Pharmacokinetic Profile of Two Active Dipyrone Metabolites, 4-Methylaminoantipyrine (MAA) and 4-Aminoantipyrine (AA), Following Intravenous Administration in Dogs: A Preliminary Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Study Preparation
2.2. Pharmacokinetic Study
2.3. Sample Extraction Procedures
2.4. Analytical UPLC-MS/MS Conditions
2.5. Validation
2.6. Pharmacokinetic Analysis
2.7. Statistical Analyses
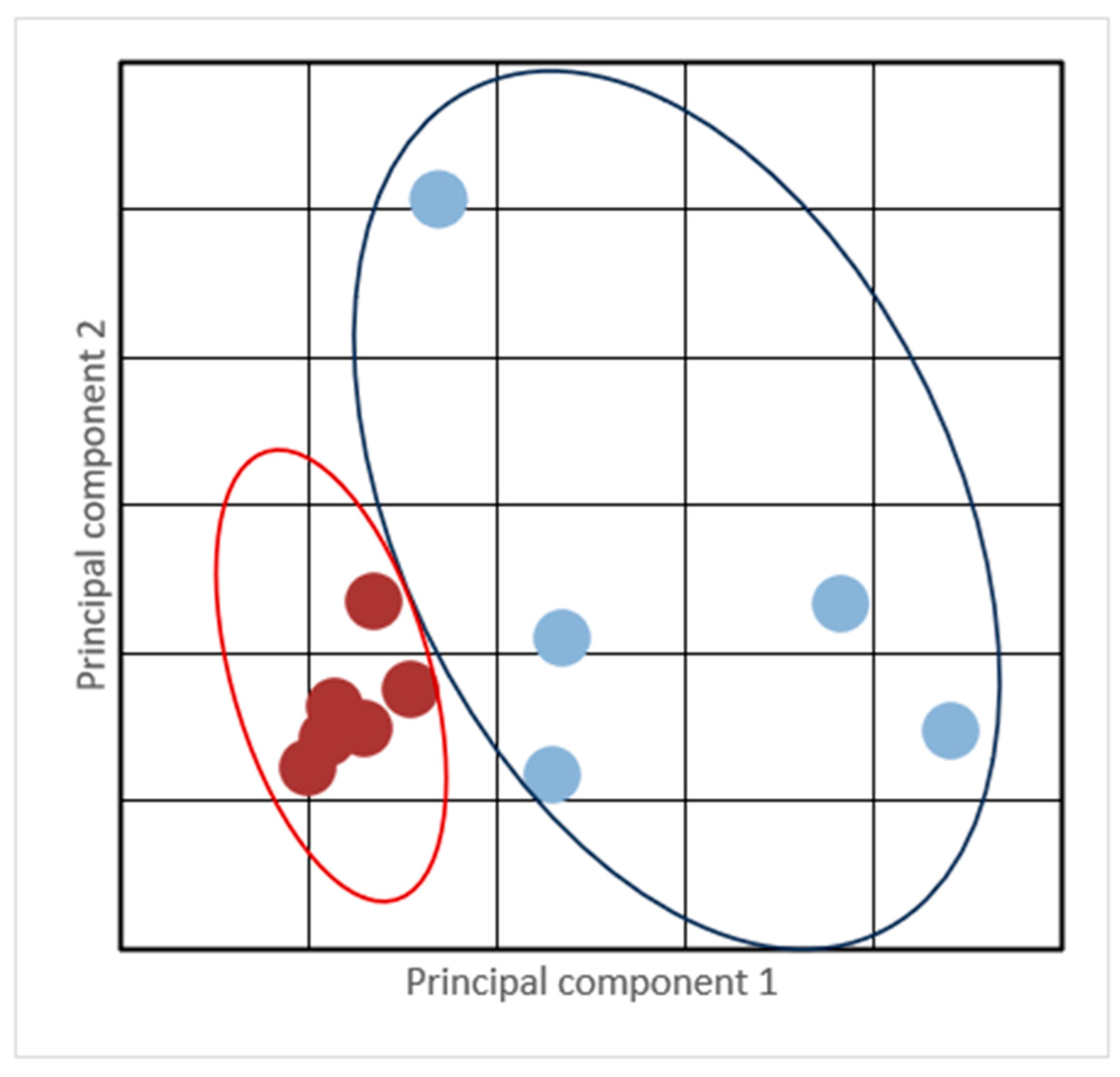
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Belshaw, Z.; Yeates, J. Assessment of quality of life and chronic pain in dogs. Vet. J. 2018, 239, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Epstein, M.E.; Rodan, I.; Griffenhagen, G.; Kadrlik, J.; Petty, M.C.; Robertson, S.A.; Simpson, W. AAHA/AAFP Pain Management Guidelines for Dogs and Cats. J. Feline Med. Surg. 2015, 17, 251–272. [Google Scholar] [CrossRef]
- Gruen, M.E.; Lascelles, B.D.X.; Colleran, E.; Gottlieb, A.; Johnson, J.; Lotsikas, P.; Marcellin-Little, D.; Wright, B. AAHA Pain Management Guidelines for Dogs and Cats. J. Am. Anim. Hosp. Assoc. 2022, 58, 55–76. [Google Scholar] [CrossRef]
- Baumgartner, C.M.; Koenighaus, H.; Ebner, J.K.; Henke, J.; Schuster, T.; Erhardt, W.D. Cardiovascular effects of dipyrone and propofol on hemodynamic function in rabbits. Am. J. Vet. Res. 2009, 70, 1407–1415. [Google Scholar] [CrossRef]
- Chandrasekharan, N.V.; Dai, H.; Roos, K.L.; Evanson, N.K.; Tomsik, J.; Elton, T.S.; Simmons, D.L. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: Cloning, structure, and expression. Proc. Natl. Acad. Sci. USA 2002, 99, 13926–13931. [Google Scholar] [CrossRef]
