The Effect of Intracameral Triamcinolone Acetonide on Controlling Common Complications following Phacoemulsification in Dogs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
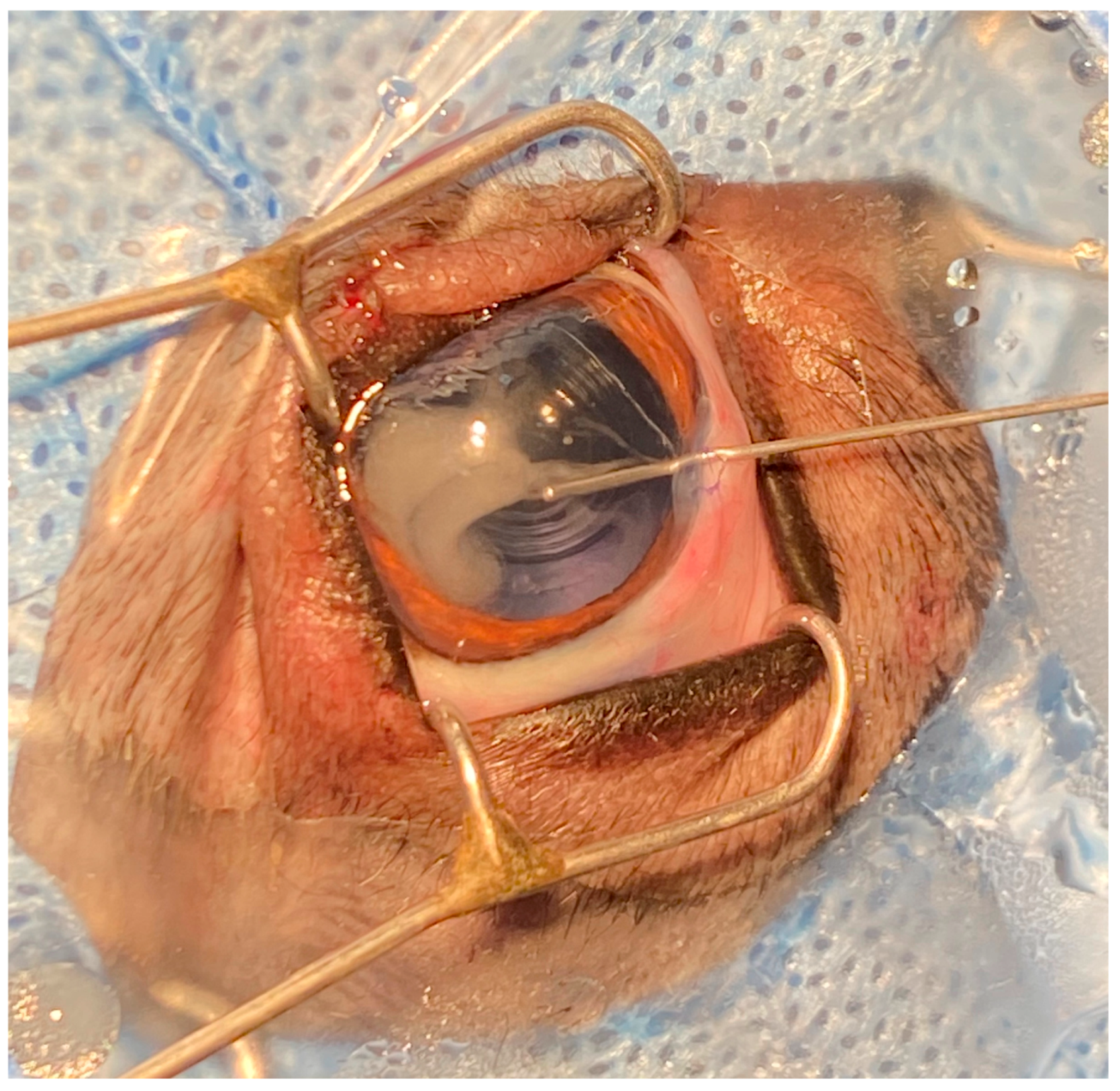
2.2. Surgical Procedures
2.3. Observation and Measurement of Postoperative Indexes
2.4. Data Analysis
3. Results
3.1. Postoperative Indexes
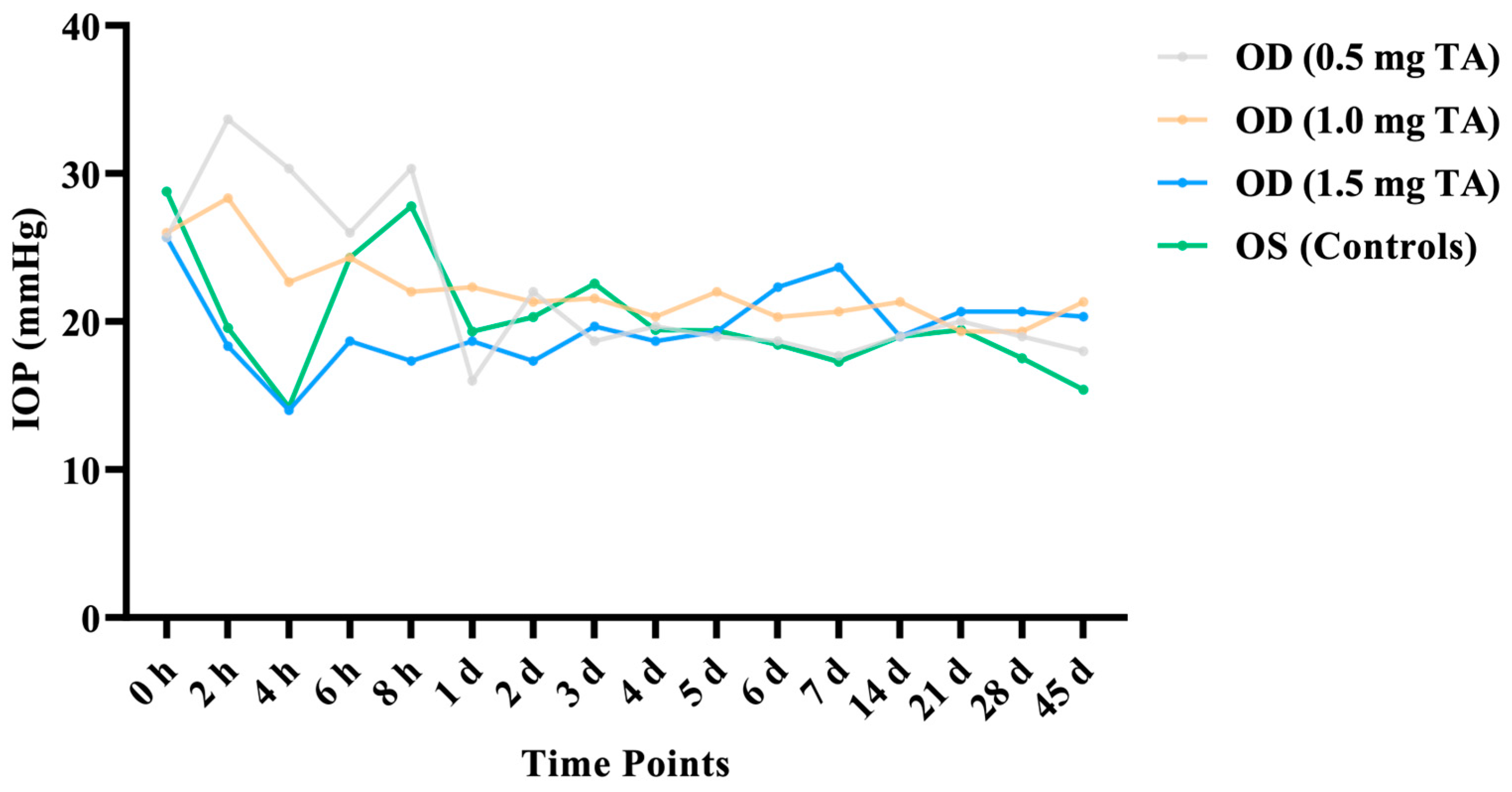
3.1.1. IOP
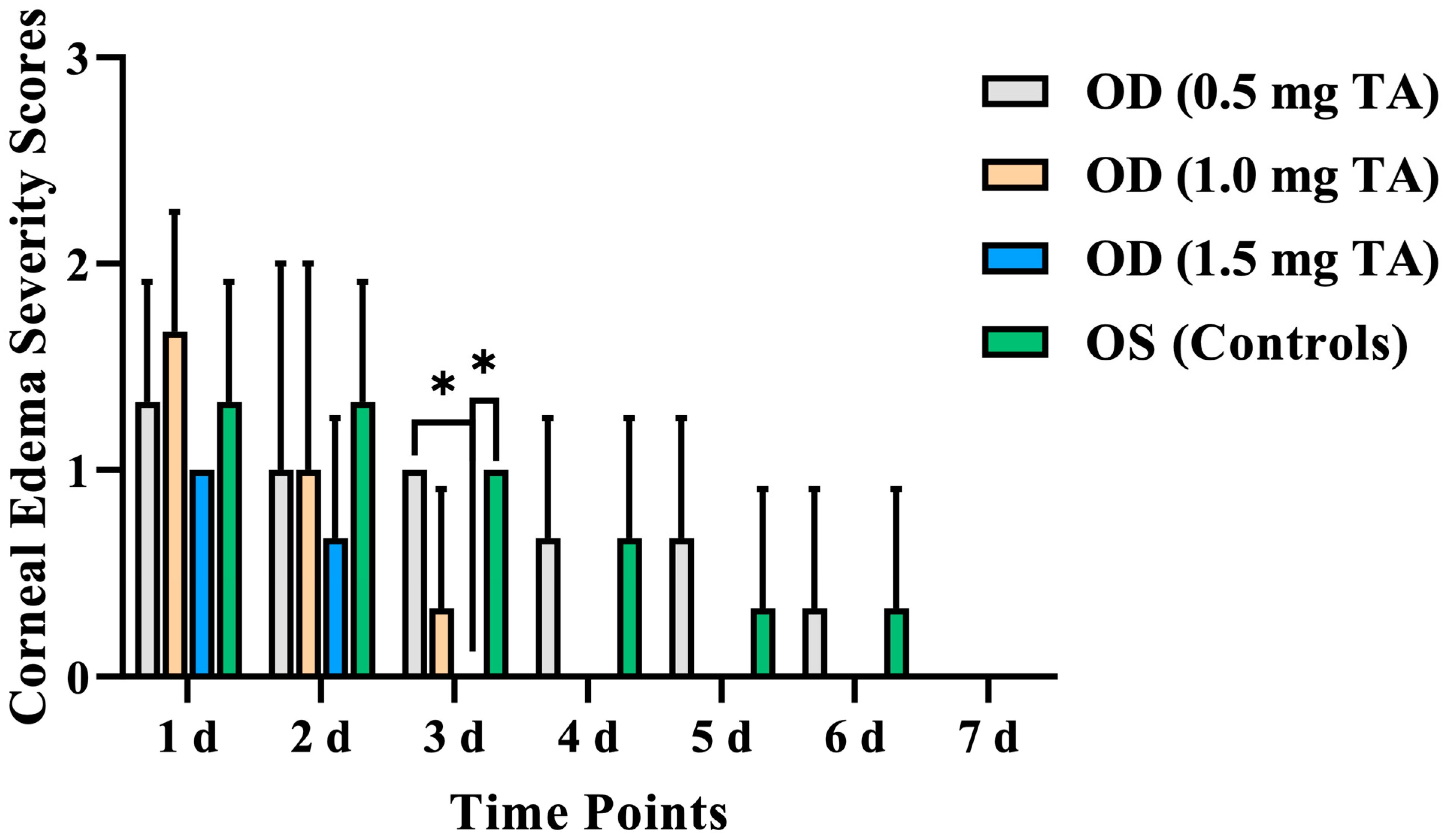
3.1.2. Corneal Edema
3.1.3. Aqueous Flare
3.2. Aqueous Humor Analysis
3.2.1. Aqueous Humor Protein Concentration
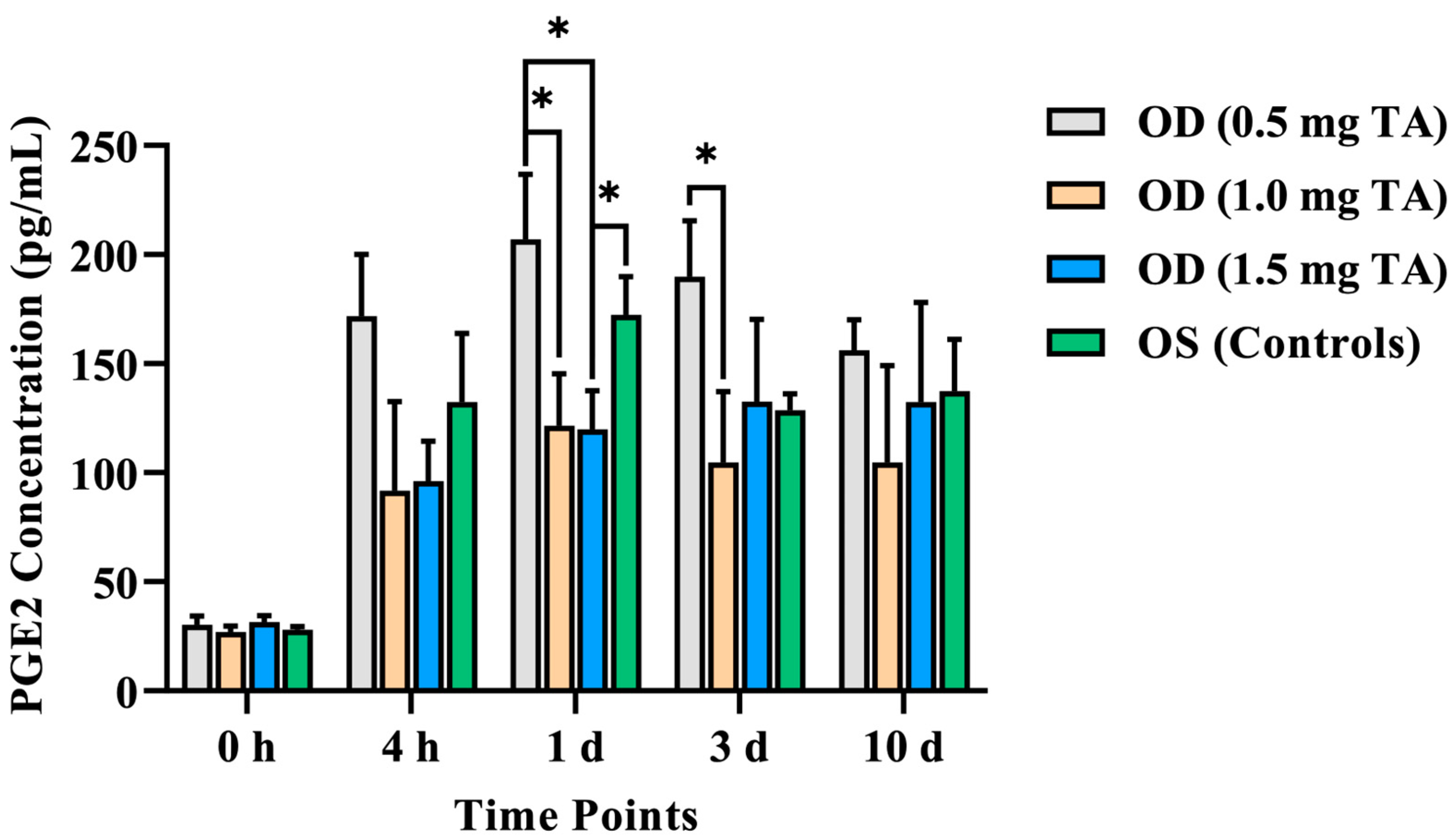
3.2.2. Aqueous Humor PGE2 Concentration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- David, J.M.; Paul, E.M.; Ron, O. Slatter’ s Fundamentals of Veterinary Ophthalmology, 6th ed.; Elsevier: St. Louis, MO, USA, 2018. [Google Scholar]
- Kirk, N.G.; Janice, P.G. Veterinary Ophthalmology Surgery; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Dowler, K.K.; Middleton, J.R.; Dufour, S.; Hood, M.A.; Giuliano, E.A. Characterization of postoperative “fibrin web” formation after canine cataract surgery. Vet. Ophthalmol. 2021, 24, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, L.A.; Nadelstein, B.; Berdoulay, A.; Spatola, R.A. Effect of immediate postoperative intracameral tissue plasminogen activator (tPA) on anterior chamber fibrin formation in dogs undergoing phacoemulsification. Vet. Ophthalmol. 2019, 22, 477–484. [Google Scholar] [CrossRef] [PubMed]
