Coagulation Status Assessment in Dogs with Chronic Enteropathy Using Viscoelastic Point-of-Care Coagulation Monitor
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Laboratory Testing
2.3. Coagulation Testing
2.4. Disease Categorization
2.5. Statistical Analysis
3. Results
3.1. Group Characteristics
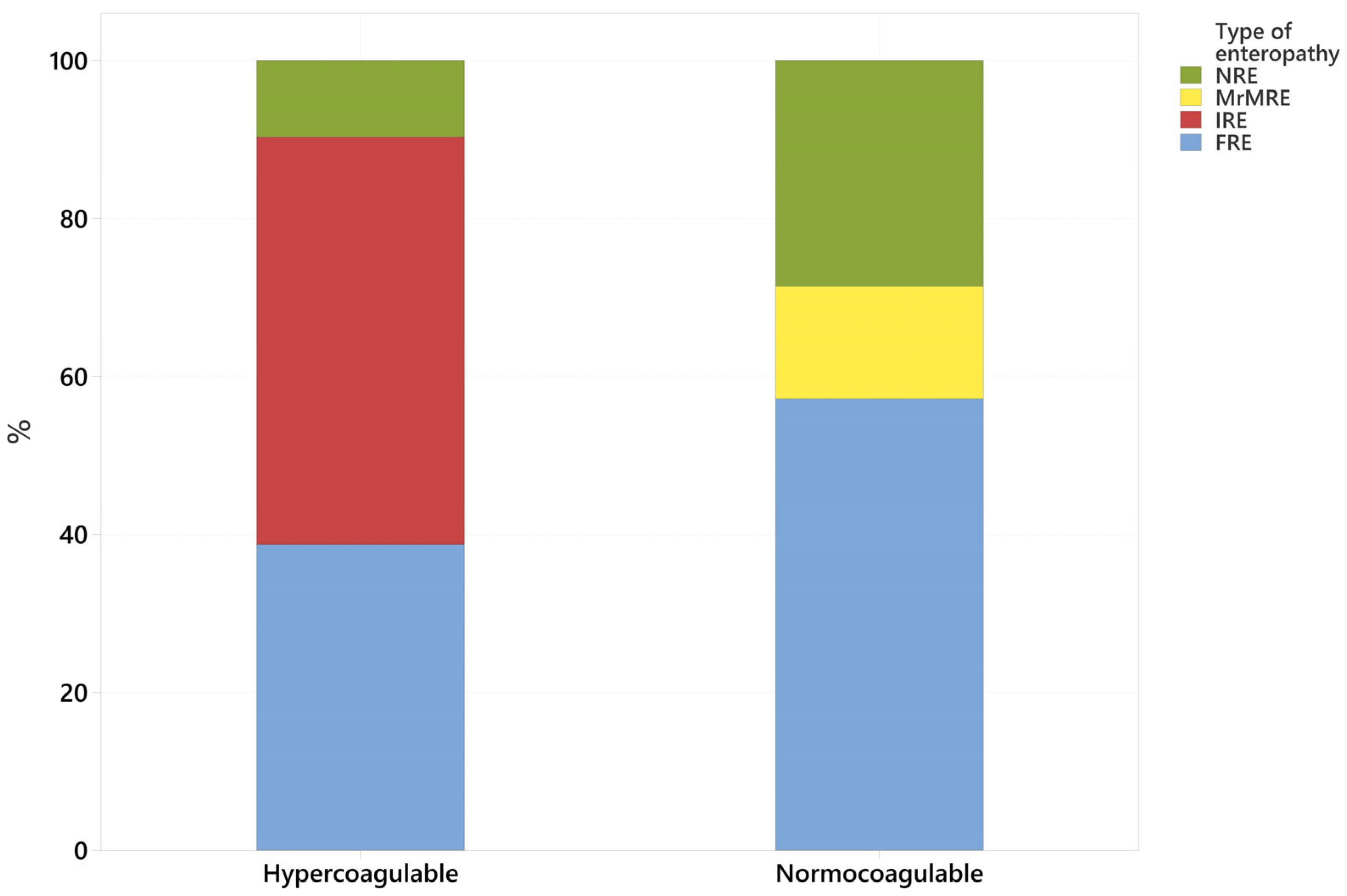
3.2. Coagulation Status (Normocoagulable vs. Hypercoagulable) Association with Type of CIE
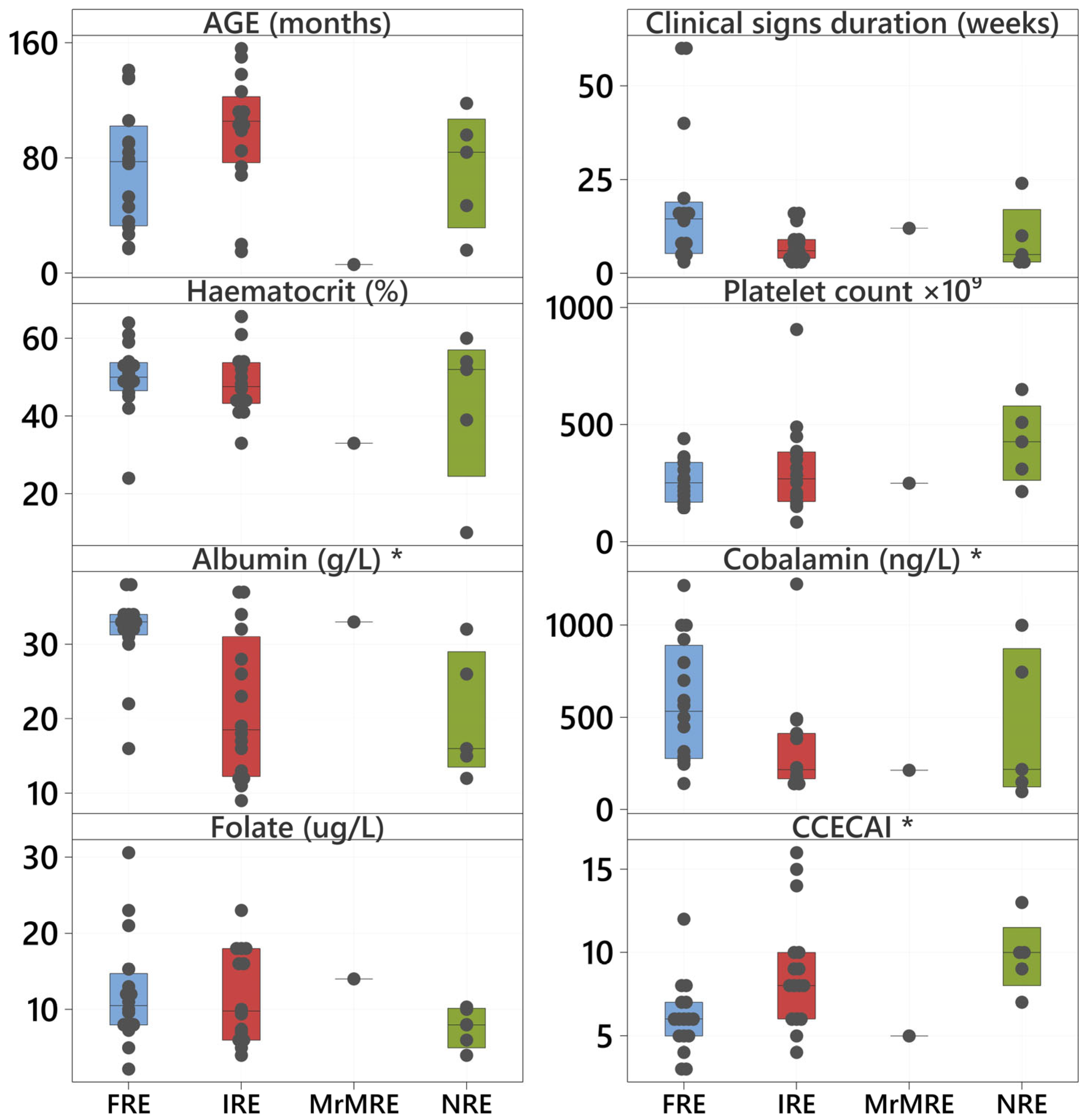