- Jasiecka, A.; Maślanka, T.; Jaroszewski, J.J. Pharmacological characteristics of metamizole. Pol. J. Vet. Sci. 2014, 17, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Hedenmalm, K.; Spigset, O. Agranulocytosis and other blood dyscrasias associated with dipyrone (metamizole). Eur. J. Clin. Pharmacol. 2002, 58, 265–274. [Google Scholar] [CrossRef]
- Currie, G.M. Pharmacology, Part 2: Introduction to Pharmacokinetics. J. Nucl. Med. Technol. 2018, 6, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, M.; Łebkowska-Wieruszewska, B.; Lisowski, A.; Owen, H.; Poapolathep, A.; Kim, T.W.; De Vito, V. Pharmacokinetic profiles of the active metamizole metabolites after four different routes of administration in healthy dogs. J. Vet. Pharmacol. Ther. 2018, 41, 428–436. [Google Scholar] [CrossRef]
- Paula, V.V.; Araújo-Silva, G.; Fernandes, N.S.; Mouta, A.N.; Nunes, T.L.; De Paiva, A.L.C.; De Macêdo, L.B.; Arcoverde, K.N.; Urizar, J.T.P. Pharmacokinetic profiles of the two major active metabolites of metamizole, 4-methylaminoantipyrine (MAA) and 4-aminoantipyrine (AA), after intravenous injection in cats. Res. Vet. Sci. 2023, 155, 156–160. [Google Scholar] [CrossRef]
- Aupanun, S.; Laus, F.; Poapolathep, A.; Owen, H.; Vullo, C.; Faillace, V.; Giorgi, M. Pharmacokinetic assessment of the marker active metabolites 4-methyl-amino-antipyrine and 4-acetyl-amino-antipyrine after intravenous and intramuscular injection of metamizole (dipyrone) in Healthy Donkeys. J. Equine Vet. Sci. 2016, 47, 55–61. [Google Scholar] [CrossRef]
- Giorgi, M.; Aupanun, S.; Lee, H.K.; Poapolathep, A.; Rychshanova, R.; Vullo, C.; Faillace, V.; Laus, F. Pharmacokinetic profiles of the active metamizole metabolites in healthy horses. J. Vet. Pharmacol. Ther. 2017, 40, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Burmańczuk, A.; Kowalski, C.; Giorgi, M.; Owen, H.; Grabowski, T. Pharmacokinetic investigations of the marker active metabolites 4-methylamino-antipyrine and 4-amino-antipyrine after intramuscular injection of metamizole in healthy piglets. J. Vet. Pharmacol. Ther. 2016, 39, 616–620. [Google Scholar] [CrossRef]
