits. Animals, 15(15), 2159. https://doi.org/10.3390/ani15152159







