Simple Summary
Veiled chameleons are native to the Arabian Peninsula, with a presence in other regions, such as the Canary Islands (Spain). The aim of this study is to analyze the existence of pathogenic bacteria in a population of this invasive reptile on Gran Canaria island. The results obtained highlight the presence of a variety of pathogens with relevance to human health, most of them related to gastrointestinal diseases. This archipelago is a biodiversity hotspot, with some endangered species living there, so the presence of veiled chameleons could be also a risk to biodiversity conservation, by the spread and/or transmission of pathogenic bacteria to the native fauna. In conclusion, the invasive veiled chameleon population in the Canary Islands should be considered as a potential risk factor for biodiversity conservation and human health.
Abstract
Veiled chameleons (Chamaeleo calyptratus) are native to the Arabian Peninsula that have been introduced as pets in many regions around the world, such as the Canary Islands (Spain). In this work, the gastrointestinal content from veiled chameleons of Gran Canaria island (Canary Islands) has been analyzed to determine the presence of zoonotic bacteria. Forty animals were analyzed using different selective culture media and PCR. The most isolated bacteria were Yersinia enterocolitica (52.4%), followed by Salmonella spp. (40.0%), with positive isolates for Salmonella Tyhpi and Salmonella Typhimurium. Pseudomonas spp. was found in 32.5% of the chameleons. More than half were positive for Pseudomonas aeruginosa. Antibiotic-resistant Staphylococcus spp. was detected in six animals plus one isolate of non-resistant Staphylococcus hominis. Multiple mycobacteria species belonging to both tuberculous and non-tuberculous complexes were identified as well as Escherichia coli carrying the stx1 and eae virulence genes with 12.5% and 7.5% prevalence, respectively. Listeria monocytogenes, Campylobacter spp., and Vibrio spp. were found in lower proportion (<5%). The results obtained indicate that veiled chameleons in Gran Canaria could be playing a role in the maintenance and dissemination of the pathogens detected, harming public health and biodiversity.
1. Introduction
The settlement of animals into new ecosystems leads to a multi-factor problem: crop loss, predation, or resource competition with the endemic species (endangered in many cases) [1] or, concerning this study, being a source of pathogens to humans, animals, and plants [1]. This last fact is especially dangerous in the case of uncommon pathogens because the local health system may not have the means and experience needed for its diagnosis and treatment [2].
The Canary Islands (Spain) are located in Norwest Africa, near the coast of Morocco (13°23′–18°80′ W and 27°37′–29°24′ N), and they present some features that make them suitable for the colonization of invasive species such as good climatic conditions, resource abundance, and lack of large predators [3].
All this, added to the high number of endemic species present in the Canary Archipelago, makes the introduction of exotic animals a risk to the local biodiversity [4]. At least 1167 exotic plant and animal species have been reported in the Canaries, of which 289 are considered invasive or potentially invasive species [5]. On Gran Canaria, the second largest island, the negative effect of exotic reptiles is well known, just as in the case of the California kingsnake (Lampropeltis californiae), which has alarmingly reduced the population of endemic lizards [6]. Apart from these snakes, the veiled chameleon, Chamaeleo calyptratus Duméril and Bibron, 1851 (Squamata, Chamaeleonidae), native to the southwest Arabian Peninsula, was introduced to the north of the island with available records reporting their entrance in 2017, firstly introduced as a pet and zoological species [7]. They are a diurnal and arboreal lizard species capable of adapting to a huge variety of ecosystems from high, dry plateaus to forests and river valleys. They have been also reported in Florida and Hawaii (USA) due to the illegal pet trade [8].
In the Canary Islands, the effect of these chameleons on the endemic invertebrates is known only because of their insect-based diet [7], but no data are available about the possible role in the transmission and maintenance of pathogenic agents. Due to this lack of information, the aim of this study was to determine the presence of pathogenic bacteria in these animals from Gran Canaria and evaluate the health risks to humans and local fauna.
2. Materials and Methods
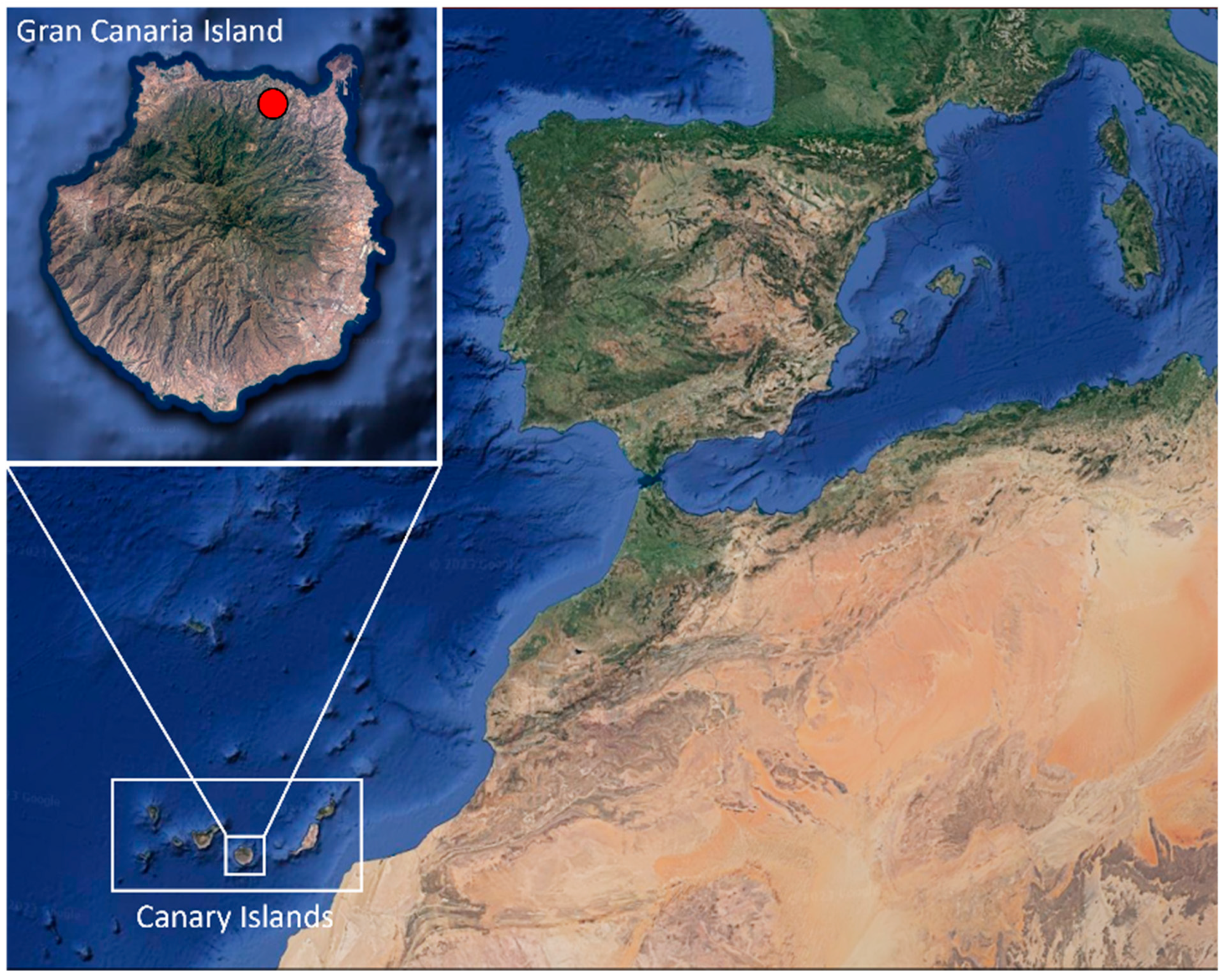
A total of 40 animals, 36 adults (11 males, 21 females, and 4 indeterminate) and 4 young individuals, from Arucas municipality, Gran Canaria (Canary Islands, Spain) (Figure 1), were donated by “Red de Alerta Temprana de Canarias para la Detección e Intervención de Especies Exóticas Invasoras” (REDEXOS) staff, after authorization of “Dirección General de Lucha Contra el Cambio Climático y Medio Ambiente” (Gobierno de Canarias, Expte. EEI-001/2016). The reptiles were sexed and necropsied. During that time, fecal matter was obtained for culture.
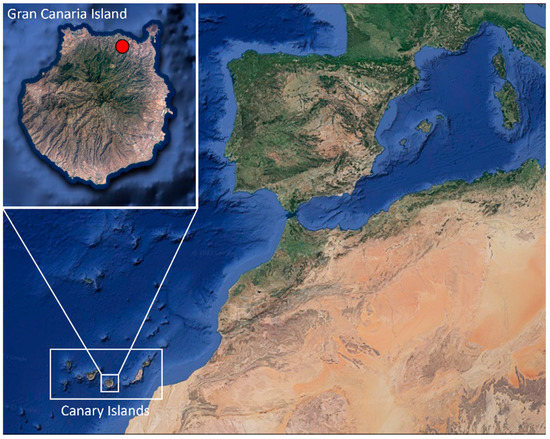
Figure 1.
Location where Chamaleo calyptratus were captured (red spot) on Gran Canaria (Canary Islands, Spain). Images obtained from Google Earth website and modified with Microsoft PowerPoint 2016 software.
2.1. Bacterial Strains
The bacterial strains used as positive controls for the assays were obtained from American Type Culture Collection (ATCC). All of them were stored at −70 °C, and incubated to make them grow for 18 to 24 h in Tryptic Soy Broth (TSB) (Labkem, Barcelona, Spain) at 37 °C under aerobic conditions, or microaerophilic conditions in the case of Campylobacter spp.
2.2. Isolation of Bacteria from the Samples
During dissections, 100 mg of intestinal content was extracted from each animal and incubated in 5 mL of Buffered Peptone Water (BPW) (Labkem, Barcelona, Spain) at 37 °C for 24 h. For the isolation of Campylobacter spp., 100 mg of intestinal content was incubated in 2 mL of BPW at 42 °C for 18 h under microaerophilic conditions while, in the case of Vibrio spp. isolation, Alkaline Peptone Water (APW) was applied instead of BPW for 8 h at 37 °C. APW was made in the laboratory from BPW, adding 1% of NaCl and increasing the pH level to 8.4.
After this first incubation, different selective culture media were used, 100 µL of peptone water culture was incubated in Baird–Parker agar (Labkem, Barcelona, Spain) for the isolation of Staphylococcus spp., Cetrimide agar (VWR International, Leuven, Belgium) for Pseudomonas spp., Cefsulodin Irgasan Novobiocin agar (CIN) (Merck, Darmstadt, Germany) for Yersinia spp., Sorbitol supplemented MacConkey agar (Scharlab, Barcelona, Spain), and Tryptone Bile X-glucuronide chromogenic agar (TBX) (Labkem, Barcelona, Spain) for Escherichia coli and Oxford agar (Labkem, Barcelona, Spain) for Listeria monocytogenes. For Vibrio spp. isolation, 100 µL of APW culture was incubated in Thiosulfate-Citrate-Bile-Saccharose agar (TCBS) (VWR International, Leuven, Belgium). All the cultures were incubated for 24 h at 37 °C except for CIN, which was incubated at 30 °C.