- Macêdo, L.B.; Mouta, A.N.; Araújo-Silva, G.; Urizar, J.T.P.; Paula, V.V. Pharmacokinetic properties of metamizole active metabolites in Northeastern Brazilian donkeys (Equus asinus). J. Vet. Pharmacol. Ther. 2021, 44, 842–849. [Google Scholar] [CrossRef]
- Bachmann, F.; Meyer, Z.U.; Schwabedissen, H.E.; Duthaler, U.; Krähenbühl, S. Cytochrome P450 1A2 is the most important enzyme for hepatic metabolism of the metamizole metabolite 4-methylaminoantipyrine. Br. J. Clin. Pharmacol. 2022, 88, 1885–1896. [Google Scholar] [CrossRef]
- Lutz, M. Metamizole (Dipyrone) and the Liver: A Review of the Literature. J. Clin. Pharmacol. 2019, 59, 1433–1442. [Google Scholar] [CrossRef]
- Levy, M.; Zylber-Katz, E.; Rosenkranz, B. Clinical pharmacokinetics of dipyrone and its metabolites. Clin. Pharmacokinet 1995, 28, 216–234. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.W.; Łebkowska-Wieruszewska, B.; Sitovs, A.; Poapolathep, A.; Owen, H.; Lisowski, A.; Abilova, Z.; Giorgi, M. Pharmacokinetic profiles of metamizole (dipyrone) active metabolites in goats and its residues in milk. J. Vet. Pharmacol. Ther. 2018, 41, 699–705. [Google Scholar] [CrossRef]
- Kalchofner, G.; Schwarz, K.S.; Wuhrmann, A.; Feldmann, R.; Hartnack, S.; Bettschart-Wolfensberger, R. Comparison of a new metamizole formulation and carprofen for extended post-operative analgesia in dogs undergoing ovariohysterectomy. Vet. J. 2015, 204, 99–104. [Google Scholar] [CrossRef]
- Agência Nacional de Vigilância Sanitária. Resolução Da Diretoria Colegiada-RDC Nº; ANVISA: Brasília, Brazil, 2017; p. 166. Available online: http://portal.anvisa.gov.br/ (accessed on 2 May 2023).