3.3. Clinical and Clinicopathological Variables Compared with Coagulation Status and Type of CIE
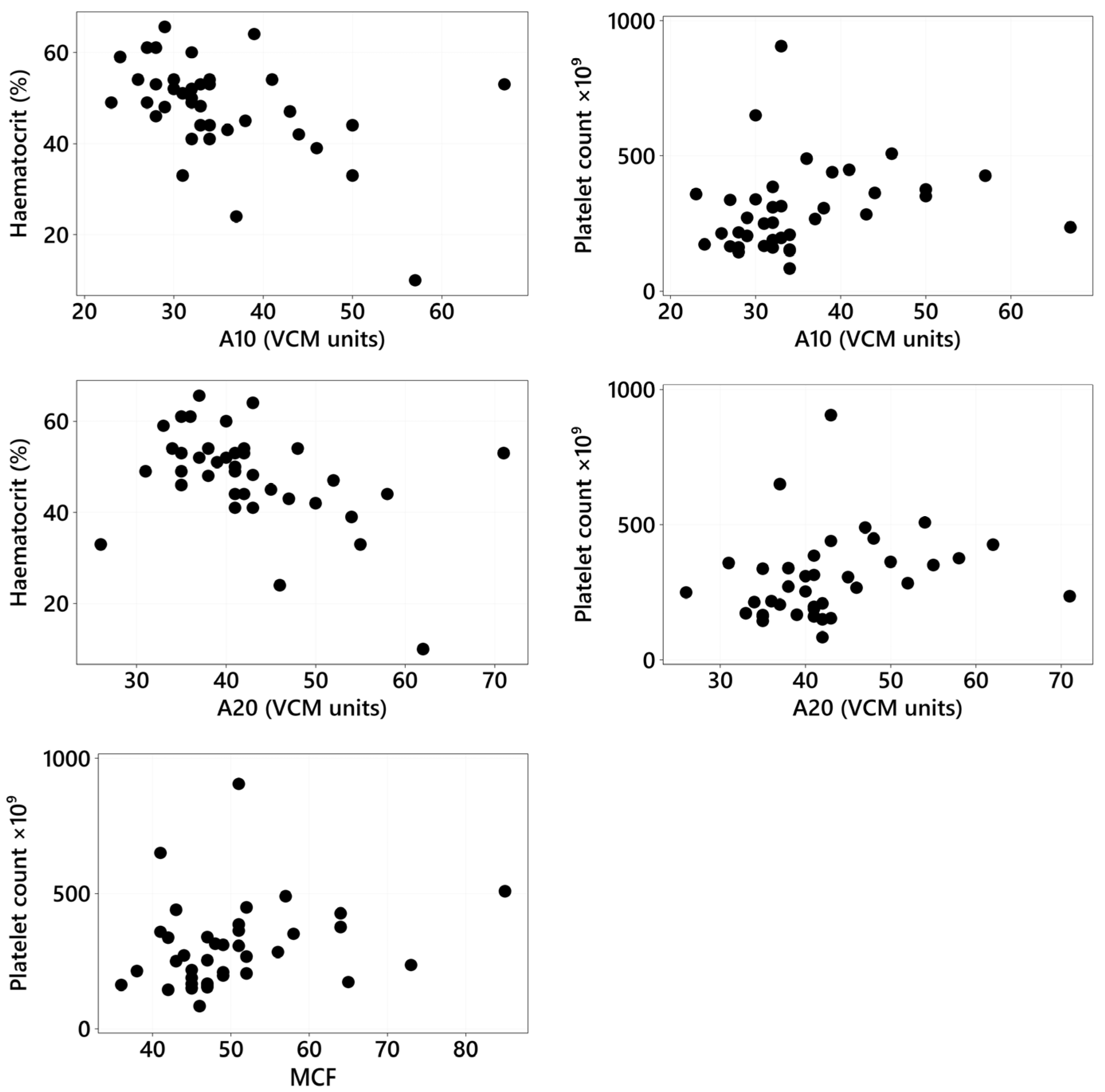
3.4. VCM Variables: Association with Clinical and Clinicopathologic Variables and Type of CIE
3.5. Suspected Thromboembolic Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Entegrion, I. VCM Vet—Real Time Hemostasis Assessment. Available online: https://vcmvet.com/ (accessed on 15 January 2025).