In the case of Salmonella spp., 500 µL of BPW culture was transferred to 4.5 mL of Rappaport–Vassiliadis Broth (VWR International, Leuven, Belgium) and stored for 20 h at 42 °C. Then, 100 µL of broth culture was later incubated in Salmonella–Shigella agar (Merck, Darmstadt, Germany) for 24 h at 37 °C.
2.3. Molecular Identification
2.3.1. DNA Extraction
For the DNA isolation of Mycobacterium spp., 1 mL of each BPW culture was taken, and for Campylobacter spp., 1 mL of BPW cultured at 42 °C under microaerophilic conditions was used. All samples were washed twice with phosphate-buffered saline (PBS) and the pellet was subjected to DNA extraction method following López et al. indications [9].
For the rest of bacteria, 5 colonies of each agar plate were suspended in 1 mL of PBS and centrifuged at 12,000× g twice. The pellet was subjected to DNA extraction following López et al. [9].
2.3.2. PCR Identification
After the DNA extraction, the most relevant zoonotic bacteria, including resistance and virulence genes, were identified using PCR techniques. All assays were performed using positive and negative controls.
For Campylobacter identification, a multiplex PCR (m-PCR) with six pairs of primers was employed for genus confirmation and identification of Campylobacter coli, Campylobacter fetus, Campylobacter jejuni, Campylobacter lari, and Campylobacter upsaliensis according to Wang et al. [10].
In the case of E. coli, virulence genes were analyzed: stx1 and stx2 genes, responsible for Shiga-like toxins synthesis, and eae gene, which codifies for intimin, following the protocol described by Blanco et al. [11].
Listeria monocytogenes was identified through the confirmation of the suspicious colonies grown in Oxford agar, by a simple PCR of a region of the iap gene, which codifies the p60 invasion-associated protein, as described by Jaton et al. [12].
Following the process described by Kim et al. [13], m-PCR was carried out for mycobacteria identification and to differentiate the Mycobacterium tuberculosis complex from the atypical mycobacteria group.
UV fluorescent colonies in Cetrimide agar were tested for Pseudomonas aeruginosa through simultaneous amplification of oprI and oprL genes, which codify for lipoproteins, as described by De Vos et al. [14].
Regarding the determination of Salmonella serotypes important to human health, Guimarães de Freitas et al. [15] protocol was followed. One m-PCR allows the detection of all bacteria belonging to the genus, and the identification of Salmonella Enteritidis and Salmonella Typhi serotypes. A second PCR was utilized to identify Salmonella Typhimurium serotype.
A single m-PCR was used for the identification of Staphylococcus aureus, Staphylococcus epidermidis, Staphylococcus haemolyticus, Staphylococcus hominis, Staphylococcus lugdunensis, and Staphylococcus saprophyticus species as well as the detection of antibiotic-resistant genes (methicillin and mupirocin) following Campos-Peña et al. [16].
For the most frequent Vibrio species, a PCR assay was performed to detect the genus according to Liu et al. [17]. The positive samples were subjected to more specific PCR to identify Vibrio cholerae, Vibrio parahaemolyticus, and Vibrio vulnificus, as described by Neogi et al. [18].
In the case of Yersinia enterocolitica, two pairs of primers were used to detect pathogenic and non-pathogenic strains, according to Wannet et al. [19].
The results of all PCR assays were evaluated with agarose gel electrophoresis (Fisher Bioreagents, Madrid, Spain), and the size of the amplification products was estimated by comparison with molecular size markers (SiZer-100 DNA Marker, iNtRON Biotechnology, Seongnam-Si, Republic of Korea; and AmpliSize Molecular Ruler, Bio-Rad, Hercules, CA, USA). Real-Safe (Durviz SL, Valencia, Spain) was used as a DNA stain and a ChemiDocTM XRS+ (Bio-Rad, Hercules, CA, USA) system for the visualization of the amplicons.
2.4. Co-Infection Index (Ic)
The co-infection index (Ic) developed by Ginsberg [20] quantifies the deviation of the number of mixed infections from independence. It is defined as the difference between the number of co-infections and the expected number due to chance alone, as a percentage of the totality of the infected animals.
where O = number of observed co-infections; E = expected number of C. calyptratus with coinfections due to chance alone; N = total number of C. calyptratus infected by either or both microorganisms.
where a = number of chameleons infected by both bacteria (equals O); b = number of chameleons infected only with microorganism 1; c = number of chameleons infected only with microorganism 2; and d = number of chameleons not infected with microorganism 1 nor 2. The Ic value is positive if the number of real co-infections is greater than that expected or negative if it is less. The significance of the co-infection index was calculated by chi-square test.
Ic = [(O − E)/N] × 100
E = (a + b) (a + c)/(a + b + c + d); N = a + b + c
2.5. Statistical Analysis
The statistical Windows software “SPSS” version 25.0 (IBM Corporation, Armonk, NY, USA) was used to compare the prevalence between sex and age of the studied individuals. For that purpose, chi-square test and Fisher’s exact test were applied with a stabled p-value of 0.05.
3. Results
Twenty-eight out of forty studied animals (70.0%) showed positive results for at least one of the investigated bacteria. The most frequent pathogen was Y. enterocolitica, confirmed in 52.4% of the isolates, followed by Salmonella spp. (40.0%) and Pseudomonas spp. (32.5%). Table 1 describes all positive isolates.

Table 1.
Percentage of isolated bacteria from Chamaeleo calyptratus from Gran Canaria (Canary Islands, Spain).
3.1. Campylobacter spp.
Among the five Campylobacter species sought, just C. lari was identified in a male chameleon, and a female was positive for a Campylobacter species that was not included in the assays. None of the young individuals were infected with this group of bacteria.
3.2. Escherichia coli Virulence Genes (stx1, stx2, and eae)
Escherichia coli containing virulence genes was identified in three (7.5%) chameleons, two individuals carrying the stx1 gene and another one carrying the eae gene. The three animals were females, not being found in males or young individuals. The stx2 gene was not identified, nor was the coexistence of E. coli with more than one gene in the same animal.
3.3. Listeria monocytogenes
The iap gene amplification allowed the identification of two females positive for L. monocytogenes. None of the males or young individuals were infected with this bacterium.
3.4. Mycobacterium spp.
Five chameleons (12.5%) were positive for Mycobacterium spp. The amplification only of the rpoB gene in three of them (7.5%) indicates an infection with Mycobacterium microti, included within the tuberculous complex, or with a non-tuberculous mycobacterium. In the other two cases, the amplification of RD1 and rpoB genes suggests a Mycobacterium bovis infection, a tuberculous mycobacterium. There were no significant differences between infected males and females or adult and young individuals (p > 0.05).
3.5. Pseudomonas spp.
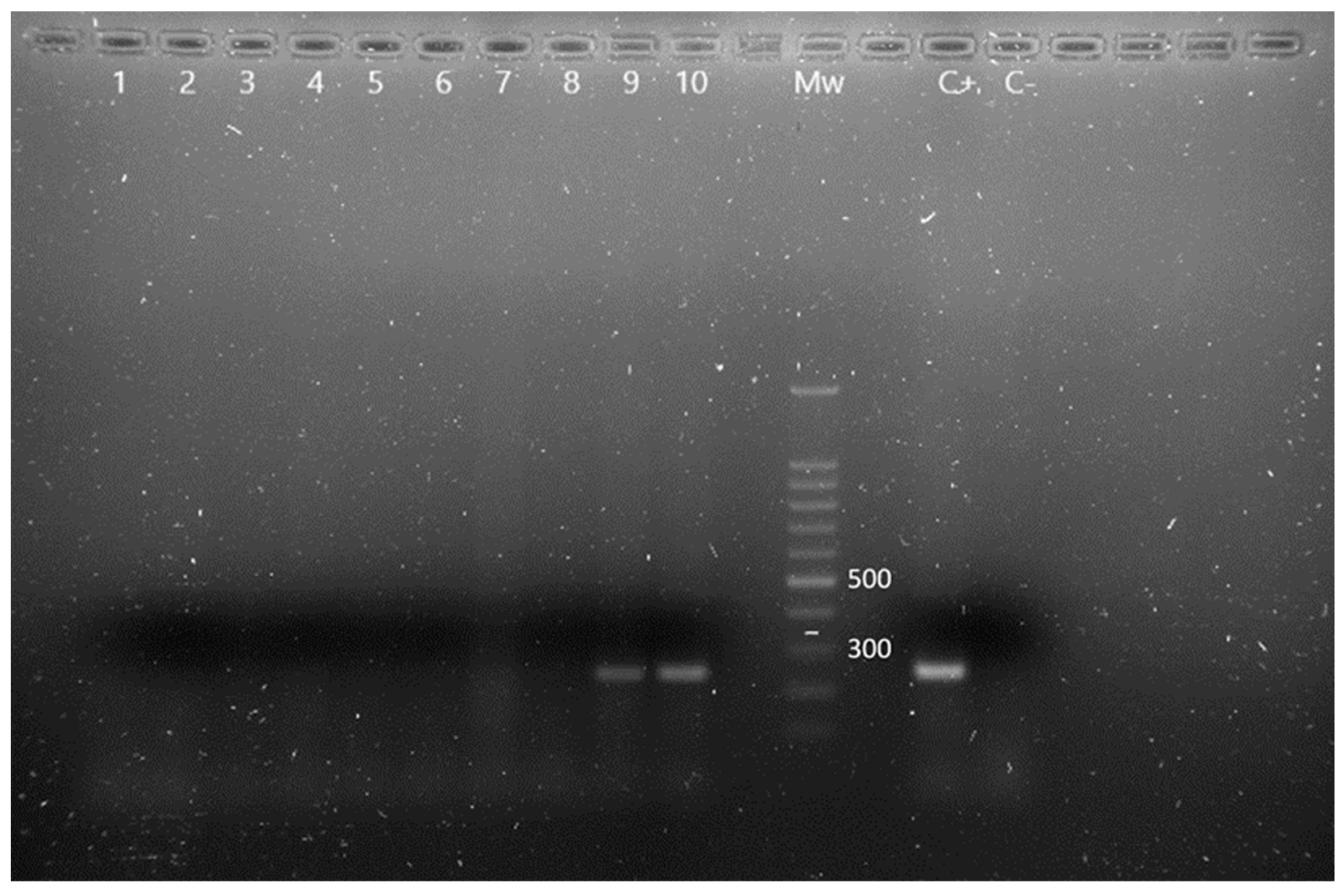
Thirteen animals were positive for Pseudomonas spp., being identified P. aeruginosa in eight of them, by simultaneous oprI and oprL gene amplifications (Figure 2). There were no significant prevalence differences between males and females or adult and young individuals (p > 0.05). The obtained results, classified according to age and sex, are described in Table 2.