- Arcoverde, K.N.; Alves, L.S.A.; Cavalcante, J.M.; Maranhão, A.C.P.M.; Jurema, A.P.; Araújo-Silva, G.; Urizar, J.T.P.; De Paula, V.V. Pharmacotherapeutic monitoring of dipyrone in northeastern Brazilian donkeys (Equus asinus). Res. Vet. Sci. 2023, 164, 105034. [Google Scholar] [CrossRef]
- Araújo-Silva, G.; De Macêdo, L.B.; Mouta, A.N.; De Oliveira, M.G.C.; Arcoverde, K.N.; Solon, L.G.S.; Perez-Urizar, J.T.; De Paula, V.V. Tramadol and M1 Bioavailability Induced by Metamizole Co-Administration in Donkeys (Equus asinus). Animals 2024, 14, 929. [Google Scholar] [CrossRef] [PubMed]
- Imagawa, V.H.; Fantoni, D.T.; Tatarunas, A.C.; Mastrocinque, S.; Almeida, T.F.; Ferreira, F.; Posso, I.P. The use of different doses of metamizol for post-operative analgesia in dogs. Vet. Anaesth. Analg. 2011, 38, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Zanuzzo, F.S.; Teixeira-Neto, F.J.; Teixeira, L.R.; Diniz, M.S.; Souza, V.L.; Thomazini, C.M.; Steagall, P.V. Analgesic and antihyperalgesic effects of dipyrone, meloxicam or a dipyrone-meloxicam combination in bitches undergoing ovariohysterectomy. Vet. J. 2015, 205, 33–37. [Google Scholar] [CrossRef]
- Hinz, B.; Cheremina, O.; Bachmakov, J.; Renner, B.; Zolk, O.; Fromm, M.F.; Brune, K. Dipyrone elicits substantial inhibition of peripheral cyclooxygenases in humans: New insights into the pharmacology of old analgesic. FASEB J. 2007, 21, 2343–2351. [Google Scholar] [CrossRef] [PubMed]
- Knights, K.M.; Mangoni, A.A.; Miners, J.O. Defining the COX inhibitor selectivity of NSAIDs: Implications for understanding toxicity. Expert. Rev. Clin. Pharmacol. 2010, 3, 769–776. [Google Scholar] [CrossRef]
