Concentration of Macroelements and Trace Elements in Farmed Fallow Deer Antlers Depending on Age
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
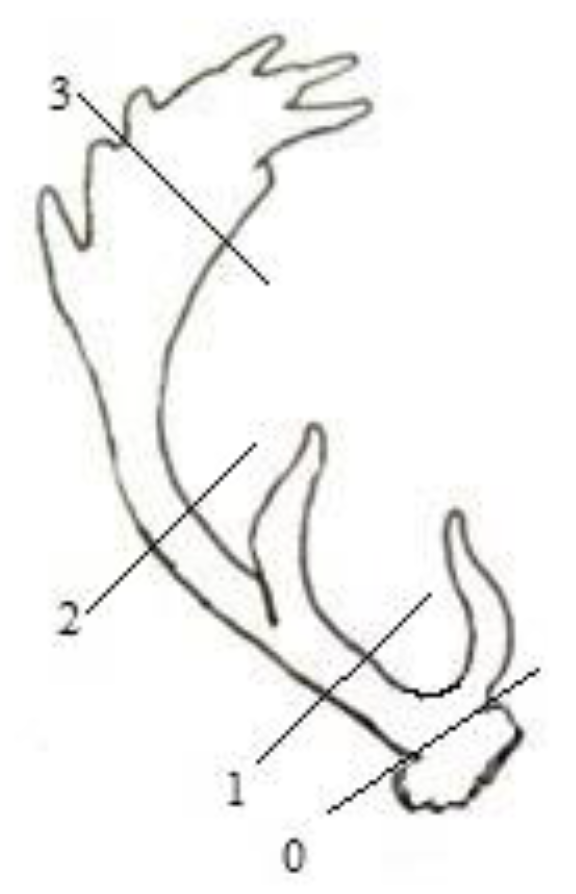
2.2. Sampling
2.3. Analysis of Macro- and Microelement Concentrations in Antlers
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Landete-Castillejos, T.; Estevez, J.A.; Ceacero, F.; García, A.J.; Gallego, L. A review of factors affecting antler composition and mechanics. Front. Biosci. 2012, E4, 2328–2339. [Google Scholar] [CrossRef]
- Gómez, J.A.; Ceacero, F.; Landete-Castillejos, T.; Gaspar-López, E.; García, A.J.; Gallego, L. Factors affecting antler investment in Iberian red deer. Anim. Prod. Sci. 2012, 52, 867–873. [Google Scholar] [CrossRef]
- Gaspar-López, E.; Landete-Castillejos, T.; Gallego, L.; García, A.J. Antler growth rate in yearling Iberian red deer (Cervus elaphus hispanicus). Eur. J. Wildl. Res. 2008, 54, 753–755. [Google Scholar] [CrossRef]
- Rolf, H.J.; Fischer, K.; Düwel, F.W.; Kauer, F.; Enderle, A. Histomorphology and Physiology of “Living” Hard Antlers: Evidence for a Substance Transport into Polished Antlers via the Vascular System. In Antler Science and Product Technology; Part I., Antler Biology; Sim, J.S., Sunwoo, H.H., Hudson, R.J., Jeon, B.T., Eds.; ASPT Research Center, University of Alberta: Edmonton, AB, Canada, 2001. [Google Scholar]
- Landete-Castillejos, T.; Currey, J.D.; Ceacero, F.; García, A.J.; Gallego, L.; Gomez, S. Does nutrition affect bone porosity and mineral tissue distribution in deer antlers? The relationship between histology, mechanical properties and mineral composition. Bone 2012, 50, 245–254. [Google Scholar] [CrossRef]
- Banks, W.; Epling, J.; Kainer, R.; Davis, R. Antler growth and osteoporosis, I. Morphological and morphometric changes in the costal compacta during the antler growth cycle. Anat. Rec. 1968, 162, 387–397. [Google Scholar] [CrossRef]
- Borsy, A.; Podani, J.; Stéger, V.; Balla, B.; Horváth, A.; Kósa, J.P.; Gyurján, I., Jr.; Molnár, A.; Szabolcsi, Z.; Szabó, L.; et al. Identifying novel genes involved in both deer physiological and human pathological osteoporosis. Mol. Genet. Genom. 2009, 281, 301–313. [Google Scholar] [CrossRef]
- Zannèse, A.; Morellet, N.; Targhetta, C.; Coulon, A.; Fuser, S.; Hewison, A.J.M.; Ramanzin, M. Spatial structure of roe deer populations: Towards defining management units at a landscape scale. J. Appl. Ecol. 2006, 43, 1087–1097. Available online: https://www.jstor.org/stable/4123801 (accessed on 1 August 2022). [CrossRef]
- Stéger, V.; Molnár, A.; Borsy, A.; Gyurján, I.; Szabolcsi, Z.; Dancs, G.; Molnár, J.; Papp, P.; Nagy, J.; Puskás, L.; et al. Antler development and coupled osteoporosis in the skeleton of red deer Cervus elaphus: Expression dynamics for regulatory and effector genes. Mol. Genet. Genom. 2010, 284, 273–287. [Google Scholar] [CrossRef]
- Ceacero, F. Long or heavy? Physiological constraints in the evolution of antlers. J. Mamm. Evol. 2015, 23, 2209–2216. [Google Scholar] [CrossRef]
- Ceacero, F.; Pluháček, J.; Landete-Castillejos, T.; García, A.J.; Gallego, L. Inter-Specific Differences in the Structure and Mechanics But Not the Chemical Composition of Antlers in Three Deer Species. In Annales Zoologici Fennici; Finnish Zoological and Botanical Publishing Board: Helsinki, Finland, 2015; Volume 52, pp. 368–376. [Google Scholar] [CrossRef]
- Gaspar-López, E.; García, A.J.; Landete-Castillejos, T.; Carrion, D.; Estévez, J.A.; Gallego, L. Growth of the first antler in Iberian red deer (Cervus elaphus hispanicus). Eur. J. Wildl. Res. 2008, 54, 1–5. [Google Scholar] [CrossRef]