- da Rocha Lima, B.; Pichi, F.; Nucci, P.; Srivastava, S.K.; Lowder, C.Y. Fibrin reaction after uveitic cataract surgery: Treatment and prevention. Eur. J. Ophthalmol. 2014, 24, 626–628. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.S.; Dong, N.; Xu, B.; Liu, J.; Xiao, L. Efficacy and safety of intracameral triamcinolone acetonide to control postoperative inflammation after phacotrabeculectomy. J. Cataract Refract. Surg. 2013, 39, 1691–1697. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Dong, L.C.; Liu, H. Study on the effect of triamcinolone acetonide on inflammatory response after cataract surgery in elderly patients. J. Hunan Norm. Univ. 2020, 17, 128–130. [Google Scholar]
- Mohamed, T.A.; Soliman, W.; Fathalla, A.M. Effect of intracameral triamcinolone acetonide on postoperative intraocular inflammation in pediatric traumatic cataract. Eur. J. Ophthalmol. 2016, 26, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Ozge, G.; Ayyildiz, O.; Kucukevcilioglu, M.; Mumcuoglu, T. Comparison of intracameral dexamethasone and intracameral triamcinolone acetonide injection at the end of phacoemulsification surgery. Indian J. Ophthalmol. 2015, 63, 287. [Google Scholar] [CrossRef]
- Bin, L.; Tong, Z.; Meng, C.; Hu, L.I.; Jie, W.U.; Ophthalmology, D.O. Effect of Triamcinolone Acetonide on TNF-α, IL-1β and IL-6 Levels in the Aqueous Fluid of Patients with Age-related Cataract. Prog. Mod. Biomed. 2018, 18, 721–724. [Google Scholar]
- Karalezli, A.; Borazan, M.; Akova, Y.A. Intracameral triamcinolone acetonide to control postoperative inflammation following cataract surgery with phacoemulsification. Acta Ophthalmol. 2008, 86, 183–187. [Google Scholar] [CrossRef]
- Li, J.; Heinz, C.; Zurek-Imhoff, B.; Heiligenhaus, A. Intraoperative intraocular triamcinolone injection prophylaxis for post-cataract surgery fibrin formation in uveitis associated with juvenile idiopathic arthritis. J. Cataract Refract. Surg. 2006, 32, 1535–1539. [Google Scholar] [CrossRef]