- Mazaleuskaya, L.L.; Ricciotti, E. Druggable Prostanoid Pathway. Adv. Exp. Med. Biol. 2020, 1274, 29–54. [Google Scholar] [CrossRef]
- Escobar, W.; Ramirez, K.; Avila, C.; Limongi, R.; Vanegas, H.; Vazquez, E. Metamizol, a non-opioid analgesic, acts via endocannabinoids in the PAG-RVM axis during inflammation in rats. Eur. J. Pain. 2012, 6, 676–689. [Google Scholar] [CrossRef]
- Pereira, M.A.; Campos, K.D.; Gonçalves, L.A.; Dos Santos, R.S.; Flôr, P.B.; Ambrósio, A.M.; Otsuki, D.A.; Matera, J.M.; Gomes, C.O.; Fantoni, D.T. Cyclooxygenases 1 and 2 inhibition and analgesic efficacy of dipyrone at different doses or meloxicam in cats after ovariohysterectomy. Vet. Anaesth. Analg. 2020, 48, 7–16. [Google Scholar] [CrossRef]
- Chae, H.; Cho, S.E.; Park, H.D.; Chun, S.; Lee, Y.W.; Yun, Y.M.; Song, S.H.; Lee, S.G.; Lee, K.; Song, J.; Lee, S.Y. Clinical Mass Spectrometry Research Committee of Korean Society of Clinical Chemistry. Use of Liquid Chromatography-Tandem Mass Spectrometry for Clinical Testing in Korean Laboratories: A Questionnaire Survey. Ann. Lab. Med. 2019, 39, 447–453. [Google Scholar] [CrossRef]
- Carrasco-Portugal, M.C.; Flores-Murrieta, F.J. Gender Differences in the Pharmacokinetics of Oral Drugs. Sci. Rep. 2011, 2, 31–41. [Google Scholar] [CrossRef]
- Fleischer, S.; Sharkey, M.; Mealey, K.; Ostrander, E.A.; Martinez, M. Pharmacogenetic and Metabolic Differences Between Dog Breeds: Their Impact on Canine Medicine and the Use of the Dog as a Preclinical Animal Model. AAPS J. 2008, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Fux, D.; Metzner, M.; Brandl, J.; Feist, M.; Behrendt-Wippermann, M.; Von Thaden, A.; Baumgartner, C. Pharmacokinetics of metamizole (dipyrone) as an add-on in calves undergoing umbilical surgery. PLoS ONE 2022, 15, e0265305. [Google Scholar] [CrossRef] [PubMed]