- Dryden, G.M. Quantitative nutrition of deer: Energy, protein and water. Anim. Prod. Sci. 2011, 51, 292–302. [Google Scholar] [CrossRef]
- Dryden, G.M. Nutrition of antler growth in deer. Anim. Prod. Sci. 2016, 56, 962–970. [Google Scholar] [CrossRef]
- Landete-Castillejos, T.; García, A.; Gallego, L. Body weight, early growth and antler size influence antler bone mineral composition of Iberian Red Deer (Cervus elaphus hispanicus). Bone 2007, 40, 230–235. [Google Scholar] [CrossRef]
- Tajchman, K.; Ukalska-Jaruga, A.; Bogdaszewski, M.; Pecio, M.; Dziki-Michalska, K. Accumulation of toxic elements in bone and bone marrow of deer living in various ecosystems. A case study of farmed and wild-living deer. Animals 2020, 10, 2151. [Google Scholar] [CrossRef]
- Tajchman, K.; Ukalska-Jaruga, A.; Bogdaszewski, M.; Pecio, M.; Janiszewski, P. Comparison of the accumulation of macro- and microelements in bone marrow and bone of wild and farmed red deer (Cervus elaphus). BMC Vet. Res. 2021, 17, 324. [Google Scholar] [CrossRef]
- Steiner-Bogdaszewska, Ż.; Tajchman, K.; Ukalska-Jaruga, A.; Florek, M.; Pecio, M. The Mineral Composition of Bone Marrow, Plasma, Bones and the First Antlers of Farmed Fallow Deer. Animals 2022, 12, 2764. [Google Scholar] [CrossRef]
- Landete-Castillejos, T.; Molina-Quilez, I.; Estevez, J.A.; Ceacero, F.; Garcia, A.J.; Gallego, L. Alternative hypothesis for the origin of osteoporosis: The role of Mn. Front Biosci. 2012, E4, 1385–1390. [Google Scholar] [CrossRef]
- Jugdaohsingh, R.; Tucker, K.L.; Qiao, N.; Cupples, L.A.; Kiel, D.P.; Powell, J.J. Dietary silicon intake is positively associated with bone mineral density in men and premenopausal women of the Framingham offspring cohort. J. Bone Miner. Res. 2004, 19, 297–307. [Google Scholar] [CrossRef]
- Nieves, J.W. Osteoporosis: The role of micronutrients. Am. J. Clin. Nutr. 2005, 81, 1232S–1239S. [Google Scholar] [CrossRef]
- Huttunen, M.M.; Pietilä, P.E.; Vijakainen, H.T.; LambergAllardt, C.J.E. Prolonged increase in dietary phosphate intake alters bone mineralization in adult male rats. J. Nutr. Biochem. 2006, 17, 479–484. [Google Scholar] [CrossRef]
- Palacios, C. The role of nutrients in bone health, from A to Z. Crit. Rev. Food Sci. Nutr. 2006, 46, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Seaborn, C.D.; Nielsen, F.H. Silicon deprivation decreases collagen formation in wounds and bone, and ornithine transaminase enzyme activity in liver. Biol. Trace Element Res. 2002, 89, 251–261. [Google Scholar] [CrossRef]
- Armstrong, T.A.; Flowers, W.L.; Spears, J.W.; Nielsen, F.H. Long term effects of boron supplementation on reproductive characteristics and bone mechanical properties in gilts. J. Anim. Sci. 2002, 80, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Davison, K.S.; Siminoski, K.; Adachi, J.D.; Hanley, D.A.; Goltzman, D.; Hodsman, A.B.; Josse, R.; Kaiser, S.; Olszynski, W.P.; Papaioannou, A. Bone strength: The whole is greater than the sum of its parts. Semin. Arthritis Rheum. 2006, 36, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Gómez, J.A. Crecimiento Corporal y Desarrollo de la Cuerna Hasta los dos Años y Medio de Vida en el Ciervo Ibérico (Cervus elaphus hispanicus). Factores Condicionantes. Ph.D. Thesis, Universidad de Castilla-La Mancha, Albacete, Spain, 2004. Available online: https://dialnet.unirioja.es/servlet/tesis?codigo=104853 (accessed on 1 August 2022).