- Munger, R.J. Veterinary ophthalmology in laboratory animal studies. Vet. Ophthalmol. 2002, 5, 167–175. [Google Scholar] [CrossRef]
- Xiong, J.W.; Gu, J.F.; Shi, W.R.; Wu, S.Q.; Marhaba, S.; Zhu, Y.Z.; Mo, X.F. The protective effects of leonurine against acute endotoxin induced uveitis in rats. Fudan Univ. J. Med. Sci. 2018, 45, 291–329. [Google Scholar]
- Diakonis, V.F.; Anagnostopoulos, A.G.; Moutsiopoulou, A.; Yesilirmak, N.; Cabot, F.; Waren, D.P.; O’ Brien, T.P.; Yoo, S.H.; Weinstock, R.J.; Donaldson, K.E. The Effect of NSAID Pretreatment on Aqueous Humor Prostaglandin E2 Concentration in Eyes Undergoing Femtosecond Laser-Assisted Capsulotomy. J. Ophthalmol. 2018, 2018, 1891249. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.Q.; Zhang, S.X. Several issues for the prevention and treatment of steroid induced glaucoma. Ophthalmol. China. 2011, 20, 17–20. [Google Scholar]
- Gomez-Sanchez, E.P.; Romero, D.G.; De, R.A.F.; Warden, M.P.; Zygmunt, K.; Gomez-Sanchez, C.E. Hexose-6-phosphate dehydrogenase and 11beta-hydroxysteroid dehydrogenase-1 tissue distribution in the rat. Endocrinology. 2008, 149, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.H.; Ke, M.; Wu, S.Y.; Zhang, J.; Zheng, T. Exploration of the preventive effect of triamcinolone acetonide on posterior capsular opacification. Chin. J. Pract. Ophthalmol. 2017, 35, 1045–1049. [Google Scholar]
- Ren, Y.; Du, S.; Zheng, D.; Shi, Y.; Pan, L.; Yan, H. Intraoperative intravitreal triamcinolone acetonide injection for prevention of postoperative inflammation and complications after phacoemulsification in patients with uveitic cataract. BMC Ophthalmol. 2021, 21, 245. [Google Scholar] [CrossRef] [PubMed]