- Giantin, M.; Carletti, M.; Capolongo, F.; Pegolo, S.; Lopparelli, R.M.; Gusson, F. Effect of breed upon cytochromes P450 and phase II enzyme expression in cattle liver. Drug Metab. Dispos. 2008, 36, 885–893. [Google Scholar] [CrossRef]
- Lebkowska-Wieruszewska, B.; Kim, T.W.; Chea, B.; Owen, H.; Poapolathep, A.; Giorgi, M. Pharmacokinetic profiles of the two major active metabolites of metamizole (dipyrone) in cats following three different routes of administration. J. Vet. Pharmacol. Ther. 2017, 41, 334–339. [Google Scholar] [CrossRef] [PubMed]
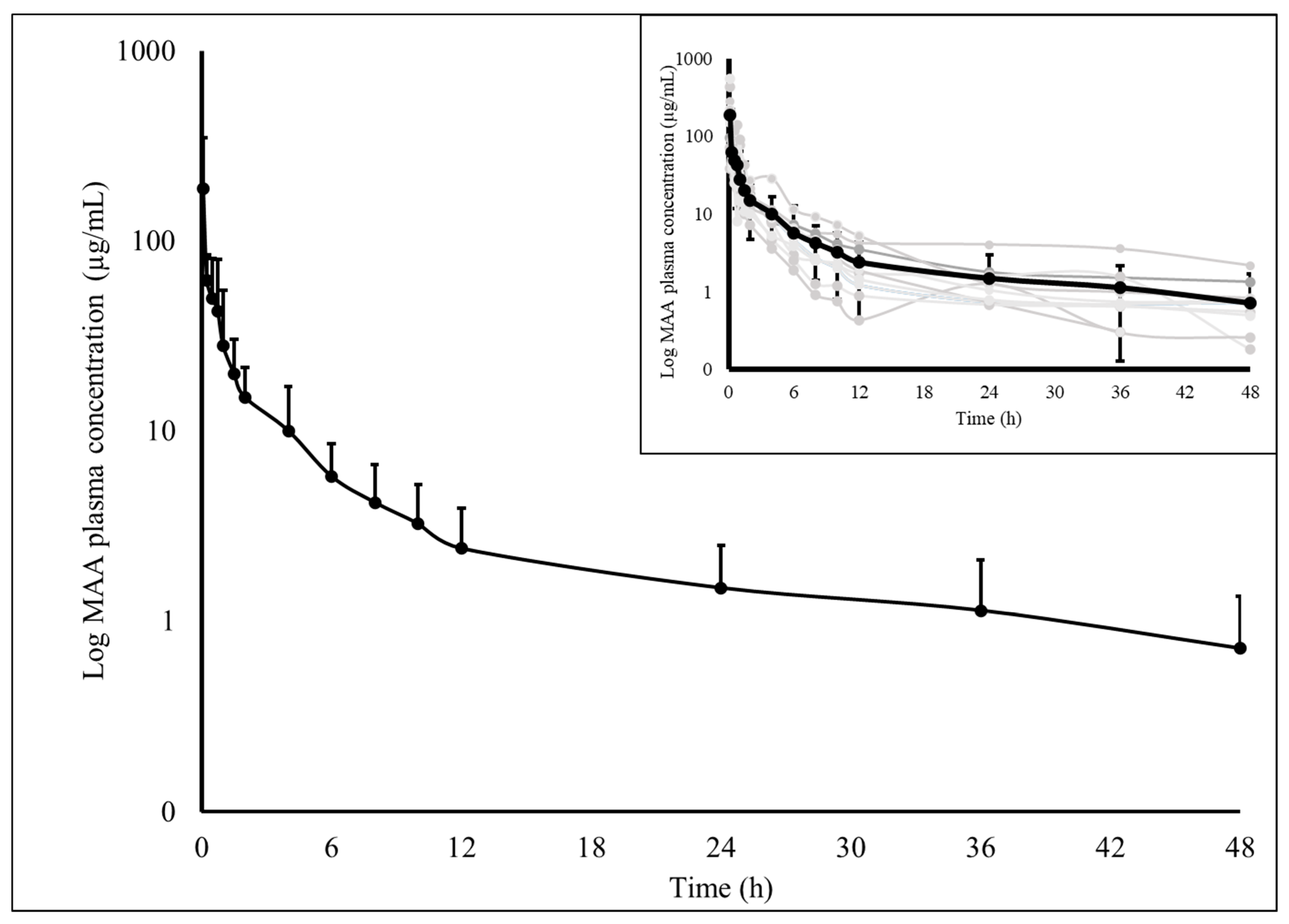
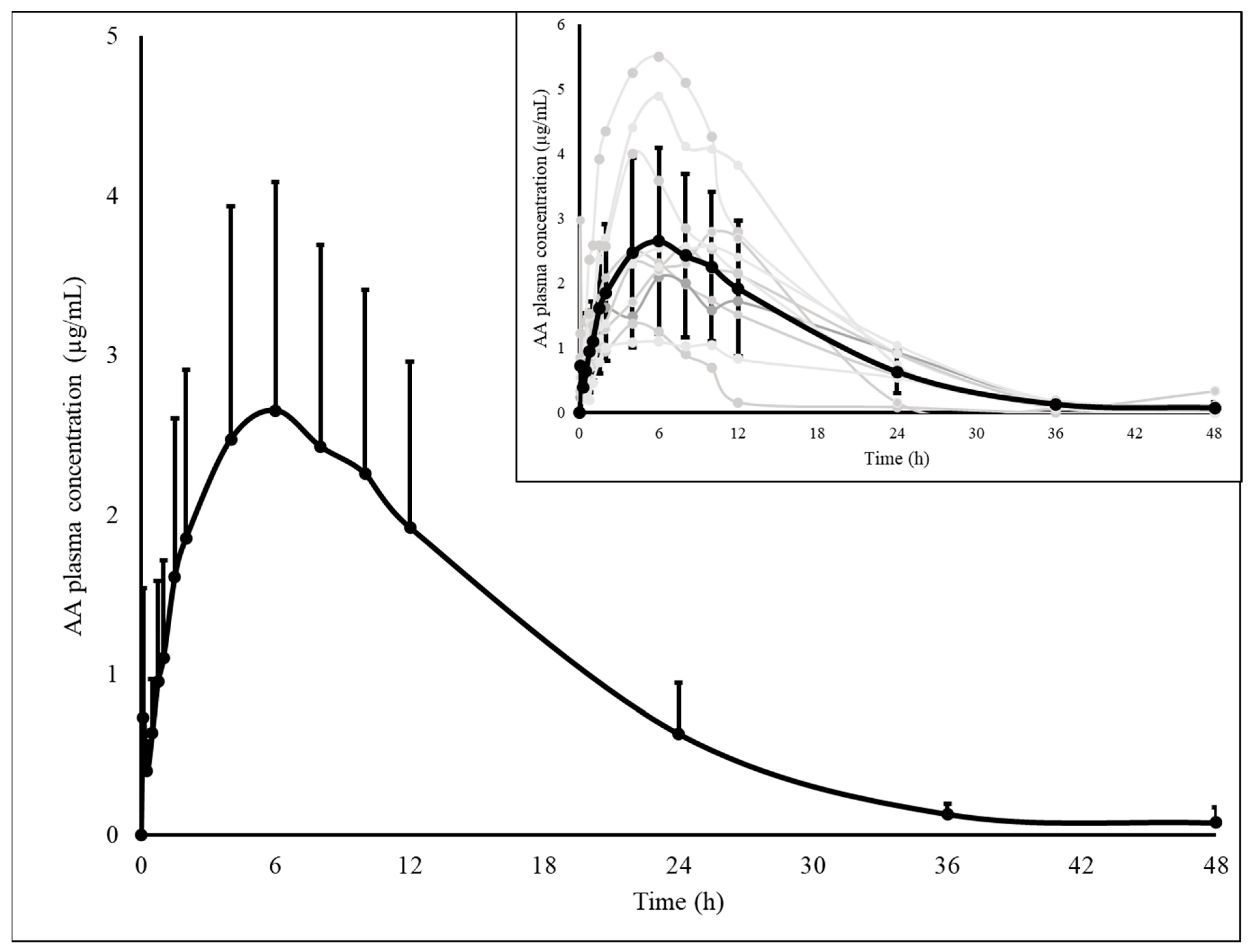

| MAA | MAA (Giorgi et al., 2018) [9] | AA | AA (Giorgi et al., 2018) [9] | ||
|---|---|---|---|---|---|
| Parameter | Unit | Means ± SD | Means ± SD | Means ± SD | Means ± SD |
| Lambda_z | 1/h | 0.06 ± 0.08 | 0.13 ± 0.04 | 0.11 ± 0.03 | 0.09 ± 0.03 |
| T ½ | h | 26.39 ± 19.29 | 5.94 ± 2.54 | 6.72 ± 1.66 | 8.05 ± 2.56 |
| Cmax | µg/mL | 203.68 ± 159.24 | 21.80 ± 2.45 | 2.80 ± 1.43 | 1.29 ± 0.21 |
| C0 | µg/mL | 409.23 ± 524.72 | - | - | - |
| Tmax | h | - | - | 6.18 ± 2.27 | 5.33 ± 1.63 |
| AUC 0-t | µg/mL × h | 205.71 ± 108.18 | 45.34 ± 9.64 | 43.87 ± 18.94 | 17.97 ± 2.91 |
| AUC 0-inf_obs | µg/mL × h | 240.02 ± 127.38 | 46.79 ± 9.15 | - | 21.08 ± 4.34 |
| AUC 0-t/0-inf_obs | 0.88 ± 0.12 | - | - | - | |
| AUMC 0-inf_obs | µg/mL × h2 | 5164.94 ± 5735.48 | 246.57 ± 31.01 | 540.32 ± 245.01 | 273.97 ± 117.74 |
| MRT 0-inf_obs | h | 18.88 ± 17.07 | 5.37 ± 0.81 | 12.09 ± 2.79 | 12.62 ± 3.13 |