- Burton, A.G.; Jandrey, K.E. Use of Thromboelastography in Clinical Practice. Vet. Clin. N. Am.-Small Anim. Pract. 2020, 50, 1397–1409. [Google Scholar] [CrossRef] [PubMed]
- Rosati, T.; Jandrey, K.E.; Burges, J.W.; Kent, M.S. Establishment of a Reference Interval for a Novel Viscoelastic Coagulometer and Comparison with Thromboelastography in Healthy Cats. Vet. Clin. Pathol. 2020, 49, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Buriko, Y.; Chalifoux, N.V.; Clarkin-Breslin, R.; Silverstein, D.C. Comparison of a Viscoelastic Point-of-Care Coagulation Monitor with Thromboelastography in Sick Dogs with Hemostatic Abnormalities. Vet. Clin. Pathol. 2023, 52, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Buriko, Y.; Drobatz, K.; Silverstein, D.C. Establishment of Normal Reference Intervals in Dogs Using a Viscoelastic Point-of-Care Coagulation Monitor and Its Comparison with Thromboelastography. Vet. Clin. Pathol. 2020, 49, 567–573. [Google Scholar] [CrossRef]
- Goodwin, L.V.; Goggs, R.; Chan, D.L.; Allenspach, K. Hypercoagulability in Dogs with Protein-Losing Enteropathy. J. Vet. Intern. Med. 2011, 25, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Dixon, A.; Hall, E.J.; Adamantos, S.; Kathrani, A.; McGrath, C.; Black, V. Hypercoagulability in Dogs with Chronic Enteropathy and Association with Serum Albumin Concentration. J. Vet. Intern. Med. 2021, 35, 860–866. [Google Scholar] [CrossRef]
- Min, S.; Wesselowski, S.R.; Nabity, M.B.; Yankin, I. Pulmonary Hypertension Is Associated with Hypocoagulability in Dogs: A Retrospective Analysis of 66 Cases (2013–2021). Am. J. Vet. Res. 2024, 85, ajvr.23.11.0252. [Google Scholar] [CrossRef]
- Dionne, T.L.; Ishak, A.M.; Cochran, L.A. Point-of-Care Global Coagulation Assay Parameters in Normal Dogs and Dogs with Primary Immune-Mediated Hemolytic Anemia. J. Vet. Emerg. Crit. Care 2023, 33, 81–90. [Google Scholar] [CrossRef]
- Wang, W.H.; Lynch, A.M.; Balko, J.A.; Duffy, D.J.; Robertson, J.B.; Posner, L.P. Point-of-Care Viscoelastic Coagulation Assessment in Healthy Dogs during the Perianesthetic Period. BMC Vet. Res. 2022, 18, 346. [Google Scholar] [CrossRef]
- Morris, L.; Galezowski, A.; Atilla, A.; Menard, J. Short-duration peripherally inserted central catheters do not alter viscoelastic parameters in healthy dogs. Canine Vet. J. 2024, 65, 692–697. [Google Scholar]
- Chang, J.; Jandrey, K.E.; Burges, J.W.; Kent, M.S. Comparison of Healthy Blood Donor Greyhounds and Non-Greyhounds Using a Novel Point-of-Care Viscoelastic Coagulometer. J. Vet. Emerg. Crit. Care 2021, 31, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Hennink, I.; Peters, L.; van Geest, G.; Adamik, K.N. Evaluation of a Viscoelastic Coagulation Monitoring System (VCM Vet®) and Its Correlation with Thromboelastometry (ROTEM®) in Diseased and Healthy Dogs. Animals 2023, 13, 405. [Google Scholar] [CrossRef]
- Moses, I.A.; Hallowell, T.C.; Johnson, J.A. Feline Cardiomyopathy Patients Do Not Exhibit Evidence of Hypercoagulability Using a Novel Viscoelastic Coagulation Monitor. Am. J. Vet. Res. 2024, 1, 1–10. [Google Scholar] [CrossRef]
- Moreno, D.; Cosford, K.; Snead, E.; Carr, A. Assessment of Hemostasis in Hyperthyroid and Euthyroid Cats Using Two Viscoelastic Assays and Platelet Aggregometry. J. Vet. Intern. Med. 2024, 38, 1377–1383. [Google Scholar] [CrossRef]
- Fudge, J.M.; Cano, K.S.; Page, B.; Jeffery, U. Comparison of Viscoelastic Test Results from Blood Collected near Simultaneously from the Jugular and Saphenous Veins in Cats. J. Feline Med. Surg. 2021, 23, 598–603. [Google Scholar] [CrossRef]
- Yozova, I.D.; Kent, M.S.; Jandrey, K.E. Effects of a Single Subcutaneous Dose of Enoxaparin on Veterinary Viscoelastic Coagulation Monitor Variables in Healthy Cats: Double Blind, Placebo Controlled Cross-over Trial. J. Vet. Intern. Med. 2023, 37, 133–139. [Google Scholar] [CrossRef]
- Fudge, J.M.; Page, B.; Mackrell, A.; Lee, I.; Jeffery, U. Blood Loss and Coagulation Profile in Pregnant and Non-Pregnant Queens Undergoing Elective Ovariohysterectomy. J. Feline Med. Surg. 2021, 23, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Dandrieux, J.R.S. Inflammatory Bowel Disease versus Chronic Enteropathy in Dogs: Are They One and the Same? J. Small Anim. Pract. 2016, 57, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Dupouy-Manescau, N.; Méric, T.; Sénécat, O.; Drut, A.; Valentin, S.; Leal, R.O.; Hernandez, J. Updating the Classification of Chronic Inflammatory Enteropathies in Dogs. Animals 2024, 14, 681. [Google Scholar] [CrossRef] [PubMed]
- Dandrieux, J.R.S.; Mansfield, C.S. Chronic Enteropathy In Canines: Prevalence, Impact And Management Strategies. Vet. Med. Res. Rep. 2019, 10, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Holmberg, J.; Pelander, L.; Ljungvall, I.; Harlos, C.; Spillmann, T.; Häggström, J. Chronic Enteropathy in Dogs-Epidemiologic Aspects and Clinical Characteristics of Dogs Presenting at Two Swedish Animal Hospitals. Animals 2022, 12, 1507. [Google Scholar] [CrossRef] [PubMed]
- Simpson, K.W.; Jergens, A.E. Pitfalls and Progress in the Diagnosis and Management of Canine Inflammatory Bowel Disease. Vet. Clin. N. Am.-Small Anim. Pract. 2011, 41, 381–398. [Google Scholar] [CrossRef] [PubMed]