- Gómez, J.A.; Landete-Castillejos, T.; García, A.J.; Gaspar-López, E.; Estévez, J.A.; Gallego, L. Lactation growth influences mineral composition of first antler in Iberian red deer (Cervus elaphus hispanicus). Wildl. Biol. 2008, 14, 331–338. [Google Scholar] [CrossRef]
- Cappelli, J.; Garcia, A.; Ceacero, F.; Gomez, S.; Luna, S.; Gallego, L.; Gambin, P.; Landete-Castillejos, T. Manganese Supplementation in Deer under Balanced Diet Increases Impact Energy and Contents in Minerals of Antler Bone Tissue. PLoS ONE 2015, 10, e0132738. [Google Scholar] [CrossRef]
- Currey, J.D.; Landete-Castillejos, T.; Estevez, J.; Ceacero, F.; Olguin, A.; Garcia, A.; Gallego, L. The mechanical properties of red deer antler bone when used in fighting. J. Exp. Biol. 2009, 212, 3985–3993. [Google Scholar] [CrossRef]
- Malo, A.F.; Roldan, E.R.; Garde, J.; Soler, A.J.; Gomendio, M. Antlers honestly advertise sperm production and quality. Proc. R. Soc. B Biol. Sci. 2005, 272, 149–157. [Google Scholar] [CrossRef]
- Baxter, B.J.; Andrews, R.N.; Barrell, G.K. Bone turnover associated with antler growth in red deer (Cervus elaphus). Anat. Rec. 1999, 256, 14–19. [Google Scholar] [CrossRef]
- Currey, J.D. Mechanical properties and adaptations of some less familiar bony tissues. J. Mech. Behav. Biomed. Mater. 2010, 3, 357–572. [Google Scholar] [CrossRef]
- Currey, J.D. Mechanical properties of bone with greatly differing functions. J. Biomech. 1979, 12, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Clutton-Brock, T.H.; Albon, S.D.; Gibson, R.M.; Guinness, F.E. The logical stag: Adaptive aspects of fighting in red deer (Cervus elaphus L.). Anim. Behav. 1979, 27, 211–215. [Google Scholar] [CrossRef]
- Johnson, H.E.; Bleich, V.C.; Krausman, P.R.; Koprowski, J.L. Effects of antler breakage on mating behavior in male tule elk (Cervus elaphus nannoides). Eur. J. Wildl. Res. 2007, 53, 9–15. [Google Scholar] [CrossRef]
- Goss, R.J. Deer Antlers Regeneration, Function and Evolution; Elsevier Inc.: Amsterdam, The Netherlands, 1983. [Google Scholar]
- Landete-Castillejos, T.; Kierdorf, H.; Gómez, S.; Luna, S.; García, A.J.; Cappelli, J.; Pérez-Serrano, M.; Pérez Barberia, J.; Gallego, L.; Kierdorf, U. Antlers-Evolution, development, structure, composition, and biomechanics of an outstanding type of bone. Bone 2019, 128, 115046. [Google Scholar] [CrossRef] [PubMed]
- Kierdorf, U.; Schultz, M.; Kierdorf, H. The consequences of living longer-Effects of an experimentally extended velvet antler phase on the histomorphologyof antler bone in fallow deer (Dama dama). J. Anat. 2021, 239, 1104–1113. [Google Scholar] [CrossRef]
- Pélabon, C.; Joly, P. What, if anything, does visual asymmetry in fallow deer antlers reveal? Anim. Behav. 2000, 59, 193–199. [Google Scholar] [CrossRef]
- Jennings, D.J.; Gammell, M.P.; Carlin, C.M.; Hayden, C.; Hayden, T.J. Effect of body weight, antler length, resource value and experience on fight duration and intensity in fallow deer. Anim. Behav. 2004, 68, 213–221. [Google Scholar] [CrossRef]