- Molleda, J.M.; Tardón, R.H.; Gallardo, J.M.; Martín-Suárez, E.M. The ocular effects of intravitreal triamcinolone acetonide in dogs. Vet. J. 2008, 176, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Jones, R., 3rd; Rhee, D.J. Corticosteroid-induced ocular hypertension and glaucoma: A brief review and update of the literature. Curr. Opin. Ophthalmol. 2006, 17, 163–167. [Google Scholar]
- Gavriş, M.; Căciulă, D.; Popa, D.; Cărăuş, C.; Căpraru, C.; Kantor, E.; Clocoţan, D. Phacoemulsification—Personal experience on my first 507 cases. Oftalmologia 2004, 48, 48–52. [Google Scholar]
- Karalezli, A.; Borazan, M.; Kucukerdonmez, C.; Akman, A.; Akova, Y.A. Effect of intracameral triamcinolone acetonide on postoperative intraocular pressure after cataract surgery. Eye 2010, 24, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.Y.; Wee, W.R.; Lee, J.H.; Kim, M.K. Short-term effect of intracameral triamcinolone acetonide on corneal endothelium using the rabbit model. Eye 2007, 21, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Allbaugh, R.A.; Wehrman, R.F.; Sebbag, L. Comparison of topically administered 0.05% difluprednate and 1% prednisolone acetate for inhibition of aqueocentesis-induced breakdown of the blood-aqueous barrier in healthy dogs. Am. J. Vet. Res. 2020, 81, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, E.K.; Meekins, J.M.; Roush, J.K.; Rankin, A.J.; KuKanich, B. Effect of oral administration of robenacoxib on inhibition of paracentesis-induced blood-aqueous barrier breakdown in healthy cats. Am. J. Vet. Res. 2018, 79, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Barar, J.; Javadzadeh, A.R.; Omidi, Y. Ocular novel drug delivery: Impacts of membranes and barriers. Expert Opin. Drug Deliv. 2008, 5, 567–581. [Google Scholar] [CrossRef] [PubMed]