- Berghoff, N.; Steiner, J.M. Laboratory Tests for the Diagnosis and Management of Chronic Canine and Feline Enteropathies. Vet. Clin. N. Am.-Small Anim. Pract. 2011, 41, 311–328. [Google Scholar] [CrossRef]
- Salavati Schmitz, S.; Gow, A.; Bommer, N.; Morrison, L.; Mellanby, R. Diagnostic Features, Treatment, and Outcome of Dogs with Inflammatory Protein-Losing Enteropathy. J. Vet. Intern. Med. 2019, 33, 2005–2013. [Google Scholar] [CrossRef]
- Peterson, P.B.; Willard, M.D. Protein-Losing Enteropathies. Vet. Clin. N. Am. Small Anim. Pract. 2003, 33, 1061–1082. [Google Scholar] [CrossRef]
- Dossin, O.; Lavoué, R. Protein-Losing Enteropathies in Dogs. Vet. Clin. N. Am. Small Anim. Pract. 2011, 41, 399–418. [Google Scholar] [CrossRef]
- Nagahara, T.; Ohno, K.; Nagao, I.; Nakagawa, T.; Yokoyama, N.; Ohmi, A.; Goto-Koshino, Y.; Chambers, J.K.; Uchida, K.; Tomiyasu, H.; et al. Changes in the Coagulation Parameters in Dogs with Protein-Losing Enteropathy between before and after Treatment. J. Vet. Med. Sci. 2021, 83, 1295–1302. [Google Scholar] [CrossRef] [PubMed]
- Wennogle, S.A.; Olver, C.S.; Shropshire, S.B. Coagulation Status, Fibrinolysis, and Platelet Dynamics in Dogs with Chronic Inflammatory Enteropathy. J. Vet. Intern. Med. 2021, 35, 892–901. [Google Scholar] [CrossRef]
- Barth, S.I.; DeMonaco, S.M.; Conner, B.J.; Wilkinson, A.R. Hypercoagulability Identified in Dogs with Chronic Enteropathy Using a Point-of-care Viscoelastic Assay. J. Small Anim. Pract. 2025. [Google Scholar] [CrossRef]
- Rose, L.J.; Dunn, M.E.; Allegret, V.; Bédard, C. Effect of Prednisone Administration on Coagulation Variables in Healthy Beagle Dogs. Vet. Clin. Pathol. 2011, 40, 426–434. [Google Scholar] [CrossRef]
- Brainard, B.M.; Meredith, C.P.; Callan, M.B.; Budsber, S.C.; Shofer, F.S.; Driessen, B.; Otto, C.M. Changes in Platelet Function, Hemostasis, and Prostaglandin Expression after Treatment with Non Steroidal Anti-Inflammatory Drugs with Various COX Selectivities in Dogs. Am. J. Vet. Res. 2007, 68, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Donahue, S.M.; Otto, C.M. Thromboelastography: A Tool for Measuring Hypercoagulability, Hypocoagulability, and Fibrinolysis. J. Vet. Emerg. Crit. Care 2005, 15, 9–16. [Google Scholar] [CrossRef]
- De Laforcade, A.; Bacek, L.; Blais, M.C.; Boyd, C.; Brainard, B.M.; Chan, D.L.; Cortellini, S.; Goggs, R.; Hoareau, G.L.; Koenigshof, A.; et al. 2022 Update of the Consensus on the Rational Use of Antithrombotics and Thrombolytics in Veterinary Critical Care (CURATIVE) Domain 1-Defining Populations at Risk. J. Vet. Emerg. Crit. Care 2022, 32, 289–314. [Google Scholar] [CrossRef] [PubMed]
- Gold, A.J.; Langlois, D.K.; Refsal, K.R. Evaluation of Basal Serum or Plasma Cortisol Concentrations for the Diagnosis of Hypoadrenocorticism in Dogs. J. Vet. Intern. Med. 2016, 30, 1798–1805. [Google Scholar] [CrossRef] [PubMed]
- Kather, S.; Grützner, N.; Kook, P.H.; Dengler, F.; Heilmann, R.M. Review of Cobalamin Status and Disorders of Cobalamin Metabolism in Dogs. J. Vet. Intern. Med. 2020, 34, 13–28. [Google Scholar] [CrossRef]
- Cobalamin Information-Gastrointestinal Laboratory. Available online: https://vetmed.tamu.edu/gilab/research/cobalamin-information/ (accessed on 8 February 2025).
- Serum Cobalamin (Vitamin B12) and Folate-Gastrointestinal Laboratory. Available online: https://vetmed.tamu.edu/gilab/service/assays/b12folate/ (accessed on 1 February 2025).
- Lavanya, M.; Jayanthi, C.; Alexandria, M.; Janani, V. Platelet Estimation by Manual and Automated Methods. Ann. Pathol. Lab. Med. 2019, 6, A596–A599. [Google Scholar] [CrossRef]
- Baird, T.N.; Zersen, K.M.; Guillaumin, J. Point-of-Care Viscoelastic Coagulation Monitoring Device Shows Promise for Informing Resuscitation Strategies in a Canine Hemorrhagic Shock Model. Am. J. Vet. Res. 2024, 86, ajvr.24.07.0196. [Google Scholar] [CrossRef]
- Hanel, R.M.; Chan, D.L.; Conner, B.; Gauthier, V.; Holowaychuk, M.; Istvan, S.; Walker, J.M.; Wood, D.; Goggs, R.; Wiinberg, B. Systematic Evaluation of Evidence on Veterinary Viscoelastic Testing Part 4: Definitions and Data Reporting. J. Vet. Emerg. Crit. Care 2014, 24, 47–56. [Google Scholar] [CrossRef]
- Allenspach, K.; Wieland, B.; Gröne, A.; Gaschen, F. Chronic Enteropathies in Dogs: Evaluation of Risk Factors for Negative Outcome. J. Vet. Intern. Med. 2007, 21, 700–708. [Google Scholar] [CrossRef]
- Jergens, A.E.; Schreiner, C.A.; Frank, D.E.; Niyo, Y.; Ahrens, F.E.; Eckersall, P.D.; Benson, T.J.; Evans, R. A Scoring Index for Disease Activity in Canine Inflammatory Bowel Disease. J. Vet. Intern. Med. 2003, 17, 291–297. [Google Scholar] [CrossRef]
- DeBerry, J. Hypoproteinemia and Hyperproteinemia. In Ettinger’s Textbook of Veterinary Internal Medicine; Côté, E., Ettinger, S.J., Feldman, E.C., Eds.; Elsevier: Philadelphia, PA, USA, 2024; Volume 1, pp. 284–289. [Google Scholar]
- Harahsheh, Y.; Ho, K.M. Use of Viscoelastic Tests to Predict Clinical Thromboembolic Events: A Systematic Review and Meta-Analysis. Eur. J. Haematol. 2018, 100, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Eissa, N.; Kittana, H.; Gomes-Neto, J.C.; Hussein, H. Mucosal Immunity and Gut Microbiota in Dogs with Chronic Enteropathy. Res. Vet. Sci. 2019, 122, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Maiuolo, J.; Carresi, C.; Gliozzi, M.; Mollace, R.; Scarano, F.; Scicchitano, M.; Macrì, R.; Nucera, S.; Bosco, F.; Oppedisano, F.; et al. The Contribution of Gut Microbiota and Endothelial Dysfunction in the Development of Arterial Hypertension in Animal Models and in Humans. Int. J. Mol. Sci. 2022, 23, 3698. [Google Scholar] [CrossRef] [PubMed]