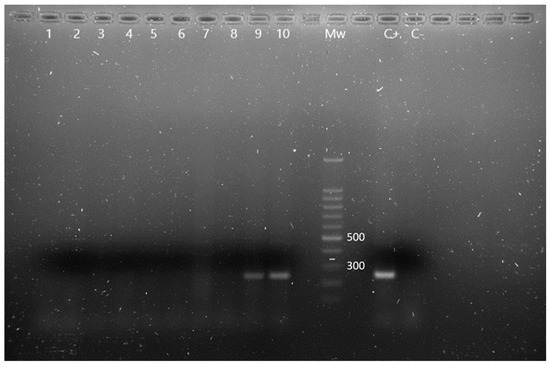
Figure 2.
m-PCR results for the detection of Pseudomonas spp. and P. aeruginosa in Chamaeleo calyptratus from Gran Canaria (Canary Islands, Spain). Lane 1 to 8: negative samples for Pseudomonas spp., lane 9 and 10: OprI gen amplification fragments characteristic of Pseudomonas spp., Mw: molecular size marker (SiZer-100 DNA Marker, iNtRON Biotechnology), C+: positive control, C−: negative control.

Table 2.
Prevalence of Pseudomonas spp. in Chamaeleo calyptratus from Gran Canaria (Canary Islands, Spain).
3.6. Salmonella spp.
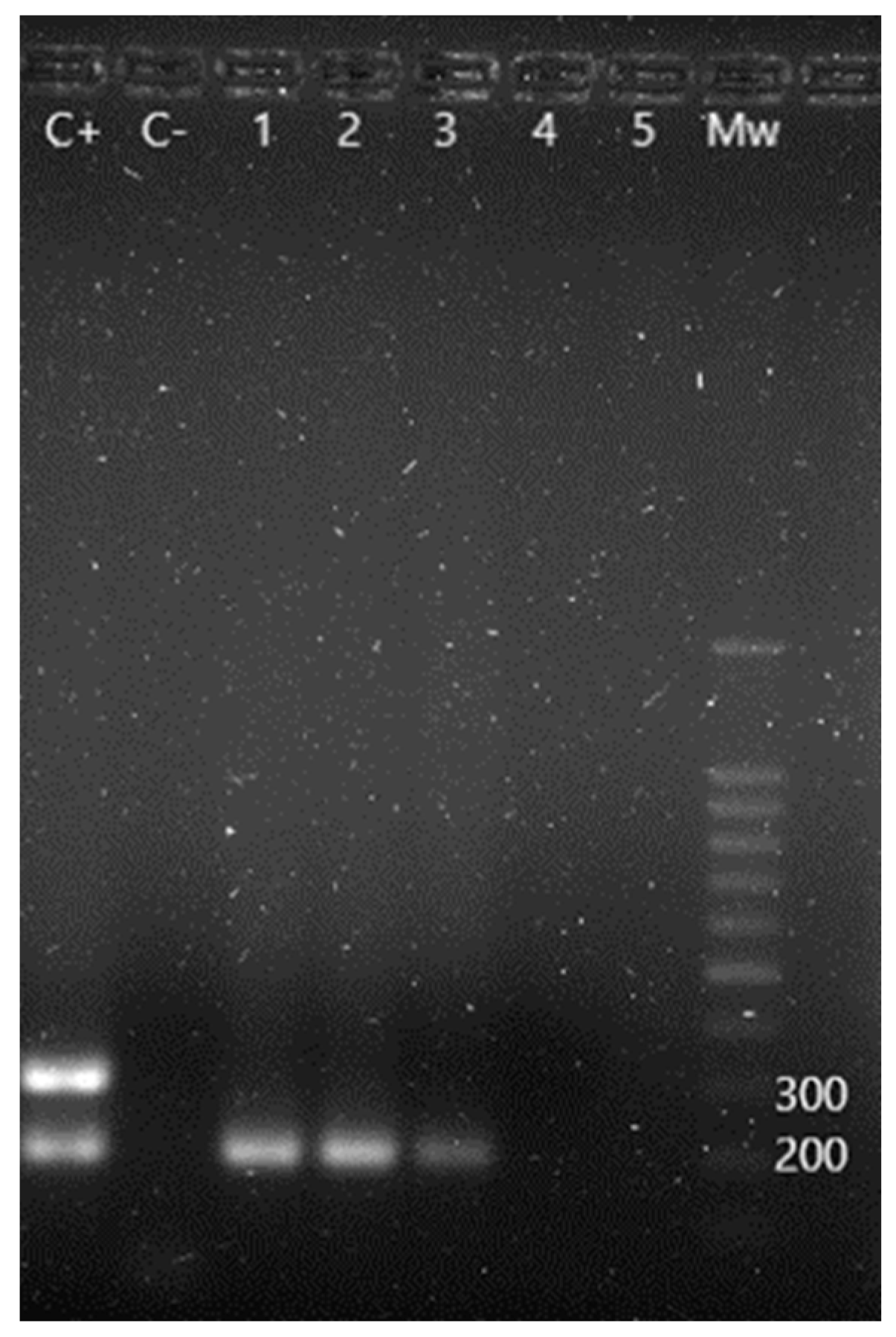
Sixteen out of forty animals (40.0%) presented positive results for Salmonella spp., having found S. Typhi in two of them and S. Typhimurium in another two, apart from one case of coinfection with both serotypes (Figure 3). Salmonella Enteritidis was not detected. No significant difference prevalences were found, according to sex and age. Table 3 shows the results obtained regarding this bacteria genus.
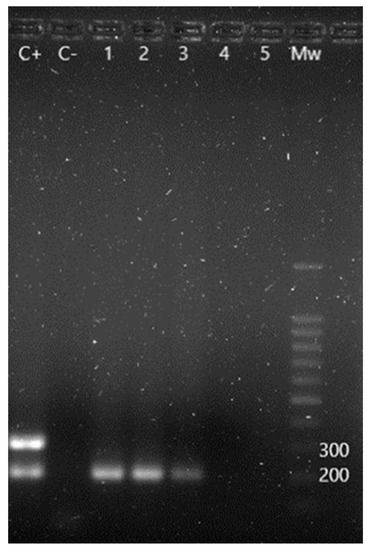
Figure 3.
m-PCR results for the detection of Salmonella spp. and its serotypes. C+: S. Enteritidis positive control showing a 204 bp amplification fragment corresponding to Salmonella spp. and a 304 bp fragment corresponding to S. Enteritidis serotype. C−: negative control. Lane 1 to 3: positive samples for Salmonella spp. Lane 4 and 5: negative samples. Mw: molecular size marker (SiZer-100 DNA Marker, iNtRON Biotechnology).

Table 3.
Prevalence of Salmonella spp. in Chamaeleo calyptratus from Gran Canaria (Canary Islands, Spain).
3.7. Staphylococcus spp.
Among the six Staphylococcus species searched, just S. hominis was found in one of the studied animals (2.5%). Despite this, multiple Staphylococcus spp. carrying antibiotic-resistant genes that could not be identified at the species level were detected, including one isolate with both genes. Statistical tests showed no significant differences between males and females or between adults and juveniles individuals. These results are described in Table 4.

Table 4.
Prevalence of Staphylococcus spp. and antibiotic-resistant genes in Chamaeleo calyptratus from Gran Canaria (Canary Islands, Spain).
3.8. Vibrio sp.
Vibrio sp. was only found in a female chameleon. One PCR assay performed after the genus confirmation showed a negative result, indicating a species different from V. vulnificus, V. parahemolyticus, and V. cholerae.
3.9. Yersinia enterocolitica
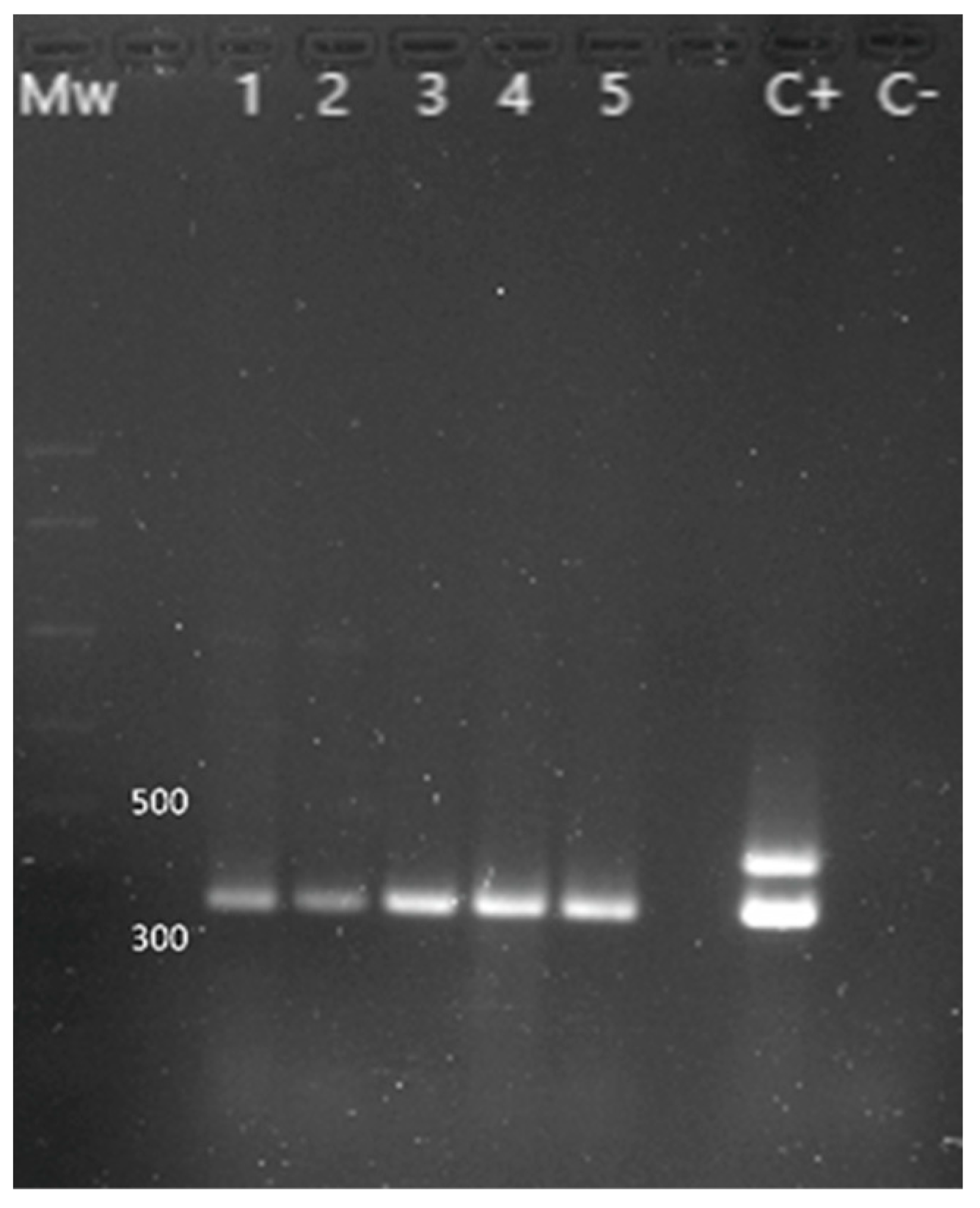
The presence of Y. enterocolitica was investigated in 21 chameleons (Figure 4), with 11 positive animals (52.4%): 7 out of 11 females (63.6% of the females studied), 2 males out of 6 (33.3% of the males studied), and 2 out of 4 juveniles (50.0% of the juveniles studied). The ail gene amplification, characteristic of pathogenic strains, was only observed in one isolate. Statistical tests did not expose significant differences when comparing the prevalence between sex and age of the individuals.