- Cleary, C.A.; Lanigan, B.; O’ Keeffe, M. Intracameral triamcinolone acetonide after pediatric cataract surgery. J. Cataract Refract. Surg. 2010, 36, 1676–1681. [Google Scholar] [CrossRef] [PubMed]
- Ben Simon, G.J.; Kenet, G.; Spierer, A. Fibrinoid reaction after lens extraction in rabbit eyes. J. Cataract Refract. Surg. 2012, 38, 890–893. [Google Scholar] [CrossRef]
- Wålinder, P.E.; Olivius, E.O.; Nordell, S.I.; Thorburn, W.E. Fibrinoid reaction after extracapsular cataract extraction and relationship to exfoliation syndrome. J. Cataract Refract. Surg. 1989, 15, 526–530. [Google Scholar] [CrossRef]
- Bynoe, L.A.; Weiss, J.N. Retinal endovascular surgery and intravitreal triamcinolone acetonide for central vein occlusion in young adults. Am. J. Ophthalmol. 2003, 135, 382–384. [Google Scholar] [CrossRef]
- Cekiç, O.; Chang, S.; Tseng, J.J.; Barile, G.R.; Weissman, H.; Del Priore, L.V.; Schiff, W.M.; Weiss, M.; Klancnik, J.M., Jr. Intravitreal triamcinolone treatment for macular edema associated with central retinal vein occlusion and hemiretinal vein occlusion. Retina 2005, 25, 846–850. [Google Scholar] [CrossRef]
- Macky, T.A.; Helmy, D.; El Shazly, N. Retinal toxicity of triamcinolone’s vehicle (benzyl alcohol): An electrophysiologic and electron microscopic study. Graefe’ s Arch. Clin. Exp. Ophthalmol. = Albrecht Von. Graefes Arch. Fur Klin. Und Exp. Ophthalmol. 2007, 245, 817–824. [Google Scholar] [CrossRef]
- Pinard, C.L.; Gauvin, D.; Moreau, M.; Martel-Pelletier, J.; Pelletier, J.P.; Troncy, E. Measurements of canine aqueous humor inflammatory mediators and the effect of carprofen following anterior chamber paracentesis. Vet. Ophthalmol. 2011, 14, 296–303. [Google Scholar] [CrossRef]