- Allenspach, K.; Culverwell, C.; Chan, D. Long-Term Outcome in Dogs with Chronic Enteropathies: 203 Cases. Vet. Record. 2016, 178, 368. [Google Scholar] [CrossRef] [PubMed]
- Münster, M.; Hörauf, A.; Bilzer, T. Assessment of Disease Severity and Outcome of Dietary, Antibiotic, and Immunosuppressive Interventions by Use of the Canine IBD Activity Index in 21 Dogs with Chronic Inflammatory Bowel Disease. Berl. Munch. Tierarztl. Wochenschr. 2006, 119, 493–505. [Google Scholar]
- Jackson, M.I.; Gaschen, F.P.; Salavati, S.; Jergens, A.E. Canine Chronic Enteropathy—Current State-of-the-Art and Emerging Concepts. Front. Vet. Sci. 2022, 9, 923013. [Google Scholar]
- Heilmann, R.M.; Steiner, J.M.; Romy Heilmann, C.M. Clinical Utility of Currently Available Biomarkers in Inflammatory Enteropathies of Dogs. J. Vet. Intern. Med. 2018, 32, 1495–1508. [Google Scholar] [CrossRef] [PubMed]
- Heilmann, R.M.; Otoni, C.C.; Jergens, A.E.; Grützner, N.; Suchodolski, J.S.; Steiner, J.M. Systemic Levels of the Anti-Inflammatory Decoy Receptor Soluble RAGE (Receptor for Advanced Glycation End Products) Are Decreased in Dogs with Inflammatory Bowel Disease. Vet. Immunol. Immunopathol. 2014, 161, 184–192. [Google Scholar] [CrossRef]
- Wennogle, S.A.; Priestnall, S.L.; Webb, C.B. Histopathologic Characteristics of Intestinal Biopsy Samples from Dogs With Chronic Inflammatory Enteropathy With and Without Hypoalbuminemia. J. Vet. Intern. Med. 2017, 31, 71–376. [Google Scholar] [CrossRef]
- Owczarek, D.; Cibor, D.; Głowacki, M.K.; Rodacki, T.; Mach, T. Inflammatory Bowel Disease: Epidemiology, Pathology and Risk Factors for Hypercoagulability. World J. Gastroenterol. 2014, 20, 53–63. [Google Scholar] [CrossRef]
- Danese, S.; Papa, A.; Saibeni, S.; Repici, A.; Malesci, A.; Vecchi, M. Inflammation and Coagulation in Inflamma-tory Bowel Disease: The Clot Thickens. Am. J. Gastroenterol. 2007, 10, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.; Breda, S.; Giordano, A.; Pengo, G.; Dall’Ara, P.; Rossi, G.; Bo, S.; Paltrinieri, S. Association between Hypocobalaminaemia and Hyperhomocysteinaemia in Dogs. Vet. Rec. 2013, 172, 365. [Google Scholar] [CrossRef] [PubMed]
- Toresson, L.; Suchodolski, J.S.; Spillmann, T.; Lopes, B.C.; Shih, J.; Steiner, J.M.; Pilla, R. The Intestinal Microbiome in Dogs with Chronic Enteropathies and Cobalamin Deficiency or Normocobalaminemia-A Comparative Study. Animals 2023, 13, 1378. [Google Scholar] [CrossRef]
- Volkmann, M.; Steiner, J.M.; Fosgate, G.T.; Zentek, J.; Hartmann, S.; Kohn, B. Chronic Diarrhea in Dogs–Retrospective Study in 136 Cases. J. Vet. Intern. Med. 2017, 31, 1043–1055. [Google Scholar] [CrossRef]
- Henry, P.M.N.; Williams, T.L. Prevalence of Neoplasia and Concurrent Diseases in Dogs and Cats with Hypercobalaminemia: A Retrospective Case–Control Study. Vet. Clin. Pathol. 2023, 52, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Da Riz, F.; Higgs, P.; Ruiz, G. Diseases Associated with Hypercobalaminemia in Dogs in United Kingdom: A Retrospective Study of 47 Dogs. Can. Vet. J. 2021, 62, 611–616. [Google Scholar]
- Ullal, T.V.; Marks, S.L.; Huebner, S.N.; Taylor, S.L.; Shelley, C.D. Association of Folate Concentrations with Clinical Signs and Laboratory Markers of Chronic Enteropathy in Dogs. J. Vet. Intern. Med. 2023, 37, 455–464. [Google Scholar] [CrossRef]
- Lehmann, E.L. Nonparametrics: Statistical Methods Based on Ranks, 1st ed.; Springer: New York, NY, USA, 2006. [Google Scholar]
- Smith, S.A.; McMichael, M.A.; Gilor, S.; Galligan, A.J.; Hoh, C.M. Correlation of Hematocrit, Platelet Concentration and Plasma Coagulation Factors with Results of TEM in Canine Whole Blood Samples. Am. J. Vet. Res. 2012, 73, 789–798. [Google Scholar] [CrossRef]
- Smith, S.A. The Cell-Based Model of Coagulation: State-Of-The-Art Review. J. Vet. Emerg. Crit. Care 2009, 19, 3–10. [Google Scholar] [CrossRef]
- Fenty, R.K.; De Laforcade, A.M.; Shaw, S.P.; Toole, T.E.O. Identification of Hypercoagulability in Dogs with Primary Immune-Mediated Hemolytic Anemia by Means of Thromboelastography. J. Am. Vet. Med. Assoc. 2011, 238, 463–467. [Google Scholar] [CrossRef]
- Brooks, A.C.; Guillaumin, J.; Cooper, E.S.; Couto, C.G. Effects of Hematocrit and Red Blood Cell-Independent Viscosity on Canine Thromboelastographic Tracings. Transfusion 2014, 54, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Blois, S.L. Chapter 171: Hyper- and Hypocoagulable States. In Ettinger’s Textbook of Veterinary Internal Medicine; Côté, E., Ettinger, S., Feldman, E., Eds.; Elsevier: Philadelphia, PA, USA, 2024; Volume 1, pp. 863–870. [Google Scholar]
- DIC and Thrombosis Cornell University College of Veterinary Medicine. Available online: https://www.vet.cornell.edu/animal-health-diagnostic-center/laboratories/comparative-coagulation/clinical-topics/dic-and-thrombosis?utm_source=chatgpt.com (accessed on 10 February 2025).