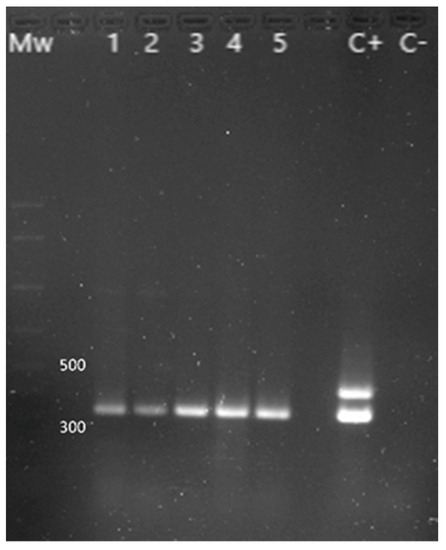
Figure 4.
PCR results for the identification of Y. enterocolitica. Mw: molecular size marker (AmpliSize Molecular Ruler, Bio-Rad). Lane 1 to 5: 16s gene (330 bp) amplification fragment characteristic of Y. enterocolitica. C+: positive control (the 425 bp fragment corresponding to the ail gene is only found in pathogenic strains). C−: negative control.
3.10. Co-Infection and Co-Infection Index (Ic)
Because Y. enterocolitica was not investigated in all animals, it was not considered for co-infection analysis. From the 28 positive animals, 8 (28.6%) were infected with just 1 investigated pathogen, 12 (39.3%) hosted 2 pathogens simultaneously, 4 (17.9%) hosted 3 pathogens, 3 (10.7%) hosted 4, and 1 animal (3.6%) hosted 5 pathogens (mupirocin-resistant Staphylococcus sp., non-pathogenic Y. enterocolitica, L. monocytogenes, P. aeruginosa, and Vibrio sp.). The most common combination (Table 5) was Pseudomonas spp. and Salmonella spp. (19.4% of all bacteria combinations), followed by Staphylococcus spp. associated with Salmonella spp. (13.9%), Mycobacterium spp. with Salmonella spp. or Pseudomonas spp. (8.3%), and, lastly, Pseudomonas spp. with Staphylococcus spp. (8.3%).

Table 5.
Percentage of co-infections in Chamaeleo calyptratus from Gran Canaria (Canary Islands, Spain).
A positive co-infection index added to a chi-square test with a p-value less than 0.05 indicates a synergic relationship between the two bacteria present in the same animal. All co-infection indexes and statistical test results are shown in Table 6.

Table 6.
Co-infection index for the different co-infections obtained from Chamaeleo calyptratus from Gran Canaria (Canary Islands, Spain).
According to the data obtained, there is a strong correlation between S. Typhi infection and the E. coli-carrying stx1 gene, while L. monocytogenes is related to the development of Staphylococcus spp.
4. Discussion
4.1. Campylobacter spp.
Campylobacter spp. are common zoonotic pathogens with relevance both in human and animal health, with rising incidence due to their ability to infect a wide variety of different species, their adaptability to new habitats, and the development of strains resistant to routine antibiotics [21]. In humans, the species that causes disease with more frequency is C. jejuni, followed by C. coli, even though more than 10 species have been identified. In reptiles, the predominant species are C. iguaniorum, C. geochelonis, and C. fetus subsp. testudinum [22,23]. Among the two positive isolates for Campylobacter spp. identified in this study, one corresponds to C. lari, a species rarely found in humans with campylobacteriosis [24], while the second species could not be identified, but it could be C. iguaniorum or C. geochelonis, both usually detected in reptiles with no data reported of causing disease in humans, although their discovery is recent and more data are needed to discard the human infection [25,26].
After an exhaustive bibliographic search, no reference was found regarding C. lari as a pathogen or commensal flora in reptiles; therefore, this could be the first case reported. Campylobacter lari is rather found in coast-related animals like shorebirds or seafood [27], so its presence in these animals could be explained by the fact that the chameleon population on Gran Canaria is located close to the coast where numerous marine animals live, especially birds like seagulls or shearwaters [28]. The cases of human infection with C. lari are not as frequent as C. jejuni or C. coli, although bacteremia cases have been reported [27,29,30] both in immunocompromised and immunocompetent individuals, which could risk the patient’s life.
4.2. Escherichia coli Virulence Genes (stx1, stx2, and eae)
Escherichia coli presence, as well as the determination of its virulence factors, like its capacity to produce toxins or its antibiotic or heat resistance, has been highly studied around the world with different samples: humans, animals, food, water, or the environment [31,32,33]. Nevertheless, few works have been performed on cold-blood animals since cattle and their derived products are the principal E. coli reservoir associated with human disease [34]. In reptiles, the E. coli prevalence and virulence are lower, but their role as reservoirs capable of maintaining the transmission of this bacterium cannot be discarded [35,36].
In this study, the number of E. coli-carrying stx1, stx2, and eae virulence genes was similar to other studies, such as Bautista Trujillo et al. [37], in which 27 out of 240 (11.25%) captive green iguanas (Iguana iguana) from Mexico were positive for these genes, with a higher prevalence of the stx1 gene (10.0%) than the stx2 (0.4%) and eae (0.83%) genes. In our study, the difference was not as remarkable (5.0% of stx1 gene prevalence against 2.5% of the eae gene), and stx2 gene amplification did not happen. On the other hand, neither in Dec et al. [35] work on reptiles from Poland nor Martínez et al. [38] work on ocellated lizards (Timon lepidus) from Spain were the stx1, stx2, or eae genes found.
Both the stx1 and stx2 genes are associated with severe symptoms like hemolytic uremic syndrome; these vary in the target organ and can cause different pathologies depending on the animal host; for example, in humans, stx2 gene expression is more often related to clinical complications than stx1 [39]. The intimin protein codified by the eae gene is involved in bacterial adherence to the intestinal epithelium and may or may not be present simultaneously with the stx1 and stx2 genes [40]. The large number of E. coli virulence genes, also divided into many subtypes with distinct particularities such as trophism for different tissues, makes stx1, stx2, and eae gene identification alone insufficient to determine the pathogenicity of the strain precisely [41].
4.3. Listeria monocytogenes
Listeriosis is an infrequent outbreak-associated disease that can cause severe health problems in humans and animals such as meningoencephalitis, septicemia, or fetal development failure if the infection affects pregnant women. It is transmitted through the ingestion of contaminated food, fundamentally meat [42,43].
Most studies regarding L. monocytogenes in animals are focused on mammals (mostly cattle) and birds, as well as food production chains [44,45]. Few authors have carried out investigations to analyze the presence of this bacterium in reptiles. Weber et al. identified 5/30 (16.7%) and 1/76 (1.3%) positive isolates of L. monocytogenes in turtles and snakes, respectively, in 1993, and 5/35 (14.29%) positive turtles in 1995 [46,47]; in both cases, the analyzed animals were pets. Similar data were published by Chen et al. showing a prevalence of 12% (2/17) in wild turtles [48]; however, the prevalence obtained by Nowakiewicz et al. in Emys orbicularis turtles was just 1.5% (2/130) [49]. Compared to those works, this study holds an intermediate place since the prevalence obtained was 5% (2/40). Equally, to humans, reptile listeriosis can be fatal and cases have been reported affecting bearded dragons (Pogona vitticeps) [50,51] and marine turtles (Caretta caretta) [52].
The ability of Listeria monocytogenes to survive in a wide range of conditions and the studies that evidence its presence in pet reptiles, like veiled chameleons, implies a potential risk of infection to humans, especially pet owners, and animal handlers.
4.4. Mycobacterium spp.
Mycobacterium spp. infection in reptiles is not as frequent as in mammals or birds, and most of the species involved belong to the atypical mycobacteria group, which rarely affects human beings, but its prevalence is globally increasing [53,54]. Commonly, non-tuberculous mycobacteria cause granulomas in different tissues of reptiles [55], even though no injuries were observed during the necropsies. Because of this, most of the studies are case reports of reptiles with granulomatosis [56,57,58], and few of the works found use samples of healthy animals.
Isolates from five different individuals were positive for Mycobacterium spp. (12.5%), having similar results as Ebani et al. [59] in healthy reptiles from Italy. In that study, a significant difference was also found between the prevalence in snakes, saurians, and chelonians, being much higher in the first ones (72.2%, 9.7%, and 15.5%, respectively).
rpoB gene amplification occurred in three bacterial isolates, indicating an infection with M. microti or non-tuberculous bacteria. It probably corresponds to mycobacteria from the outer tuberculous complex, more common in reptiles such as Mycobacterium chelonae or Mycobacterium marinum [60], with one human reptile-related case reported concerning the second one [61]. The other two samples were positive for M. bovis, a zoonotic tuberculous bacterium with relevance in human health [62], but it cannot be confirmed if M. bovis can develop inside veiled chameleons or if they are just carriers, similar to Mycobacterium tuberculosis, whose reservoir are human beings, but have been identified in a huge variety of animals [63].
4.5. Pseudomonas spp.
Bacteria of the Pseudomonas genus are widely distributed opportunistic pathogens of humans and animals. Of all of them, P. aeruginosa is the species most related to human disease, being responsible for severe nosocomial infections in patients with burns or cystic fibrosis [64]. Moreover, this microorganism usually expresses antibiotic-resistant genes, making treatment ineffective in these cases [65,66]. In reptiles, cutaneous pathology is common, with ulcers or dermatitis [67,68], but other tissues may also be harmed [69].