- Gilmour, M.A.; Payton, M.E. Comparison of the effects of IV administration of meloxicam, carprofen, and flunixin meglumine on prostaglandin E2 concentration in aqueous humor of dogs with aqueocentesis-induced anterior uveitis. Am. J. Vet. Res. 2012, 73, 698–703. [Google Scholar] [CrossRef]

| Time Points | TA Group (n = 18) | Control Group (n = 18) | ||
|---|---|---|---|---|
| OD (0.5 mg TA) (n = 6) | OD (1.0 mg TA) (n = 6) | OD (1.5 mg TA) (n = 6) | OS | |
| 0 h | 25.67 ± 4.16 | 26.00 ± 6.08 | 25.67 ± 2.52 | 28.77 ± 5.98 |
| 2 h | 33.67 ± 17.56 | 28.33 ± 7.02 | 18.33 ± 0.58 | 19.58 ± 1.42 |
| 4 h | 30.33 ± 17.21 | 22.67 ± 6.35 | 14.00 ± 3.46 | 14.20 ± 6.42 |
| 6 h | 26.00 ± 9.17 | 24.33 ± 6.35 | 18.67 ± 1.15 | 24.31 ± 4.59 |
| 8 h | 30.33 ± 17.24 | 22.00 ± 2.65 | 17.33 ± 1.53 | 27.77 ± 19.03 |
| 1 d | 16.00 ± 3.00 | 22.33 ± 3.51 | 18.67 ± 0.58 | 19.32 ± 11.21 |
| 2 d | 22.00 ± 11.79 | 21.33 ± 4.04 | 17.33 ± 2.31 | 20.31 ± 8.39 |
| 3 d | 18.67 ± 5.51 | 21.67 ± 1.53 | 19.67 ± 1.53 | 22.57 ± 3.96 |
| 4 d | 19.67 ± 1.53 | 20.33 ± 0.58 | 18.67 ± 1.53 | 19.43 ± 4.12 |
| 5 d | 19.00 ± 0.00 | 22.00 ± 3.00 | 19.33 ± 0.58 | 19.39 ± 2.17 |
| 6 d | 18.67 ± 1.15 | 20.30 ± 2.89 | 22.33 ± 2.52 | 18.43 ± 1.62 |
| 7 d | 17.67 ± 1.53 | 20.67 ± 6.66 | 23.67 ± 7.23 | 17.29 ± 2.16 |
| 14 d | 19.00 ± 1.00 | 21.33 ± 2.52 | 19.00 ± 2.00 | 19.00 ± 2.00 |
| 21 d | 20.00 ± 1.73 | 19.33 ± 0.58 | 20.67 ± 1.53 | 19.44 ± 1.06 |
| 28 d | 19.00 ± 0.00 | 19.33 ± 2.52 | 20.67 ± 1.53 | 17.53 ± 0.67 |
| 45 d | 18.00 ± 1.73 | 21.33 ± 0.58 | 20.33 ± 1.15 | 15.40 ± 8.26 |
| Time Points | TA Group (n = 18) | Control Group (n = 18) | ||