| Vd | (L/kg) | 4.38 ± 3.62 | 4.95 ± 0.99 | - | - |
| Vss | (L/kg) | - | - | 6.72 ± 3.74 | 13.85 ± 3.68 |
| Cl | (mL/h/kg) | 147.67 ± 103.85 | 552.43 ± 98.34 | 712.76 ± 45.23 | 1.224.03 ± 228.74 |
| MAA | All Dogs (n = 11) | Slow Metabolized (SL) (n = 5) | Normal/Quick Metabolized (NM) (n = 6) | |||
|---|---|---|---|---|---|---|
| Parameter | Unit | Means ± SD | Means ± SD | Median (Max–Min) | Means ± SD | Median (Max–Min) |
| Lambda_z | 1/h | 0.06 ± 0.08 | 0.02 ± 0.004 | 0.017 (0.020–0.011) | 0.09 ± 0.09 | 0.05 (0.29–0.04) |
| T ½ | h | 26.39 ± 19.29 | 44.44 ± 11.74 * | 38 (58.35–33.57) * | 11.25 ± 5.37 | 12.58 (16.8–2.35) |
| Cmax | µg/mL | 203.68 ± 159.24 | 238.29 ± 197.92 | 223.68 (548.74–44.13) | 174.84 ± 131.07 | 149.14 (428.98–71.78) |
| C0 | µg/mL | 409.23 ± 524.72 | 606.69 ± 757.10 | 434.48 (1912.98–38.00) | 244.67 ± 143.60 | 211.38 (493.71–77.45) |
| Tmax | h | - | - | - | - | |
| AUC 0-t | µg/mL×h | 205.71 ± 108.18 | 239.78 ± 88.95 | 250.42 (336.07–103.95) | 177.32 ± 122.27 | 166.84 (404.65–67.37) |
| AUC 0-inf_obs | µg/mL×h | 240.02 ± 127.38 | 306.76 ± 106.84 * | 326.29 (441.35–148.5) * | 184.41 ± 123.09 | 174.99 (407.78–69.99) |
| AUC 0-t/0-inf_obs | 0.88 ± 0.12 | 0.78 ± 0.1 * | 0.76 (0.90–0.65) * | 0.95 ± 0.04 | 0.95 (0.99–0.90) | |
| AUMC 0-inf_obs | µg/mL×h2 | 5164.94 ± 5735.48 | 9733.64 ± 5762.53 | 6899.49 (17,240.51–3583.31) | 1357.68 ± 979.22 | 1281.33 (2857.61–153.47 |
| MRT 0-inf_obs | h | 18.88 ± 17.07 | 32.62 ± 16.53 * | 32.43 (52.83–12.97) * | 7.44 ± 4.25 | 7.18 (14.09–2.02) |
| Vd | (L/kg) | 4.38 ± 3.62 | 6.32 ± 4.29 | 4.71 (13.62–2.74) | 2.77 ± 2.13 | 2.33 (6.77–1.06) |