In this study, 32.5% (13/40) of the sampled chameleons were infected with Pseudomonas spp. and 20% (8/40) with P. aeruginosa. These results are similar to Muñoz-Ibarra et al. in reptiles [66] (order Testudines and Squamata) from the Iberian Peninsula, who obtained a prevalence of 23.2% for Pseudomonas spp. and 18.0% for P. aeruginosa. Equally, Cristina et al. found 11.6% positive isolates of P. aeruginosa in snakes, lizards, and turtles from Romania [70]. The prevalence was lower in reptiles from Italy, where Ebani et al. [65] identified 22 (10.1%) positive samples for Pseudomonas spp. out of 218 animals among saurian, ophidians, and chelonids, with 4.1% of P. aeruginosa. In contrast, Andrea Sala et al. [71] described a P. aeruginosa prevalence of 59.9% in snakes from Italy, marking the variability of Pseudomonas spp. presence between different types of reptiles. All of these works were carried out with samples from captive animals or pets; hence, different results could be obtained from wild fauna. Colinon et al. [72] analyzed samples from captive and wild animals looking for the presence of P. aeruginosa and found prevalences of 87% in captive animals and 12% in wild ones; nevertheless, this difference could be due to different factors like the animal species, the geographical location, and/or human contact.
Apart from the clinical signs described in reptiles, Pseudomonas spp. can be found as regular oral and fecal microbiota, making them capable of transmitting these bacteria to animal handlers by bites or contact with feces [72].
4.6. Salmonella spp.
Reptiles are well-known carriers of Salmonella spp. in their gastrointestinal tract and numerous serotypes capable of causing disease, either in humans or reptiles, have been identified; in fact, there are many reports of reptile-associated salmonellosis from all around the world [73]. Most of the serotypes belong to Salmonella enterica subsp. Enterica, which is responsible for 99% of human infections; S. Typhi causes typhoid fever and is found typically in humans, but also produces no harm, whereas S. Typhimurium and S. Enteritidis cause gastrointestinal symptomatology in birds and mammals, including humans beings [74,75].
Salmonella spp. prevalence is highly variable between reptiles; for example, in their investigations, Bjelland et al. [73], Cota et al. [76], and Maja Lukac et al. [77] agree that chelonians are infected in a smaller proportion compared to saurian and ophidians. Moreover, in Merkevičienė et al. [78] study, a significant difference was observed between the prevalence in wild and domestic reptiles, being higher in the last ones (18.2% and 61.3%, respectively). The 40.0% prevalence for Salmonella spp. obtained in this study is comparable with Hydeskov et al. [79] in reptiles from Denmark (35.0%) and Corrente et al. in Italy with 50.5%, where all (5/5) C. calyptratus were positive [80]. In Gran Canaria, Monzón Moreno et al. reported a 100% (17/17) Salmonella spp. infection in the endemic lizard Gallotia stehlini [81], and Santana-Hernández et al. reported a prevalence of 20.5% in California Kingsnake (L. californiae) on the same island [82].
The presence of zoonotic S. Typhi and S. Typhimurium serotypes in the investigated animals demonstrates the transmission risk to people in close contact with chameleons, like animal handlers, with the possibility of developing severe disease. In addition, Salmonella species or serotypes not yet identified with the PCR assay employed could also be problematic and cause harm to humans or other animals.
4.7. Staphylococcus spp.
Staphylococcus is a broad bacterial genus forming part of normal skin and mucosa microflora of animals, birds, and reptiles [83]. In humans, the most relevant species is S. aureus because is responsible for many nosocomial infections that can risk a patient’s life, especially in the case of antibiotic-resistant strains. This bacterium can also harm animals, together with the Staphylococcus hyicus and Staphylococcus intermedius group, even though these have a limited host range compared to S. aureus [84,85]. None of the sampled animals were positive for S. aureus, which matches with other studies in reptiles such as Espinosa-Gongora et al. [86], in which no bacteria were isolated in any of the 21 samples examined from multiple reptile species, or Cristina et al. [70] work, with a S. aureus prevalence of 2.5% (1/43).
In our study, two Staphylococcus spp. isolates were positive for the methicillin-resistant gene and the other two for the mupirocin-resistant gene, and also one juvenile individual was positive for both antibiotic-resistant genes. This involves difficulty for treatment, even though the species could not be identified in the PCR assay and zoonotic transmission may not occur.
The only species identified was S. hominis in just one isolate; this microorganism is frequently found in human skin along with S. epidermidis and is a rare health problem; on the contrary, its protective role against opportunistic cutaneous infections has been studied [87,88]. However, there have been cases reported of severe infections, especially after prosthetic surgery, due to their ability to form biofilms [89,90]. In animals, its presence is uncommon and probably due to human contact [91,92,93].
4.8. Vibrio spp.
Around 80 species of Vibrio are known, of which 12 are capable of affecting humans, commonly V. cholerae, V. parahemolyticus, and V. vulnificus. They are water-associated bacteria that can grow under different temperature, pH, and salt conditions and cause human disease after eating contaminated marine-based food, particularly seafood [94], causing gastrointestinal problems with symptoms depending on the involved bacteria [95,96].
Few studies have been conducted about the presence of Vibrio spp. in non-marine animals and their role in spreading these bacteria is not clear, and vibriosis can occur possibly by the ingestion of reptile-origin food [97] or by bites [98]. Vibrio cholerae have been found both in the skin and gastrointestinal tract of soft-shell turtles [99]; hence, it is probable that the transmission to humans occurred through aquatic reptiles used as pets, but more studies are needed to prove this possibility. In Tenerife, the largest island of the Canarian archipelago, Vibrio cholerae was identified in feces from Anolis sp., another introduced reptile [100].
In terrestrial reptiles, such as veiled chameleons, Vibrio spp. infections do not seem to be frequent and their origins may be the water or food; consequently, Vibrio spp. determination in animals should be always attached to the analysis of nearly water masses. The absence in this study of species found commonly involved in human vibriosis does not discard the possibility of them causing disease since they are not the only ones capable of affecting humans, even though their low prevalence obtained in this work (2.5%) makes infections difficult.
4.9. Yersinia enterocolitica
Yersinia enterocolitica is an important zoonotic agent, which causes fever, gastrointestinal symptoms, and lymphadenopathy, and enters the organism mainly through pork meat but also milk and water. Direct person-to-person contact or via animals and their faces transmission routes have been also described [101].
Few studies have been carried out looking for Y. enterocolitica in reptiles because they are typically associated with mammals, with pigs and boars as the main reservoirs [102]. The high prevalence of Y. enterocolitica (52.4%) found in the veiled chameleons studied greatly contrasts with previous works such as Shayegani et al. [103], in which none of the reptile samples were positive for this bacterium, or Nowakiewicz et al. [49] with only 2 out of 130 turtles infected. It must be emphasized that the ail gene virulence factor associated with adhesion and invasion of the intestinal wall [104] was only identified in one of the eleven positive isolates for Y. enterocolitica; for this reason, the probability of this high prevalence for producing disease in humans is lower.
To discover why this prevalence is elevated, other animals in contact with the chameleons must be analyzed as well as the surrounding water supplies or diet to establish a clear cause. The insect-based diet of the veiled chameleons could be the reason for the difference between the reported prevalences and the one obtained in this work, because the invertebrates could be hosting the bacteria from scavenging or because of the ingestion of infected feces of mammals. This phenomenon does not occur in other reptiles such as turtles or snakes.
4.10. Summary
In the C. calyptratus investigated population, the most prevalent bacteria were Y. enterocolitica, followed by Salmonella spp. and Pseudomonas spp, with Y. enterocolitica, Salmonella spp., and E. coli being well-known zoonotic species. Lower prevalences were found in the previous few studies conducted looking for Y. enterocolitica in reptiles, while more than half of the sampled chameleons were infected with this bacterium in this study. The data obtained around the other three bacteria are quite similar to the other several works carried out.
Multi-infection in the same individual is usual in nature due to exposure to different pathogens, not only to bacteria but to viruses, fungi, or parasites, which coexist in the environment or within the food [105]. In this case, half (20/40) of the studied animals were infected with more than 1 microorganism, 8 of them with 3 or more simultaneously. Moreover, significant relationships were observed between different bacteria species, indicating that the presence of one of them facilitates the infection of another one; highlights: S. Typhi with E. coli carrying the stx1 gene and L. monocytogenes with Staphylococcus sp. Further investigation is needed to understand the mechanisms that ease these co-infections.
Even though this work did identify bacteria in reptiles not previously reported, C. lari, M. bovis, and S. Typhi, the role of the veiled chameleons in their transmission is not clear, and more studies must be conducted to confirm if those bacteria are capable of development within these animals or they have been accidentally found after having been ingested along with water or food or through contact with other animals or the environment.
The presence of all the pathogens identified in C. calyptratus from Gran Canaria, some of them with well-known zoonotic activity, could be a health risk to both human and animal populations who contact them, mainly if correct handling procedures are not applied.
5. Conclusions
An alien Chamaeleo calyptratus population studied in the Canary Islands (Spain) harbors various bacteria with importance to public health. Taking account of the zoonotic importance of many of the pathogens detected, handling C. calyptratus from Gran Canaria supposes a risk to human health. Yersinia enterocolitica, pathogenic Escherichia coli, and Salmonella spp. were detected, all of them being species with recognized zoonotic potential.
Veiled chameleons could also transmit pathogens to native animals, including endemic species, and/or spread them to the environment, so their possible role against biodiversity should be taken into account. Considering the human and veterinary health importance of the present results, even by analyzing a limited number of samples, more studies are needed with larger sample sizes to better understand the epidemiology of the pathogenic bacteria present in the invasive C. calyptratus in the Canary Islands.
Author Contributions
Conceptualization, P.F. and N.A.-A.; methodology, N.A.-A. and P.F.; formal analysis, R.P.-V.; investigation, R.P.-V.; resources, P.F.; data curation, R.P.-V.; writing—original draft preparation, R.P.-V.; writing—review and editing, all the authors; visualization, R.P.-V. and P.F; supervision, P.F.; project administration, N.A.-A. and P.F.; funding acquisition, P.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by “Consejería de Transición Ecológica, Lucha contra el Cambio Climático y Planificación Territorial” (Gobierno de Canarias)-Universidad de La Laguna agreement “Estudio de patógenos en aves migratorias y en especies exóticas en un escenario de cambio climático”. R.P.-V. was granted a predoctoral scholarship of the training program of the Department of Economy, Knowledge, and Employment of the Canary Government, co-funded by the European Social Fund (ESF) with a co-financing rate of 85% within the framework of the Canary Islands ESF Operational Program 2014–2020, priority axis 3, investment priority 10.2, specific object 10.2.1.
Institutional Review Board Statement
The animal study protocol was approved by the “Dirección General de Lucha Contra el Cambio Climático y Medio Ambiente” (Gobierno de Canarias).