- Wiinberg, B.; Jensen, A.L.; Johansson, P.I.; Rozanski, E.; Tranholm, M.; Kristensen, A.T. Thromboelastographic Evaluation of Hemostatic Function in Dogs with Disseminated Intravascular Coagulation. J. Vet. Intern. Med. 2008, 22, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Asakura, H. Classifying Types of Disseminated Intravascular Coagulation: Clinical and Animal Models. Rinsho Ketsueki 2016, 57, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Kittrell, D.; Berkwitt, L. Hypercoagulability in Dogs Pathophysiology. Compend. Contin. Educ. Vet. 2012, 34, E1–E5. [Google Scholar]
- Laurenson, M.P.; Hopper, K.; Herrera, M.A.; Johnson, E.G. Concurrent Diseases and Conditions in Dogs with Splenic Vein Thrombosis. J. Vet. Intern. Med. 2010, 24, 1298–1304. [Google Scholar] [CrossRef]
- Garcia-Sancho, M.; Saiz, A.; Rodriguez-Franco, F.; Villaescusa, A.; Rodriguez-Bertos, A. Pulmonary Thromboembolism in a Dog with Inflammatory Bowel Disease. Rev. Complut. Cienc. Vet. 2010, 4, 78–86. [Google Scholar]
- Jacinto, A.M.L.; Ridyard, A.E.; Aroch, I.; Watson, P.J.; Morrison, L.R.; Chandler, M.L.; Kuzi, S. Thromboembolism in Dogs with Protein-Losing Enteropathy with Non-Neoplastic Chronic Small Intestinal Disease. J. Am. Anim. Hosp. Assoc. 2017, 53, 185–192. [Google Scholar] [CrossRef]
- Littman, M.P.; Dambach, D.M.; Vaden, S.L.; Giger, U. Familial Protein-Losing Enteropathy and Protein-Losing Nephropathy in Soft Coated Wheaten Terriers: 222 Cases (1983–1997). J. Vet. Intern. Med. 2000, 14, 68–80. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Ishigaki, K.; Ishikawa, C.; Nakayama, T.; Asano, K.; Sakai, M. Successful Management of Portal Vein Thrombosis in a Yorkshire Terrier with Protein-Losing Enteropathy. BMC Vet. Res. 2020, 16, 418. [Google Scholar] [CrossRef]
- Wiinberg, B.; Kristensen, A.T. Thromboelastography in Veterinary Medicine. Semin. Thromb. Hemost. 2010, 36, 747–756. [Google Scholar] [CrossRef]
- Respess, M.; O’Toole, T.E.; Taeymans, O.; Rogers, C.L.; Johnston, A.; Webster, C.R.L. Portal Vein Thrombosis in 33 Dogs: 1998–2011. J. Vet. Intern. Med. 2012, 26, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Kittrell, D.; Berkwitt, L. Hypercoagulability in Dogs: Treatment. Compend. Contin. Educ. Vet. 2012, 34, E3. [Google Scholar]
- Hindmarsh, D.D.; Rutter, C.R.; Pugnetti, V.D.; Jeffery, U. Response of the VCMVet Viscoelastic Coagulation Monitor to Veterinary Environmental Simulation Challenges. Vet. Clin. Pathol. 2022, 51, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Goggs, R.; Brainard, B.; De Laforcade, A.M.; Flatland, B.; Hanel, R.; Mcmichael, M.; Wiinberg, B. Partnership on Rotational ViscoElastic Test Standardization (PROVETS): Evidence-Based Guidelines on Rotational Viscoelastic Assays in Veterinary Medicine. J. Vet. Emerg. Crit. Care 2014, 24, 1–22. [Google Scholar] [CrossRef]
| Clinical Sign | Number of Cases (n) | Percentage of Total (%) |
|---|---|---|
| Vomiting | 28 | 73.68 |
| Diarrhea | 27 | 71.05 |
| Reduced appetite | 18 | 47.37 |
| Weight loss | 11 | 28.95 |
| Hematochezia | 11 | 28.95 |
| Lethargy | 8 | 21.05 |
| Borborygmi | 7 | 18.42 |
| Abdominal discomfort | 6 | 15.79 |
| Polyuria/polydipsia | 4 | 10.53 |
| Regurgitation | 3 | 7.89 |
| Melena | 2 | 5.26 |
| Ascites | 2 | 5.26 |
| Hypersalivation | 1 | 2.63 |
| Peripheral edema | 1 | 2.63 |
| Flatulence | 1 | 2.63 |
| Case | CT (RI 241–470 s) | CFT (104–266 s) | α Angle (RI 43–64 Degrees) | A10 (RI 16–30 VCM Units) | A20 (RI 22–28 VCM Units) | MCF (RI 29–44) | VCM Result |
|---|---|---|---|---|---|---|---|
| 1 | 393 | 124 | 60 | 34 * | 43 * | 47 * | Hypercoagulable |
| 2 | 319 | 128 | 64 | 28 | 35 * | 36 | Normocoagulable |
| 3 | 378 | 163 | 56 | 27 | 35 * | 45 * | Hypercoagulable |
| 4 | 426 | 127 | 61 | 31* | 39 * | 47 * | Hypercoagulable |
| 5 | 345 | 172 | 55 | 24 | 33 * | 65 * | Hypercoagulable |
| 6 | 360 | 156 | 58 | 29 | 38 * | 44 | Normocoagulable |
| 7 | 392 | 108 | 65 * | 32 * | 41 * | 45 * | Hypercoagulable |
| 8 | 382 | 80 * | 67 * | 44 * | 50 * | 51 * | Hypercoagulable |