|---|---|---|---|---|
| OD (0.5 mg TA) (n = 6) | OD (1.0 mg TA) (n = 6) | OD (1.5 mg TA) (n = 6) | OS | |
| 1 d | 1.33 ± 0.58 | 1.67 ± 0.58 | 1.00 ± 0.00 | 1.33 ± 0.58 |
| 2 d | 1.00 ± 1.00 | 1.00 ± 1.00 | 0.67 ± 0.58 | 1.33 ± 0.58 |
| 3 d | 1.00 ± 0.00 a | 0.33 ± 0.58 | 0.00 ± 0.00 ab | 1.00 ± 0.00 b |
| 4 d | 0.67 ± 0.58 | 0.33 ± 0.58 | 0.00 ± 0.00 | 0.67 ± 0.58 |
| 5 d | 0.67 ± 0.58 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.33 ± 0.58 |
| 6 d | 0.33 ± 0.58 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.33 ± 0.58 |
| 7 d | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Time Points | TA Group (n = 18) | Control Group (n = 18) | ||
|---|---|---|---|---|
| OD (0.5 mg TA) (n = 6) | OD (1.0 mg TA) (n = 6) | OD (1.5 mg TA) (n = 6) | OS | |
| 1 d | 0.67 ± 0.58 | 1.33 ± 0.58 | 1.33 ± 0.58 | 1.33 ± 0.58 |
| 2 d | 0.67 ± 0.58 | 1.33 ± 0.58 | 1.00 ± 0.00 | 1.00 ± 1.00 |
| 3 d | 0.67 ± 0.58 | 0.67 ± 0.58 | 0.00 ± 0.00 | 0.33 ± 0.58 |
| 4 d | 0.33 ± 0.58 | 0.33 ± 0.58 | 0.00 ± 0.00 | 0.33 ± 0.58 |
| 5 d | 0.33 ± 0.58 | 0.33 ± 0.58 | 0.00 ± 0.00 | 0.33 ± 0.58 |
| 6 d | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 7 d | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Time Points | TA Group (n = 18) | Control Group (n = 18) | ||
|---|---|---|---|---|
| OD (0.5 mg TA) (n = 6) | OD (1.0 mg TA) (n = 6) | OD (1.5 mg TA) (n = 6) | OS | |
| 0 h | 1.38 ± 0.67 | 0.43 ± 0.17 | 0.54 ± 0.13 | 0.49 ± 0.16 |
| 4 h | 14.20 ± 4.50 | 16.53 ± 0.29 | 13.66 ± 9.02 | 6.94 ± 5.43 |
| 1 d | 6.29 ± 2.45 | 11.80 ± 2.71 a | 4.79 ± 1.82 ab | 21.27 ± 1.39 b |
| 3 d | 3.12 ± 1.94 | 1.88 ± 0.43 | 1.50 ± 0.20 | 3.11 ± 0.76 |
| 5 d | 2.31 ± 1.59 | 1.31 ± 0.24 | 1.20 ± 0.13 c | 2.53 ± 0.69 c |