Data Availability Statement
All data obtained are included within the article.
Acknowledgments
We want to thank the REDEXOS staff for helping to supply the chameleons.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Andersen, M.C.; Adams, H.; Hope, B.; Powell, M. Risk Assessment for Invasive Species. Risk Anal. 2004, 24, 787–793. [Google Scholar] [CrossRef]
- Najberek, K.; Olszańska, A.; Tokarska-Guzik, B.; Mazurska, K.; Dajdok, Z.; Solarz, W. Invasive alien species as reservoirs for pathogens. Ecol. Indic. 2022, 139, 108879. [Google Scholar] [CrossRef]
- Ciencia Canaria—Invasoras: Las Especies Que Acechan en Las Islas. Available online: https://www.cienciacanaria.es/secciones/a-fondo/1174-invasoras-las-especies-que-acechan-en-las-islas (accessed on 21 April 2022).
- Especies Exóticas Invasoras. Available online: https://www.gobiernodecanarias.org/medioambiente/materias/biodiversidad/especies-exoticas-invasoras/efectos-sopbre-la-biodiversidad-local/efectos-potenciales-introduccion/ (accessed on 19 May 2022).
- Efectos. Available online: https://www.gobiernodecanarias.org/medioambiente/materias/biodiversidad/especies-exoticas-invasoras/efectos-sopbre-la-biodiversidad-local/ (accessed on 21 April 2022).
- La Serpiente Invasora de Gran Canaria—#STOPCULEBRAREAL. Available online: https://www.stopculebrareal.com/info/ (accessed on 21 April 2022).
- Exos. Available online: https://www.biodiversidadcanarias.es/exos/especie/E09198 (accessed on 21 April 2022).
- Gillette, C.R.; Krysko, K.L. New County Record for The Veiled Chameleon, Chamaeleo calyptratus Duméril and Bibron 1851 (Sauria: Chamaeleonidae), in Florida. Reptiles Amphib. 2012, 19, 130–131. [Google Scholar] [CrossRef]
- López, C.; Clemente, S.; Almeida, C.; Brito, A.; Hernández, M. A genetic approach to the origin of Millepora sp. in the eastern Atlantic. Coral Reefs 2015, 34, 631–638. [Google Scholar] [CrossRef]
- Wang, G.; Clark, C.G.; Taylor, T.M.; Pucknell, C.; Barton, C.; Price, L.; Woodward, D.L.; Rodgers, F.G. Colony Multiplex PCR Assay for Identification and Differentiation of Campylobacter jejuni, C. coli, C. lari, C. upsaliensis, and C. fetus subsp. fetus. J. Clin. Microbiol. 2002, 40, 4744–4747. [Google Scholar] [CrossRef]
- Blanco, M.; Blanco, J.E.; Mora, A.; Dahbi, G.; Alonso, M.P.; González, E.A.; Bernárdez, M.I.; Blanco, J. Serotypes, virulence genes, and intimin types of shiga toxin (verotoxin)-producing Escherichia coli isolates from cattle in Spain and identification of a new intimin variant gene (eae-ξ). J. Clin. Microbiol. 2004, 42, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Jaton, K.; Sahli, R.; Bille, J. Development of Polymerase Chain Reaction assays for detection of Listeria monocytogenes in clinical cerebrospinal fluid samples. J. Clin. Microbiol. 1992, 30, 1931–1936. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Choi, Y.; Jeon, B.Y.; Jin, H.; Cho, S.N.; Lee, H. A simple and efficient Multiplex PCR assay for the identification of Mycobacterium genus and Mycobacterium tuberculosis complex to the species level. Yonsei Med. J. 2013, 54, 1220–1226. [Google Scholar] [CrossRef] [PubMed]
- De Vos, D.; Lim, A., Jr.; Pirnay, J.P.; Struelens, M.; Vandenvelde, C.; Duinslaeger, L.; Vanderkelen, A.; Cornelis, P. Direct detection and identification of Pseudomonas aeruginosa in clinical samples such as skin biopsy specimens and expectorations by Multiplex PCR Based on two outer membrane lipoprotein genes, oprI and oprL. J. Clin. Microbiol. 1997, 35, 1295–1299. [Google Scholar] [CrossRef]
- Guimarães de Freitas, C.; Patrícia Santana, A.; da Silva, P.H.C.; Salvador Picão Gonçalves, V.; Ferreira Barros, M.A.; Gonçalves Torres, F.A.; Murata, L.S.; Perecmanis, S. PCR multiplex for detection of Salmonella Enteritidis, Typhi and Typhimurium and occurrence in poultry meat. Int. J. Food Microbiol. 2010, 139, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Campos-Peña, E.; Martín-Nuñez, E.; Pulido-Reyes, G.; Martín-Padrón, J.; Caro-Carrillo, E.; Donate-Correa, J.; Lorenzo-Castrillejo, I.; Alcoba-Flórez, J.; Machín, F.; Méndez-Alvarez, S. Multiplex PCR assay for identification of six different Staphylococcus spp. and simultaneous detection of methicillin and mupirocin resistance. J. Clin. Microbiol. 2014, 52, 2698–2701. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, G.; Wang, H.; Chen, J.; Shi, X.; Zou, G.; Wei, Q.; Sun, X. Design of Vibrio 16S rRNA gene specific primers and their application in the analysis of seawater Vibrio community. J. Ocean Univ. China 2006, 5, 157–164. [Google Scholar]
- Neogi, S.B.; Chowdhury, N.; Asakura, M.; Hinenoya, A.; Haldar, S.; Saidi, S.M.; Kogure, K.; Lara, R.J.; Yamasaki, S. A highly sensitive and specific multiplex PCR assay for simultaneous detection of Vibrio cholerae, Vibrio parahaemolyticus and Vibrio vulnificus. Lett. Appl. Microbiol. 2010, 51, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Wannet, W.J.B.; Reessink, M.; Brunings, H.A.; Maas, H.M.E. Detection of pathogenic Yersinia enterocolitica by rapid and sensitive duplex PCR assay. J. Clin. Microbiol. 2001, 39, 4483–4486. [Google Scholar] [CrossRef]
- Ginsberg, H.S. Potential Effects of Mixed Infections in Ticks on Transmission Dynamics of Pathogens: Comparative Analysis of Published Records. Exp. Appl. Acarol. 2008, 46, 29–41. [Google Scholar] [CrossRef]
- Masila, N.M.; Ross, K.E.; Gardner, M.G.; Whiley, H. Zoonotic and Public Health Implications of Campylobacter Species and Squamates (Lizards, Snakes and Amphisbaenians). Pathogens 2020, 9, 799. [Google Scholar] [CrossRef]
- Fitzgerald, C. Campylobacter. Clin. Lab. Med. 2015, 35, 289–298. [Google Scholar] [CrossRef]
- De Luca, C.; Iraola, G.; Apostolakos, I.; Boetto, E.; Piccirillo, A. Occurrence and diversity of Campylobacter species in captive chelonians. Vet. Microbiol. 2020, 241, 108567. [Google Scholar] [CrossRef]
- Huang, H.; Brooks, B.W.; Lowman, R.; Carrillo, C.D. Campylobacter species in animal, food, and environmental sources, and relevant testing programs in Canada. Can. J. Microbiol. 2015, 61, 701–721. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.J.; Kik, M.; Miller, W.G.; Duim, B.; Wagenaar, J.A. Campylobacter iguaniorum sp. nov., isolated from reptiles. Int. J. Syst. Evol. Microbiol. 2015, 65, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Piccirillo, A.; Niero, G.; Calleros, L.; Pérez, R.; Naya, H.; Iraola, G. Campylobacter geochelonis sp. nov. isolated from the western Hermann’s tortoise (Testudo hermanni hermanni). Int. J. Syst. Evol. Microbiol. 2016, 66, 3468–3476. [Google Scholar] [CrossRef] [PubMed]
- Gourmelon, M.; Boukerb, A.M.; Nabi, N.; Banerji, S.; Joensen, K.G.; Serghine, J.; Cormier, A.; Megraud, F.; Lehours, P.; Alter, T.; et al. Genomic Diversity of Campylobacter lari group isolates from Europe and Australia in a One Health context. Appl. Environ. Microbiol. 2022, 88, e0136822. [Google Scholar] [CrossRef]
- Categoría:Aves—CanariWiki. Available online: https://www3.gobiernodecanarias.org/medusa/wiki/index.php?title=Categor%C3%ADa:Aves (accessed on 31 May 2023).
