Preliminary Evidence of Chlamydiosis in Koalas of the Greater Geelong Region, Victoria: A Potential Emerging Threat?
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
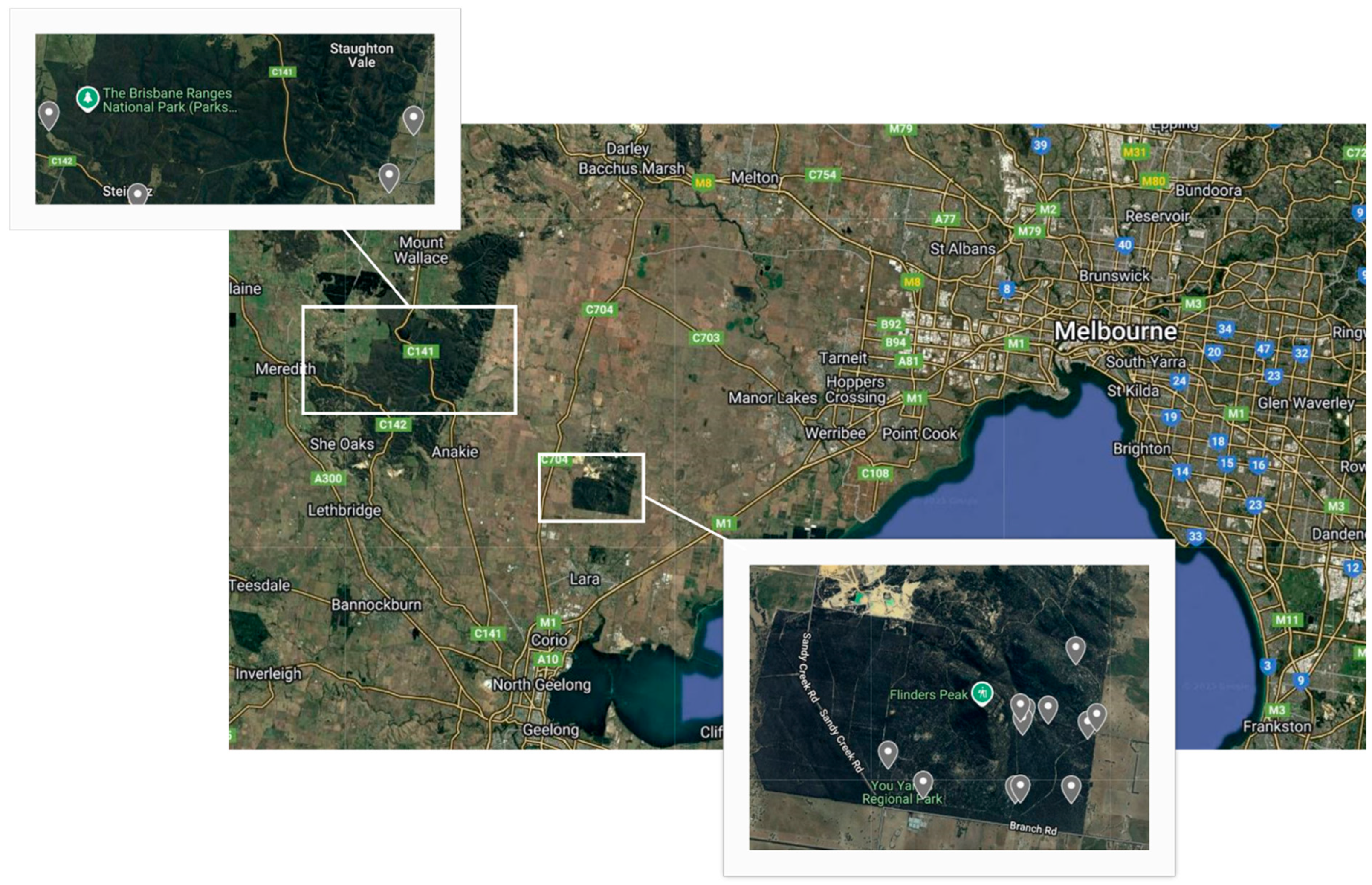
2.1. Sample Collection
2.2. DNA Extraction and Quantitative Polymerase Chain Reaction (qPCR) Analysis
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| YYRP | You Yangs Regional Park |
| BRNP | Brisbane Ranges National Park |
| DNA | Deoxyribonucleic acid |
| qPCR | Quantitative polymerase chain reaction |
| NSW | New South Wales |
| KHH | Koala Health Hub |
| PCR | Polymerase chain reaction |
| mRNA | Messenger ribonucleic acid |
| ompB | Outer membrane protein B |
| CT | Cycle threshold |
| ΔCT | Delta cycle threshold |
| KoRV | Koala retro virus |
| QC-fail | Quality control fail |
References
- Melzer, A.; Carrick, F.; Menkhorst, P.; Lunney, D.; John, B.S. Overview, Critical Assessment, and Conservation Implications of Koala Distribution and Abundance. Conserv. Biol. 2000, 14, 619–628. [Google Scholar] [CrossRef]
- Department of Climate Change Energy the Environment and Water. Koala Listing Under National Environmental Law; Department of Climate Change Energy the Environment and Water: Canberra, ACT, Australia, 2022. Available online: https://www.dcceew.gov.au/environment/biodiversity/threatened/species/koalas/listing-under-national-environmental-law (accessed on 16 April 2025).