| Cl | (mg/kg)/(ug/mL)/h | 0.15 ± 0.10 | 0.09 ± 0.04 | 0.07 (0.16–0.05) | 0.19 ± 0.12 | 0.14 (0.35–0.06) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mouta, A.N.; Arcoverde, K.N.; Fernandes, N.S.; Passos, Y.D.B.; Oliveira, C.V.A.d.; Honorato, R.A.; Araujo-Silva, G.; Paula, V.V.d. Pharmacokinetic Profile of Two Active Dipyrone Metabolites, 4-Methylaminoantipyrine (MAA) and 4-Aminoantipyrine (AA), Following Intravenous Administration in Dogs: A Preliminary Study. Animals 2025, 15, 1666. https://doi.org/10.3390/ani15111666
Mouta AN, Arcoverde KN, Fernandes NS, Passos YDB, Oliveira CVAd, Honorato RA, Araujo-Silva G, Paula VVd. Pharmacokinetic Profile of Two Active Dipyrone Metabolites, 4-Methylaminoantipyrine (MAA) and 4-Aminoantipyrine (AA), Following Intravenous Administration in Dogs: A Preliminary Study. Animals. 2025; 15(11):1666. https://doi.org/10.3390/ani15111666
Chicago/Turabian StyleMouta, Andressa N., Kathryn N. Arcoverde, Naftáli S. Fernandes, Yanna D. B. Passos, Caio V. A. de Oliveira, Robson A. Honorato, Gabriel Araujo-Silva, and Valéria V. de Paula. 2025. "Pharmacokinetic Profile of Two Active Dipyrone Metabolites, 4-Methylaminoantipyrine (MAA) and 4-Aminoantipyrine (AA), Following Intravenous Administration in Dogs: A Preliminary Study" Animals 15, no. 11: 1666. https://doi.org/10.3390/ani15111666
APA StyleMouta, A. N., Arcoverde, K. N., Fernandes, N. S., Passos, Y. D. B., Oliveira, C. V. A. d., Honorato, R. A., Araujo-Silva, G., & Paula, V. V. d. (2025). Pharmacokinetic Profile of Two Active Dipyrone Metabolites, 4-Methylaminoantipyrine (MAA) and 4-Aminoantipyrine (AA), Following Intravenous Administration in Dogs: A Preliminary Study. Animals, 15(11), 1666. https://doi.org/10.3390/ani15111666