- Martinot, M.; Jaulhac, B.; Moog, F.; De Martino, S.; Kehrli, P.; Monteil, H.; Piemont, Y. Campylobacter lari bacteremia. Clin. Microbiol. Infect. 2001, 7, 96–97. [Google Scholar] [CrossRef]
- Krause, R.; Ramschak-Schwarzer, S.; Gorkiewicz, G.; Schnedl, W.J.; Feierl, G.; Wenisch, C.; Reisinger, E.C. Recurrent septicemia due to Campylobacter fetus and Campylobacter lari in an immunocompetent patient. Infection 2002, 30, 171–174. [Google Scholar] [CrossRef]
- Jang, J.; Hur, H.G.; Sadowsky, M.J.; Byappanahalli, M.N.; Yan, T.; Ishii, S. Environmental Escherichia coli: Ecology and public health implications—A review. J. Appl. Microbiol. 2017, 123, 570–581. [Google Scholar] [CrossRef]
- Kolenda, R.; Burdukiewicz, M.; Schierack, P. A systematic review and meta-analysis of the epidemiology of pathogenic Escherichia coli of calves and the role of calves as reservoirs for human pathogenic E. coli. Front. Cell. Infect. Microbiol. 2015, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Guragain, M.; Schmidt, J.W.; Dickey, A.M.; Bosilevac, J.M. Distribution of extremely heat-resistant Escherichia coli in the beef production and processing continuum. J. Food. Prot. 2023, 86, 100031. [Google Scholar] [CrossRef]
- Karmali, M.A.; Gannon, V.; Jan, M.; Sargeant, J.M. Verocytotoxin-producing Escherichia coli (VTEC). Vet. Microbiol. 2010, 140, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Dec, M.; Stepien-Pysniak, D.; Szczepaniak, K.; Turchi, B.; Urban-Chmiel, R. Virulence profiles and antibiotic susceptibility of Escherichia coli strains from pet reptiles. Pathogens 2022, 11, 127. [Google Scholar] [CrossRef] [PubMed]
- Gopee, N.V.; Adesiyun, A.A.; Caesar, K. A longitudinal study of Escherichia coli strains isolated from captive mammals, birds, and reptiles in Trinidad. J. Zoo Wildl. Med. 2000, 31, 353–360. [Google Scholar]
- Bautista-Trujillo, G.U.; Gutiérrez-Miceli, F.A.; Mandujano-García, L.; Oliva-Llaven, M.A.; Ibarra-Martínez, C.; Mendoza-Nazar, P.; Ruiz-Sesma, B.; Tejeda-Cruz, C.; Pérez-Vázquez, L.C.; Pérez-Batrez, J.E.; et al. Captive green iguana carries diarrheagenic Escherichia coli pathotypes. Front. Vet. Sci. 2020, 7, 99. [Google Scholar] [CrossRef]
- Martínez, R.; Sánchez, S.; Alonso, J.M.; Herrera-León, S.; Rey, J.; Echeita, M.A.; Morán, J.M.; García-Sánchez, A. Salmonella spp. and Shiga toxin-producing Escherichia coli prevalence in an ocellated lizard (Timon lepidus) research center in Spain. Foodborne Pathog. Dis. 2011, 8, 1309–1311. [Google Scholar] [CrossRef]
- Bryan, A.; Youngster, I.; McAdam, A.J. Shiga Toxin Producing Escherichia coli. Clin. Lab. Med. 2015, 35, 247–272. [Google Scholar] [CrossRef]
- Vieira, M.A.; Andrade, J.R.; Trabulsi, L.R.; Rosa, A.C.; Días, A.M.; Ramos, S.R.; Frankel, G.; Gomes, T.A. Phenotypic and genotypic characteristics of Escherichia coli strains of non-enteropathogenic E. coli (EPEC) serogroups that carry eae and lack the EPEC adherence factor and Shiga toxin DNA probe sequences. J. Infect. Dis. 2001, 183, 762–772. [Google Scholar] [CrossRef]
- Balière, C.; Rincé, A.; Delannoy, S.; Fach, P.; Gourmelon, M. Molecular profiling of Shiga toxin-producing Escherichia coli and enteropathogenic E. coli strains isolated from French coastal environments. Appl. Environ. Microbiol. 2016, 82, 3913–3927. [Google Scholar] [CrossRef] [PubMed]
- Disson, O.; Moura, A.; Lecuit, M. Making sense of the biodiversity and virulence of Listeria monocytogenes. Trends Microbiol. 2021, 29, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Boland, J.A.; Kuhn, M.; Berche, P.; Chakraborty, T.; Domínguez-Bernal, G.; Goebel, W.; González-Zorn, B.; Wehland, J.; Kreft, J. Listeria pathogenesis and molecular virulence determinants. Clin. Microbiol. Rev. 2001, 14, 584–640. [Google Scholar] [CrossRef] [PubMed]
- Schoder, D.; Guldimann, C.; Märtlbauer, E. Asymptomatic carriage of Listeria monocytogenes by animals and humans and its impact on the food chain. Foods 2022, 11, 3472. [Google Scholar] [CrossRef] [PubMed]
- Schönberg, A.; Gerigk, K. Listeria in effluents from the food-processing industry. Rev. Sci. Tech. 1991, 10, 787–797. [Google Scholar] [CrossRef]
- Weber, A.; Prell, A.; Potel, J.; Schäfer, R. Vorkommen von Listeria monocytogenes bei Schlangen, Schildkröten, Echsen und Amphibien in der Heimtierhaltung [Occurrence of Listeria monocytogenes in snakes, tortoises, lizards and amphibians raised as pets]. Berl. Munch. Tierarztl. Wochenschr. 1993, 106, 293–295. [Google Scholar] [PubMed]
- Weber, A.; Potel, J.; Schäfer-Schmidt, R.; Prell, A.; Datzmann, C. Untersuchungen zum Vorkommen von Listeria monocytogenes in Kotproben von Haus- und Heimtieren [Studies on the occurrence of Listeria monocytogenes in fecal samples of domestic and companion animals]. Zentralblatt Hyg. Umweltmed. 1995, 198, 117–123. [Google Scholar]
- Chen, T.; Orsi, R.H.; Chen, R.; Gunderson, M.; Roof, S.; Wiedmann, M.; Childs-Sanford, S.E.; Cummings, K.J. Characterization of Listeria monocytogenes isolated from wildlife in central New York. Vet. Med. Sci. 2022, 8, 1319–1329. [Google Scholar] [CrossRef]
- Nowakiewicz, A.; Ziółkowska, G.; Zięba, P.; Dziedzic, B.M.; Gnat, S.; Wójcik, M.; Dziedzic, R.; Kostruba, A. Aerobic bacterial microbiota isolated from the cloaca of the European pond turtle (Emys orbicularis) in Poland. J. Wildl. Dis. 2015, 51, 255–259. [Google Scholar] [CrossRef]
- Matt, C.L.; Ramachandran, A.; Allison, R.W.; Wall, C.R.; Dieterly, A.M.; Brandão, J. Listeria monocytogenes in an inland bearded dragon (Pogona vitticeps). J. Exot. Pet Med. 2019, 30, 76–81. [Google Scholar] [CrossRef]
- Girling, S.J.; Fraser, M.A. Listeria monocytogenes septicaemia in an inland bearded dragon, Pogona vitticeps. J. Herpetol. Med. Surg. 2004, 14, 6–9. [Google Scholar] [CrossRef]
- Di Renzo, L.; De Angelis, M.E.; Torresi, M.; Di Lollo, V.; Di Teodoro, G.; Averaimo, D.; Defourny, S.V.P.; Di Giacinto, F.; Profico, C.; Olivieri, V.; et al. First Report of Septicaemic Listeriosis in a loggerhead sea turtle (Caretta caretta) stranded along the adriatic coast: Strain detection and sequencing. Animals 2022, 12, 2364. [Google Scholar] [CrossRef]
- Mitchell, M.A. Mycobacterial infections in reptiles. Vet. Clin. North. Am. Exot. Anim. Pract. 2012, 15, 101–111. [Google Scholar]
- Tortoli, E. Microbiological features and clinical relevance of new species of the genus Mycobacterium. Clin. Microbiol. Rev. 2014, 27, 727–752. [Google Scholar] [CrossRef] [PubMed]
- Ebani, V.V. Domestic reptiles as source of zoonotic bacteria: A mini review. Asian Pac. J. Trop. Med. 2017, 10, 723–728. [Google Scholar] [CrossRef]
- Soldati, G.; Lu, Z.H.; Vaughan, L.; Polkinghorne, A.; Zimmermann, D.R.; Huder, J.B.; Pospischil, A. Detection of mycobacteria and chlamydiae in granulomatous inflammation of reptiles: A retrospective study. Vet. Pathol. 2004, 41, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Ullmann, L.S.; das Neves Dias-Neto, R.; Cagnini, D.Q.; Yamatogi, R.S.; Oliveira-Filho, J.P.; Nemer, V.; Teixeira, R.H.; Biondo, A.W.; Araújo, J.P., Jr. Mycobacterium genavense infection in two species of captive snakes. J. Venom. Anim. Toxins Incl. Trop. Dis. 2016, 18, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Reavill, D.R.; Schmidt, R.E. Mycobacterial lesions in fish, amphibians, reptiles, rodents, lagomorphs, and ferrets with reference to animal models. Vet. Clin. N. Am. Exot. Anim. Pract. 2012, 15, 25–40. [Google Scholar] [CrossRef]
- Ebani, V.V.; Fratini, F.; Bertelloni, F.; Cerri, D.; Tortoli, E. Isolation and identification of mycobacteria from captive reptiles. Res. Vet. Sci. 2012, 93, 1136–1138. [Google Scholar] [CrossRef]
- Maluta, A.; Zając, M.; Krajewska-Wędzina, M.; Wasyl, D.; Heckers, K.; Didkowska, A.; Anusz, K. Mixed infection of Mycobacterium szulgai, M. lentiflavum, and Gram-negative bacteria as a cause of death in a brown caiman Caiman crocodylus: A case report. Vet. Sci. 2022, 9, 133. [Google Scholar] [CrossRef] [PubMed]
- Bouricha, M.; Castan, B.; Duchene-Parisi, E.; Drancourt, M. Mycobacterium marinum infection following contact with reptiles: Vivarium granuloma. Int. J. Infect. Dis. 2014, 21, 17–18. [Google Scholar] [CrossRef] [PubMed]
- Thoen, C.; Lobue, P.; de Kantor, I. The importance of Mycobacterium bovis as a zoonosis. Vet. Microbiol. 2006, 112, 339–345. [Google Scholar] [CrossRef]
- Une, Y.; Mori, T. Tuberculosis as a zoonosis from a veterinary perspective. Comp. Immunol. Microbiol. Infect. Dis. 2007, 30, 415–425. [Google Scholar] [CrossRef]
- Mena, K.D.; Gerba, C.P. Risk assessment of Pseudomonas aeruginosa in water. Rev. Environ. Contam. Toxicol. 2009, 201, 71–115. [Google Scholar]
- Ebani, V.V.; Fratini, F.; Ampola, M.; Rizzo, E.; Cerri, D.; Andreani, E. Pseudomonas and Aeromonas isolates from domestic reptiles and study of their antimicrobial in vitro sensitivity. Vet. Res. Commun. 2008, 32, 195–198. [Google Scholar] [CrossRef]
- Muñoz-Ibarra, E.; Molina-López, R.A.; Durán, I.; Garcias, B.; Martín, M.; Darwich, L. Antimicrobial resistance in bacteria isolated from exotic pets: The situation in the Iberian Peninsula. Animals 2022, 12, 1912. [Google Scholar] [CrossRef]
- Ladyman, J.M.; Kuchling, G.; Burford, D.; Boardman, W.; Raidal, S.R. Skin disease affecting the conservation of the western swamp tortoise (Pseudemydura umbrina). Aust. Vet. J. 1998, 76, 743–745. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Wu, Q.; Qin, X.; Yang, C.; Luo, S.; He, J.; Cheng, Q.; Wu, Z. Identification of Pseudomonas aeruginosa from the skin ulcer disease of crocodile lizards (Shinisaurus crocodilurus) and probiotics as the control measure. Front. Vet. Sci. 2022, 9, 850684. [Google Scholar] [CrossRef] [PubMed]