- Department of Energy Environment and Climate Action. Victorian Koala Management Strategy; Victorian Government: Melbourne, VIC, Australia, 2023.
- Menkhorst, P. Hunted marooned re-introduced contracepted: Ahistory of Koala management in Victoria. In Too Close for Comfort: Contentious Issues in Human-Wildlife Encounters; Lunney, D., Munn, A., Meikle, W., Eds.; Royal Zoological Society of New South Wales: Sydney, NSW, Australia, 2008. [Google Scholar]
- Whisson, D.A.; Dixon, V.; Taylor, M.L.; Melzer, A. Failure to respond to food resource decline has catastrophic consequences for koalas in a high-density population in southern Australia. PloS ONE 2016, 11, e0144348. [Google Scholar] [CrossRef] [PubMed]
- Wedrowicz, F.; Wright, W.; Schlagloth, R.; Santamaria, F.; Cahir, F. Landscape, koalas and people: A historical account of koala populations and their environment in South Gippsland. Aust. Zool. 2017, 38, 518–536. [Google Scholar] [CrossRef]
- Menkhorst, P.W. Mammals of Victoria: Distribution, Ecology and Conservation; Oxford University Press: Oxford, UK, 1995. [Google Scholar]
- Whisson, D.A.; Ashman, K.R. When an iconic native animal is overabundant: The koala in southern Australia. Conserv. Sci. Pract. 2020, 2, e188. [Google Scholar] [CrossRef]
- Santamaria, F.; Schlagloth, R. The effect of Chlamydia on translocated Chlamydia-naïve koalas: A case study. Aust. Zool. 2016, 38, 192–202. [Google Scholar] [CrossRef]
- Martin, R.W. Overbrowsing decline of a population of the koala Phascolarctos cinereus in Victoria, III. Population dynamics. Wildl. Res. 1985, 12, 377–385. [Google Scholar] [CrossRef]
- Ramsey, D.S.; Brown, G.W.; Tolsma, A.D. Towards a Habitat Condition Assessment Method for Guiding the Management of Overabundant Koala Populations; Arthur Rylah Institute for Environmental Research Technical Report Series No. 272; Department of Environment, Land, Water and Planning: Heidelberg, VIC, Australia, 2016.
- Quigley, B.L.; Timms, P. Helping koalas battle disease—Recent advances in Chlamydia and koala retrovirus (KoRV) disease understanding and treatment in koalas. FEMS Microbiol. Rev. 2020, 44, 583–605. [Google Scholar] [CrossRef]
- Higgins, D.P.; Hemsley, S.; Canfield, P.J. Immuno-histochemical demonstration of the role of Chlamydiaceae in renal, uterine and salpingeal disease of the koala, and demonstration of Chlamydiaceae in novel sites. J. Comp. Pathol. 2005, 133, 164–174. [Google Scholar] [CrossRef]
- Johnston, S.D.; Deif, H.H.; McKinnon, A.; Theilemann, P.; Griffith, J.E.; Higgins, D.P. Orchitis and epididymitis in koalas (Phascolarctos cinereus) infected with Chlamydia pecorum. Vet. Pathol. 2015, 52, 1254–1257. [Google Scholar] [CrossRef]
- Legione, A.R.; Patterson, J.L.S.; Whiteley, P.L.; Amery-Gale, J.; Lynch, M.; Haynes, L.; Gilkerson, J.R.; Polkinghorne, A.; Devlin, J.M.; Sansom, F.M. Sansom, Identification of unusual Chlamydia pecorum genotypes in Victorian koalas (Phascolarctos cinereus) and clinical variables associated with infection. J. Med. Microbiol. 2016, 65, 420–428. [Google Scholar] [CrossRef]