| 9 | 314 | 97 * | 64 | 43 * | 52 * | 56 * | Hypercoagulable |
| 10 | 306 | 110 | 65 * | 32 * | 40 * | 47 * | Hypercoagulable |
| 11 | 206 * | 108 | 63 | 30 | 38 * | 47 * | Hypercoagulable |
| 12 | 305 | 104 | 63 | 38 * | 45 * | 51 * | Hypercoagulable |
| 13 | 333 | 126 | 50 | 31 * | 26 | 43 | Normocoagulable |
| 14 | 179 * | 56 * | 72 * | 57 * | 62 * | 64 * | Hypercoagulable |
| 15 | 106 * | 111 | 59 | 50 * | 58 * | 64 * | Hypercoagulable |
| 16 | 283 | 131 | 58 | 32 * | 41 * | 51 * | Hypercoagulable |
| 17 | 320 | 160 | 51 | 36 * | 47 * | 57 * | Hypercoagulable |
| 18 | 303 | 125 | 60 | 33 * | 41 * | 48 * | Hypercoagulable |
| 19 | 281 | 94 * | 69 * | 34 * | 42 * | 46 * | Hypercoagulable |
| 20 | 284 | 122 | 59 | 33 * | 41 * | 49 * | Hypercoagulable |
| 21 | 262 | 66 * | 69 * | 67 * | 71 * | 73 * | Hypercoagulable |
| 22 | 425 | 125 | 59 | 37 * | 46 * | 52 * | Hypercoagulable |
| 23 | 362 | 152 | 55 | 32 * | 41 * | 47 * | Hypercoagulable |
| 24 | 333 | 147 | 54 | 30 | 37 * | 41 | Normocoagulable |
| 25 | 326 | 80 * | 73 * | 34 * | 42 * | 45 * | Hypercoagulable |
| 26 | 464 | 196 | 47 | 26 | 34 * | 38 | Normocoagulable |
| 27 | 354 | 121 | 61 | 33 * | 43 * | 51 * | Hypercoagulable |
| 28 | 169 * | 117 | 62 | 28 | 35 * | 42 | Hypercoagulable |
| 29 | 310 | 120 | 65 * | 29 | 37 * | 52 * | Hypercoagulable |
| 30 | 350 | 86 * | 65 * | 50 * | 55 * | 58 * | Hypercoagulable |
| 31 | 186 * | 54 * | 76 * | 46 * | 54 * | 85 * | Hypercoagulable |
| 32 | 233 * | 80 * | 70 * | 41 * | 48 * | 52 * | Hypercoagulable |
| 33 | 287 | 106 | 66 * | 34 * | 42 * | 49 * | Hypercoagulable |
| 34 | 417 | 165 | 54 | 27 | 35 * | 42 | Normocoagulable |
| 35 | 330 | 146 | 58 | 28 | 36 * | 45 * | Hypercoagulable |
| 36 | 267 | 110 | 66 * | 32 * | 40 * | 49 * | Hypercoagulable |
| 37 | 414 | 175 | 56 | 23 | 31 * | 41 | Normocoagulable |
| 38 | 255 | 75 * | 73 * | 39 * | 43 * | 43 | Hypercoagulable |
| Case | Age (months) | Sex and Neuter Status | Duration of Clinical Signs (weeks) | Hct (37–55%) | Platelet Count (140–700 × 109/L) | Albumin (24–40 g/L) | Cobalamin (400–907 ng/L) | Folate (7.7–24.4 ng/L) | CCECAI Score | VCM Result | CIE Type |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 68 | MN | 4 | 41 | 154 | 28 | 171 * | 18 | 5–mild | Hypercoagulable | IRE |
| 2 | 32 | FN | 40 | 53 | 162 | 33 | 448 | 10 | 6—moderate | Normocoagulable | FRE |
| 3 | 99 | FN | 16 | 61 | 166 | 26 | 185 * | 7 * | 8—moderate | Hypercoagulable | IRE |
| 4 | 17 | ME | 5 | 51 | 167 | 31 | 1000 * | 11 | 6—moderate | Hypercoagulable | FRE |
| 5 | 107 | FN | 16 | 59 | 173 | 32 | 593 | 8 | 7—moderate | Hypercoagulable | FRE |
| 6 | 135 | FN | 20 | 48 | 271 | 38 | 265 * | 21 | 8—moderate | Normocoagulable | FRE |
| 7 | 112 | FN | 8 | 41 | 189 | 32 | 413 | 6 * | 8—moderate | Hypercoagulable | IRE |
| 8 | 18 | FN | 16 | 42 | 363 | 16 * | 1000 * | 12 | 12—very severe | Hypercoagulable | FRE-PLE |
| 9 | 108 | FN | 4 | 47 | 284 | 11 * | 217 * | 5 * | 14—very severe | Hypercoagulable | IRE-PLE |
| 10 | 15 | FN | 4 | 52 | 253 | 23 * | 140 * | 6 * | 6—moderate | Hypercoagulable | IRE-PLE |
| 11 | 79 | ME | 15 | 54 | 339 | 33 | 500 | 5 * | 6—moderate | Hypercoagulable | FRE |
| 12 | 76 | MN | 60 | 45 | 307 | 33 | 1216 * | 13 | 8—moderate | Hypercoagulable | FRE |
| 13 | 6 | FE | 12 | 37 | 250 | 33 | 213 * | 14 | 5—mild | Normocoagulable | MrMRE |
| 14 | 84 | ME | 5 | 10 * | 427 | 32 | 149 * | 10 | 9—severe | Hypercoagulable | NRE |
| 15 | 150 | FE | 8 | 44 | 376 | 34 | 493 | 16 | 6—moderate | Hypercoagulable | IRE |
| 16 | 126 | FN | 9 | 50 | 386 | 16 * | 164 * | 23 | 6—moderate | Hypercoagulable | IRE-PLE |
| 17 | 111 | FN | 16 | 43 | 490 | 18 * | 385 * | 18 | 9—severe | Hypercoagulable | IRE-PLE |
| 18 | 85 | MN | 9 | 44 | 314 | 12 * | 140 * | 18 | 10—severe | Hypercoagulable | IRE-PLE |
| 19 | 103 | MN | 14 | 54 | 84 * | 37 | 1223 * | 16 | 8—moderate | Hypercoagulable | IRE |
| 20 | 36 | ME | 60 | 53 | 197 | 30 | 923 * | 12 | 5—mild | Hypercoagulable | FRE |
| 21 | 141 | FN | 14 | 53 | 236 | 38 | 287 * | 8 | 3—insignificant | Hypercoagulable | FRE |