- Seixas, R.; Pissarra, H.; Santos, J.; Bernardino, R.; Fernandes, T.; Correia, J.; Vilela, C.L.; Oliveira, M. Severe fibrinonecrotic enteritis caused by Pseudomonas aeruginosa in a captive monitor lizard (Varanus niloticus). J. Zoo Wildl. Med. 2014, 45, 410–412. [Google Scholar] [CrossRef] [PubMed]
- Cristina, R.T.; Kocsis, R.; Dégi, J.; Muselin, F.; Dumitrescu, E.; Tirziu, E.; Herman, V.; Darău, A.P.; Oprescu, I. Pathology and Prevalence of Antibiotic-Resistant Bacteria: A Study of 398 Pet Reptiles. Animals 2022, 12, 1279. [Google Scholar] [CrossRef]
- Sala, A.; Di Ianni, F.; Pelizzone, I.; Bertocchi, M.; Santospirito, D.; Rogato, F.; Flisi, S.; Spadini, C.; Iemmi, T.; Moggia, E.; et al. The prevalence of Pseudomonas aeruginosa and multidrug resistant Pseudomonas aeruginosa in healthy captive ophidian. PeerJ 2019, 7, e6706. [Google Scholar] [CrossRef]
- Colinon, C.; Jocktane, D.; Brothier, E.; Rossolini, G.M.; Cournoyer, B.; Nazaret, S. Genetic analyses of Pseudomonas aeruginosa isolated from healthy captive snakes: Evidence of high inter- and intrasite dissemination and occurrence of antibiotic resistance genes. Environ. Microbiol. 2010, 12, 716–729. [Google Scholar] [CrossRef]
- Bjelland, A.M.; Sandvik, L.M.; Skarstein, M.M.; Svendal, L.; Debenham, J.J. Prevalence of Salmonella serovars isolated from reptiles in Norwegian zoos. Acta Vet. Scand. 2020, 62, 3. [Google Scholar] [CrossRef]
- McWhorter, A.; Owens, J.; Valcanis, M.; Olds, L.; Myers, C.; Smith, I.; Trott, D.; McLelland, D. In vitro invasiveness and antimicrobial resistance of Salmonella enterica subspecies isolated from wild and captive reptiles. Zoonoses Public Health 2021, 68, 402–412. [Google Scholar] [CrossRef]
- Chlebicz, A.; Śliżewska, K. Campylobacteriosis, salmonellosis, yersiniosis, and listeriosis as zoonotic foodborne diseases: A review. Int. J. Environ. Res. Public Health 2018, 15, 863. [Google Scholar] [CrossRef]
- Cota, J.B.; Carvalho, A.C.; Dias, I.; Reisinho, A.; Bernardo, F.; Oliveira, M. Salmonella spp. in pet reptiles in Portugal: Prevalence and chlorhexidine gluconate antimicrobial efficacy. Antibiotics 2021, 10, 324. [Google Scholar] [CrossRef]
- Lukac, M.; Pedersen, K.; Prukner-Radovcic, E. Prevalence of Salmonella in captive reptiles from Croatia. J. Zoo Wildl. Med. 2015, 46, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Merkevičienė, L.; Butrimaitė-Ambrozevičienė, Č.; Paškevičius, G.; Pikūnienė, A.; Virgailis, M.; Dailidavičienė, J.; Daukšienė, A.; Šiugždinienė, R.; Ruzauskas, M. Serological variety and antimicrobial resistance in Salmonella isolated from reptiles. Biology 2022, 11, 836. [Google Scholar] [CrossRef] [PubMed]
- Hydeskov, H.B.; Guardabassi, L.; Aalbaek, B.; Olsen, K.E.; Nielsen, S.S.; Bertelsen, M.F. Salmonella prevalence among reptiles in a zoo education setting. Zoonoses Public Health 2013, 60, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Corrente, M.; Madio, A.; Friedrich, K.G.; Greco, G.; Desario, C.; Tagliabue, S.; D’Incau, M.; Campolo, M.; Buonavoglia, C. Isolation of Salmonella strains from reptile faeces and comparison of different culture media. J. Appl. Microbiol. 2004, 96, 709–715. [Google Scholar] [CrossRef]
- Monzón Moreno, C.; Ojeda Vargas, M.M.; Echeita, A.; Usera, M.A. Occurrence of Salmonella in cold-blooded animals in Gran Canaria, Canary Islands, Spain. Antonie Van Leeuwenhoek 1995, 68, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Santana-Hernández, K.M.; Rodríguez-Ponce, E.; Medina, I.R.; Acosta-Hernández, B.; Priestnall, S.L.; Vega, S.; Marin, C.; Cerdà-Cuéllar, M.; Marco-Fuertes, A.; Ayats, T.; et al. One Health Approach: Invasive California Kingsnake (Lampropeltis californiae) as an Important Source of Antimicrobial Drug-Resistant Salmonella Clones on Gran Canaria Island. Animals 2023, 13, 1790. [Google Scholar] [CrossRef]
- Cristina, R.T.; Janos, D. Multiresistant Staphylococcus intermedius isolated from otitis externa in dogs and them human owners—A practical approach. Afr. J. Pharm. Pharmacol. 2013, 7, 1351–1356. [Google Scholar] [CrossRef]
- Graveland, H.; Duim, B.; van Duijkeren, E.; Heederik, D.; Wagenaar, J.A. Livestock-associated methicillin-resistant Staphylococcus aureus in animals and humans. Int. J. Med. Microbiol. 2011, 301, 630–634. [Google Scholar] [CrossRef]
- Fitzgerald, J.R. Evolution of Staphylococcus aureus during human colonization and infection. Infect. Genet. Evol. 2014, 21, 542–547. [Google Scholar] [CrossRef]
- Espinosa-Gongora, C.; Chrobak, D.; Moodley, A.; Bertelsen, M.F.; Guardabassi, L. Occurrence and distribution of Staphylococcus aureus lineages among zoo animals. Vet. Microbiol. 2012, 158, 228–231. [Google Scholar] [CrossRef]
- Severn, M.M.; Williams, M.R.; Shahbandi, A.; Bunch, Z.L.; Lyon, L.M.; Nguyen, A.; Zaramela, L.S.; Todd, D.A.; Zengler, K.; Cech, N.B.; et al. The ubiquitous human skin commensal Staphylococcus hominis protects against opportunistic pathogens. mBio 2022, 13, e0093022. [Google Scholar] [CrossRef]
- Nakatsuji, T.; Chen, T.H.; Narala, S.; Chun, K.A.; Two, A.M.; Yun, T.; Shafiq, F.; Kotol, P.F.; Bouslimani, A.; Melnik, A.V.; et al. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci. Transl. Med. 2017, 9, eaah4680. [Google Scholar] [CrossRef]
- Uddin, O.; Hurst, J.; Alkayali, T.; Schmalzle, S.A. Staphylococcus hominis cellulitis and bacteremia associated with surgical clips. IDCases 2022, 27, e01436. [Google Scholar] [CrossRef]
- Aykut, A.; Sevik, M.O.; Şan, B.; Şahin, Ö. Staphylococcus hominis: A rare cause of endophthalmitis. Arq. Bras. Oftalmol. 2022, 86, 281–283. [Google Scholar] [CrossRef] [PubMed]
- El-Jakee, J.K.; Aref, N.E.; Gomaa, A.; El-Hariri, M.D.; Galal, H.M.; Omar, S.A.; Samir, A. Emerging of coagulase negative staphylococci as a cause of mastitis in dairy animals: An environmental hazard International. J. Vet. Med. Sci. 2013, 1, 74–78. [Google Scholar] [CrossRef]
- Loncaric, I.; Tichy, A.; Handler, S.; Szostak, M.P.; Tickert, M.; Diab-Elschahawi, M.; Spergser, J.; Künzel, F. Prevalence of methicillin-resistant Staphylococcus sp. (MRS) in different companion animals and determination of risk factors for colonization with MRS. Antibiotics 2019, 8, 36. [Google Scholar] [CrossRef]
- Ocloo, R.; Nyasinga, J.; Munshi, Z.; Hamdy, A.; Marciniak, T.; Soundararajan, M.; Newton-Foot, M.; Ziebuhr, W.; Shittu, A.; Revathi, G.; et al. Epidemiology and antimicrobial resistance of staphylococci other than Staphylococcus aureus from domestic animals and livestock in Africa: A systematic review. Front. Vet. Sci. 2022, 9, 1059054. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.D.; Pruzzo, C.; Vezzulli, L.; Kaper, J.B. Vibrio Species. In Food Microbiology: Fundamentals and Frontiers, 4th ed.; Doyle, M.P., Buchanan, R.L., Eds.; ASM Press: Washington, DC, USA, 2013; pp. 401–439. [Google Scholar]
- Baker-Austin, C.; Stockley, L.; Rangdale, R.; Martinez-Urtaza, J. Environmental occurrence and clinical impact of Vibrio vulnificus and Vibrio parahaemolyticus: A European perspective. Environ. Microbiol. Rep. 2010, 2, 7–18. [Google Scholar] [CrossRef]
- Fernández, F.S.; Alonso, G. Cólera y Vibrio cholerae [Cholera and Vibrio cholerae]. INHRR 2009, 40, 50–69. [Google Scholar]
- Magnino, S.; Colin, P.; Dei-Cas, E.; Madsen, M.; McLauchlin, J.; Nöckler, K.; Maradona, M.P.; Tsigarida, E.; Vanopdenbosch, E.; Van Peteghem, C. Biological risks associated with consumption of reptile products. Int. J. Food Microbiol. 2009, 134, 163–175. [Google Scholar] [CrossRef]
- Lopardo, A.H. Microbiología de las infecciones posteriores a mordeduras [Microbiology of post-bite infections. Med. Infant. 2018, 24, 38–45. [Google Scholar]
- Wang, J.; Yan, M.; Gao, H.; Lu, X.; Kan, B. Vibrio cholerae colonization of soft-shelled turtles. Appl. Environ. Microbiol. 2017, 83, 713–717. [Google Scholar] [CrossRef]
- Abreu-Acosta, N.; Pino-Vera, R.; Izquierdo-Rodríguez, E.; Afonso, O.; Foronda, P. Zoonotic Bacteria in Anolis sp., an Invasive Species Introduced to the Canary Islands (Spain). Animals 2023, 13, 414. [Google Scholar] [CrossRef] [PubMed]
- Leon-Velarde, C.G.; Jun, J.W.; Skurnik, M. Yersinia phages and food safety. Viruses 2019, 11, 1105. [Google Scholar] [CrossRef] [PubMed]
- Terech-Majewska, E.; Pajdak, J.; Platt-Samoraj, A.; Szczerba-Turek, A.; Bancerz-Kisiel, A.; Grabowska, K. Characterization of Yersinia enterocolitica strains potentially virulent for humans and animals in river water. J. Appl. Microbiol. 2016, 121, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Shayegani, M.; Stone, W.B.; DeForge, I.; Root, T.; Parsons, L.M.; Maupin, P. Yersinia enterocolitica and related species isolated from wildlife in New York State. Appl. Environ. Microbiol. 1986, 52, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Syczyło, K.; Platt-Samoraj, A.; Bancerz-Kisiel, A.; Szczerba-Turek, A.; Lipczyńska, K.; Jabłoński, A.; Procajło, Z.; Szweda, W. Monitoring of Yersinia enterocolitica strains from free-living animals using different methods. Pol. J. Vet. Sci. 2016, 19, 221–223. [Google Scholar] [CrossRef] [PubMed]
- Viney, M.E.; Graham, A.L. Patterns and processes in parasite co-infection. Adv. Parasitol. 2013, 82, 321–369. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).