| 10 d | 1.23 ± 1.15 | 2.76 ± 1.51 | 0.87 ± 0.33 d | 2.16 ± 0.32 d |
| Time Points | TA Group (n = 18) | Control Group (n = 18) | ||
|---|---|---|---|---|
| OD (0.5 mg TA) (n = 6) | OD (1.0 mg TA) (n = 6) | OD (1.5 mg TA) (n = 6) | OS | |
| 0 h | 30.35 ± 3.82 | 26.95 ± 2.7 | 31.61 ± 2.93 | 27.94 ± 1.62 |
| 4 h | 171.70 ± 28.4 | 91.67 ± 40.99 | 96.24 ± 18.19 | 132.41 ± 31.52 |
| 1 d | 206.97 ± 28.72 ab | 121.51 ± 23.88 a | 119.80 ± 17.85 bc | 172.48 ± 17.32 c |
| 3 d | 189.83 ± 25.77 d | 104.64 ± 32.65 d | 132.55 ± 37.73 | 128.66 ± 7.56 |
| 10 d | 156.13 ± 13.92 | 104.67 ± 44.36 | 132.40 ± 45.67 | 137.49 ± 23.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Lu, D.; Pang, M.; Li, J.; Liu, Y.; Shi, H.; Liu, G.; Jin, Y. The Effect of Intracameral Triamcinolone Acetonide on Controlling Common Complications following Phacoemulsification in Dogs. Animals 2024, 14, 547. https://doi.org/10.3390/ani14040547
Liu Z, Lu D, Pang M, Li J, Liu Y, Shi H, Liu G, Jin Y. The Effect of Intracameral Triamcinolone Acetonide on Controlling Common Complications following Phacoemulsification in Dogs. Animals. 2024; 14(4):547. https://doi.org/10.3390/ani14040547
Chicago/Turabian StyleLiu, Zichen, Di Lu, Mo Pang, Jing Li, Yue Liu, Hao Shi, Gang Liu, and Yipeng Jin. 2024. "The Effect of Intracameral Triamcinolone Acetonide on Controlling Common Complications following Phacoemulsification in Dogs" Animals 14, no. 4: 547. https://doi.org/10.3390/ani14040547
APA StyleLiu, Z., Lu, D., Pang, M., Li, J., Liu, Y., Shi, H., Liu, G., & Jin, Y. (2024). The Effect of Intracameral Triamcinolone Acetonide on Controlling Common Complications following Phacoemulsification in Dogs. Animals, 14(4), 547. https://doi.org/10.3390/ani14040547