| 22 | 53 | MN | 8 | 24 * | 267 | 22 * | 273 * | 23 | 4—mild | Hypercoagulable | FRE-PLE |
| 23 | 90 | FN | 5 | 49 | 161 | 33 | 141 * | 8 | 6—moderate | Hypercoagulable | FRE |
| 24 | 47 | FN | 10 | 52 | 650 | 26 | 218 * | 6 * | 10—severe | Normocoagulable | NRE |
| 25 | 20 | FN | 3 | 44 | 150 | 17 * | 167 * | 10 | 13—very severe | Hypercoagulable | IRE-PLE |
| 26 | 118 | MN | 3 | 54 | 214 | 12 * | 97 * | 4 * | 12—very severe | Normocoagulable | NRE-PLE |
| 27 | 103 | ME | 3 | 48 | 906 * | 13 * | N/A | N/A | 16—very severe | Hypercoagulable | IRE-PLE |
| 28 | 27 | ME | 4 | 46 | 144 | 32 | 315 * | 30.6 * | 6—moderate | Hypercoagulable | FRE |
| 29 | 138 | FN | 6 | 65 | 205 | 19 * | 228 * | 9.4 | 8—moderate | Hypercoagulable | IRE-PLE |
| 30 | 74 | ME | 4 | 37 | 351 | 9 * | 182 * | 4.2 * | 14—very severe | Hypercoagulable | IRE-PLE |
| 31 | 96 | MN | 24 | 39 | 509 | 15 * | 1000 * | 8 | 13—very severe | Hypercoagulable | NRE-PLE |
| 32 | 112 | FN | 3 | 54 | 449 | 12 * | 224 * | 7.4 * | 10—severe | Hypercoagulable | IRE-PLE |
| 33 | 156 | MN | 4 | 53 | 209 | 37 | 485 | 9.8 | 4—mild | Hypercoagulable | IRE |
| 34 | 136 | ME | 6 | 49 | 337 | 32 | 565 | 7.3 * | 5—mild | Normocoagulable | FRE |
| 35 | 84 | FN | 3 | 61 | 217 | 34 | 246 * | 9.6 | 7—moderate | Hypercoagulable | FRE |
| 36 | 16 | FE | 3 | 60 | 310 | 16 * | 745 | 10.3 | 10—severe | Hypercoagulable | NRE-PLE |
| 37 | 46 | ME | 8 | 49 | 359 | 34 | 797 | 15.3 | 5—mild | Normocoagulable | FRE |
| 38 | 91 | FN | 16 | 64 | 440 | 34 | 700 | 2.2 * | 3—insignificant | Hypercoagulable | FRE |
| CIE Type | CIE Subtype | Number of Dogs | Age (months) | Duration of Signs (weeks) | Hct (%) | Platelet Count (×109/L) | Albumin (g/L) | Cobalamin (ng/L) | Folate (ng/L) | CCECAI Score |
|---|---|---|---|---|---|---|---|---|---|---|
| FRE | PLE | 2 | 18–53 | 8–16 | 24–42 | 267–363 | 16–22 | 273–1000 | 12–23 | 4–12 |
| Non-PLE | 14 | 17–141 | 3–60 | 45–64 | 144–440 | 31–38 | 141–1216 | 2.2–30.6 | 3–8 | |
| IRE | PLE | 10 | 15–138 | 4–16 | 43–65 | 150–906 | 9–23 | 140–385 | 4.2–26 | 6–16 |
| Non-PLE | 6 | 68–156 | 4–16 | 41–61 | 84–376 | 26–37 | 171–1223 | 6–18 | 4–8 | |
| MrMRE | Non-PLE | 1 | 68 | 12 | 41 | 154 | 28 | 213 | 18 | 5 |
| NRE | PLE | 3 | 47–84 | 3–24 | 39–60 | 427–650 | 12–16 | 97–1000 | 4–10.3 | 10–13 |
| Non-PLE | 2 | 16–118 | 5–10 | 10–52 | 214–509 | 26–32 | 149–218 | 6–10 | 6–10 |
| CIE Subtype | Number of Dogs (%) | Coagulation Status |
|---|---|---|
| FRE | 16 (42.1%) | 12 hypercoagulable (75%) 4 normocoagulable (25%) |
| IRE | 16 (42.1%) | 16 hypercoagulable (100%) 0 normocoagulable (0%) |
| MrMRE | 1 (2.6%) | 1 normocoagulable (100%) |
| NRE | 5 (13.2%) | 3 hypercoagulable (60%) 2 normocoagulable (40%) |
| Parameter | FRE | IRE | NRE | p-Value |
|---|---|---|---|---|
| Albumin (g/L) | 33 (16–38) | 18 (9–37) | 16 (12–32) | 0.005 |
| Cobalamin (ng/L) | 532 (141–1216) | 217 (140–1223) | 218 (97–1000) | 0.028 |
| CCECAI | 6 (3–12) (insignificant to very severe) | 8 (4–16) (mild to very severe) | 10 (7–13) (severe to very severe) | 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marín Lucas, M.J.; Sparks, T.; Rosa, C. Coagulation Status Assessment in Dogs with Chronic Enteropathy Using Viscoelastic Point-of-Care Coagulation Monitor. Animals 2025, 15, 1571. https://doi.org/10.3390/ani15111571
Marín Lucas MJ, Sparks T, Rosa C. Coagulation Status Assessment in Dogs with Chronic Enteropathy Using Viscoelastic Point-of-Care Coagulation Monitor. Animals. 2025; 15(11):1571. https://doi.org/10.3390/ani15111571
Chicago/Turabian StyleMarín Lucas, María José, Tim Sparks, and Chantal Rosa. 2025. "Coagulation Status Assessment in Dogs with Chronic Enteropathy Using Viscoelastic Point-of-Care Coagulation Monitor" Animals 15, no. 11: 1571. https://doi.org/10.3390/ani15111571
APA StyleMarín Lucas, M. J., Sparks, T., & Rosa, C. (2025). Coagulation Status Assessment in Dogs with Chronic Enteropathy Using Viscoelastic Point-of-Care Coagulation Monitor. Animals, 15(11), 1571. https://doi.org/10.3390/ani15111571