- Polkinghorne, A.; Hanger, J.; Timms, P. Recent advances in understanding the biology, epidemiology and control of chlamydial infections in koalas. Vet. Microbiol. 2013, 165, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Hulse, L.; Beagley, K.; Larkin, R.; Nicolson, V.; Gosálvez, J.; Johnston, S. The effect of Chlamydia infection on koala (Phascolarctos cinereus) semen quality. Theriogenology 2021, 167, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Robbins, A.; Hanger, J.; Jelocnik, M.; Quigley, B.L.; Timms, P. Longitudinal study of wild koalas (Phascolarctos cinereus) reveals chlamydial disease progression in two thirds of infected animals. Sci. Rep. 2019, 9, 13194. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, C.M.; Schmertmann, L.J.; Higgins, D.P.; Casteriano, A.; Irinyi, L.; Mella, V.S.A.; Crowther, M.S.; Meyer, W.; Krockenberger, M.B. Genetic differences in Chlamydia pecorum between neighbouring sub-populations of koalas (Phascolarctos cinereus). Vet. Microbiol. 2019, 231, 264–270. [Google Scholar] [CrossRef]
- Patterson, J.L.S.; Lynch, M.; Anderson, G.A.; Noormohammadi, A.H.; Legione, A.; Gilkerson, J.R.; Devlin, J.M. Prevalence and clinical significance of chlamydia infection in island and mainland populations of Victorian koalas (Phascolarctos cinereus). J. Wildl. Dis. 2015, 51, 309–317. [Google Scholar] [CrossRef]
- Every, K. Evaluation of a decline in population of the koala, Phascolarctos cinereus (Goldfuss) in Ventnor Reserve, Phillip I., Vic., by means of a triple-count technique. Wildl. Res. 1986, 13, 517–525. [Google Scholar] [CrossRef]
- Wedrowicz, F.; Mosse, J.; Wright, W.; Hogan, F.E. Using non-invasive sampling methods to determine the prevalence and distribution of Chlamydia pecorum and koala retrovirus in a remnant koala population with conservation importance. Wildl. Res. 2018, 45, 366–380. [Google Scholar] [CrossRef]
- Adams-Hosking, C.; McBride, M.F.; Baxter, G.; Burgman, M.; de Villiers, D.; Kavanagh, R.; Lawler, I.; Lunney, D.; Melzer, A.; Menkhorst, P.; et al. Use of expert knowledge to elicit population trends for the koala (Phascolarctos cinereus). Divers. Distrib. 2016, 22, 249–262. [Google Scholar] [CrossRef]
- Duffy, J.; Stragliotto, T.; Mella, V.S.A. On the nose: Validating a novel, non-invasive method to identify individual koalas using unique nose patterns. Wildl. Res. 2024, 51, 1–8. [Google Scholar] [CrossRef]
- Mella, V.S.A.; Orr, C.; Hall, L.; Velasco, S.; Madani, G. An insight into natural koala drinking behaviour. Ethology 2020, 126, 858–863. [Google Scholar] [CrossRef]
- Flanagan, C.; Krockenberger, M.B.; Van Stan, J.T.; Duffy, J.; Mella, V.S.A. Koalas (Phascolarctos cinereus) and stemflow: Drinking more than just water. Austral Ecol. 2025, 50, e70076. [Google Scholar] [CrossRef]
- Grogan, L.F.; Berger, L.; Rose, K.; Grillo, V.; Cashins, S.D.; Skerratt, L.F. Surveillance for emerging biodiversity diseases of wildlife. PLoS Pathog. 2014, 10, e1004015. [Google Scholar] [CrossRef] [PubMed]
- Ashman, K.R.; Watchorn, D.J.; Whisson, D.A. Prioritising research efforts for effective species conservation: A review of 145 years of koala research. Mammal Rev. 2019, 49, 189–200. [Google Scholar] [CrossRef]
- Cristescu, R.H.; Miller, R.L.; Schultz, A.J.; Hulse, L.; Jaccoud, D.; Johnston, S.; Hanger, J.; Booth, R.; Frère, C.H. Developing noninvasive methodologies to assess koala population health through detecting Chlamydia from scats. Mol. Ecol. Resour. 2019, 19, 957–969. [Google Scholar] [CrossRef]
- NSW Parliament. Koala Populations and Habitat in New South Wales—Report 3; Parliament of New South Wales: Sydney, NSW, Australia, 2020.
- Simpson, S.J.; Higgins, D.P.; Timms, P.; Mella, V.S.A.; Crowther, M.S.; Fernandez, C.M.; McArthur, C.; Phillips, S.; Krockenberger, M.B. Efficacy of a synthetic peptide Chlamydia pecorum major outer membrane protein vaccine in a wild koala (Phascolarctos cinereus) population. Sci. Rep. 2023, 13, 15087. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Envronment for Statistical Computing, R Foundation for Statistical Computing: Vienna, Austria, 2024.
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Narayan, E. Physiological stress levels in wild koala sub-populations facing anthropogenic induced environmental trauma and disease. Sci. Rep. 2019, 9, 6031. [Google Scholar] [CrossRef]
- Fabijan, J.; Caraguel, C.; Jelocnik, M.; Polkinghorne, A.; Boardman, W.S.J.; Nishimoto, E.; Johnsson, G.; Molsher, R.; Woolford, L.; Timms, P.; et al. Chlamydia pecorum prevalence in South Australian koala (Phascolarctos cinereus) populations: Identification and modelling of a population free from infection. Sci. Rep. 2019, 9, 6261. [Google Scholar] [CrossRef]
- Tarlinton, R.; Meers, J.; Hanger, J.; Young, P. Real-time reverse transcriptase PCR for the endogenous koala retrovirus reveals an association between plasma viral load and neoplastic disease in koalas. J. Gen. Virol. 2005, 86, 783–787. [Google Scholar] [CrossRef]
- Turner, P.J.; Scott, J.K.; Spafford, H. The ecological barriers to the recovery of bridal creeper (Asparagus asparagoides (L.) Druce) infested sites: Impacts on vegetation and the potential increase in other exotic species. Austral Ecol. 2008, 33, 713–722. [Google Scholar] [CrossRef]
- Roberts, D. A history of boneseed control in the You Yangs Regional Park, Victoria. Plant Prot. Q. 2008, 23, 51. [Google Scholar]
- Fernandez, C.M.; Krockenberger, M.B.; Ho, S.Y.; Crowther, M.; Mella, V.S.; Jelocnik, M.; Wilmott, L.; Higgins, D. Novel typing scheme reveals emergence and genetic diversity of Chlamydia pecorum at the local management scale across two koala populations. Vet. Microbiol. 2024, 293, 110085. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.; Giffard, P.; Timms, P. Outer membrane protein a gene sequencing demonstrates the polyphyletic nature of koala Chlamydia pecorum isolates. Syst. Appl. Microbiol. 1997, 20, 187–200. [Google Scholar] [CrossRef]
- Wan, C.; Loader, J.; Hanger, J.; Beagley, K.; Timms, P.; Polkinghorne, A. Using quantitative polymerase chain reaction to correlate Chlamydia pecorum infectious load with ocular, urinary and reproductive tract disease in the koala (Phascolarctos cinereus). Aust. Vet. J. 2011, 89, 409–412. [Google Scholar] [CrossRef]
- Jackson, M.; White, N.; Giffard, P.; Timms, P. Epizootiology of Chlamydia infections in two free-range koala populations. Vet. Microbiol. 1999, 65, 255–264. [Google Scholar] [CrossRef]
- Nyari, S.; Waugh, C.A.; Dong, J.; Quigley, B.L.; Hanger, J.; Loader, J.; Polkinghorne, A.; Timms, P. Epidemiology of chlamydial infection and disease in a free-ranging koala (Phascolarctos cinereus) population. PLoS ONE 2017, 12, e0190114. [Google Scholar] [CrossRef]
- Fernandez, C.M.; Krockenberger, M.B.; Crowther, M.; Mella, V.S.; Wilmott, L.; Higgins, D.P. Genetic markers of Chlamydia pecorum virulence in ruminants support short term host-pathogen evolutionary relationships in the koala, Phascolarctos cinereus. Infect. Genet. Evol. 2023, 116, 105527. [Google Scholar] [CrossRef]
| Beta-Actin | Chlamydia pecorum | Chlamydia Genus (23S) | Result |
|---|---|---|---|
| CT ≤ 32 | no amplification | no amplification | Negative |
| CT ≤ 32 | CT ≤ 34 | CT ≤ 34 | Positive |
| CT ≤ 32 | CT 34–40 | CT 34–40 | Weak positive |
| CT > 32 | CT 34–40 | CT 34–40 | Weak positive |
| CT ≤ 32 | no amplification | CT 34+ | Suspect |
| CT > 32 | no amplification | no amplification | QC-fail |
| no amplification | no amplification | no amplification | QC-fail |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kramer, G.; Duffy, J.; Mella, V.S.A. Preliminary Evidence of Chlamydiosis in Koalas of the Greater Geelong Region, Victoria: A Potential Emerging Threat? Animals 2025, 15, 2048. https://doi.org/10.3390/ani15142048
Kramer G, Duffy J, Mella VSA. Preliminary Evidence of Chlamydiosis in Koalas of the Greater Geelong Region, Victoria: A Potential Emerging Threat? Animals. 2025; 15(14):2048. https://doi.org/10.3390/ani15142048
Chicago/Turabian StyleKramer, Gianna, Janine Duffy, and Valentina S. A. Mella. 2025. "Preliminary Evidence of Chlamydiosis in Koalas of the Greater Geelong Region, Victoria: A Potential Emerging Threat?" Animals 15, no. 14: 2048. https://doi.org/10.3390/ani15142048
APA StyleKramer, G., Duffy, J., & Mella, V. S. A. (2025). Preliminary Evidence of Chlamydiosis in Koalas of the Greater Geelong Region, Victoria: A Potential Emerging Threat? Animals, 15(14), 2048. https://doi.org/10.3390/ani15142048