- Jennings, D.J.; Gammell, M.P.; Carlin, C.M.; Hayden, T.J. Does Lateral Presentation of the Palmate Antlers During Fights by Fallow Deer (Dama dama L.) Signify Dominance or Submission? Ethology 2002, 108, 389–401. [Google Scholar] [CrossRef]
- Evans, L.A.; McCutcheon, A.L.; Dennis, G.R.; Mulley, R.C.; Wilson, M.A. Pore size analysis of fallow deer (Dama dama) antler bone. J. Mater. Sci. 2005, 40, 5733–5739. [Google Scholar] [CrossRef]
- Szuwart, T.; Kierdorf, H.; Kierdorf, U.; Clemen, G. Histochemical and Ultrastructural Studies of Cartilage Resorption and Acid Phosphatase Activity During Antler Growth in Fallow Deer (Dama dama). Anat. Rec. 2002, 268, 66–72. [Google Scholar] [CrossRef]
- Kierdorf, U.; Stoffels, E.; Stoffels, D.; Kierdorf, H.; Szuwart, T.; Clemen, G. Histological Studies of Bone Formation During Pedicle Restoration and Early Antler Regeneration in Roe Deer and Fallow Deer. Anat. Rec. Part A 2003, 273A, 741–751. [Google Scholar] [CrossRef]
- Kierdorf, U.; Kierdorf, H.; Schultz, M.; Rolf, H.J. Histological Structure of Antlers in Castrated Male Fallow Deer (Dama dama). Anat. Rec. Part A 2004, 281A, 1352–1362. [Google Scholar] [CrossRef] [PubMed]
- Tajchman, K.; Bogdaszewski, M.; Kowalczuk-Vasilev, E.; Dąbrowski, R. Impact of the day length and total protein content in the diet of farmed fallow deer (Dama dama) on their plasma mineral level and haematological indices. Appl. Ecol. Environ. Res. 2019, 17, 14729–14750. [Google Scholar] [CrossRef]
- Ny, V.; Kotrba, R.; Cappelli, J.; Bures, D.; Clar, M.A.; Garcia, A.J.; Landete-Castillejos, T.; Barton, L.; Ceacero, F. Effects of Lisyne and Methionine supplementation on first antler growth in fallow deer (Dama dama). Small Rumin. Res. 2020, 187, 106119. [Google Scholar] [CrossRef]
- DEFRA Code of Recommendations for the Welfare of Farmed Deer. 2022. Available online: http://www.defra.gov.uk/animalh/welfare/farmed/othersps/deer/pb0055/deercode.htm (accessed on 20 February 2022).
- FEDFA Federation of European Deer Farmers Associations. 2022. Available online: https://www.fedfa.com/ (accessed on 20 February 2022).
- Mattiello, S. Welfare issues of modern deer farming. Ital. J. Anim. Sci. 2009, 8, 205–217. [Google Scholar] [CrossRef]
- Nowicka, W.; Machoy, Z.; Gutowska, I.; Noceń, I.; Piotrowska, S.; Chlubek, D. Contents of Calcium, Magnesium, and Phosphorus in Antlers and Cranial Bones of the European Red Deer (Cervus elaphus) from Different Region in Western Poland. Pol. J. Environ. Stud. 2006, 15, 297–301. [Google Scholar]
- Grace, N.D.; Wilson, P.R.; Nicol, A.M. The copper nutrition of grazing deer. The copper nutrition of grazing deer. Deer nutrition symposium: The nutrition and management of deer on grazing systems. In Proceedings of the New Zealand Grassland Association Symposium, Canterbury, New Zealand, 8–9 November 2002; Lincoln University: Lincoln, UK; pp. 113–119. [Google Scholar]
- Geist, V.; Bayer, M. Sexual dimorphism in the Cervidae and its relation to habitat. J. Zool. 1988, 214, 45–53. [Google Scholar] [CrossRef]
- Estevez, J.A.; Landete-Castillejos, T.; Martinez, A.; Garcia, A.J.; Ceacero, F.; Gaspar-Lopez, E.; Calatayud, A.; Gallego, L. Antler mineral composition of Iberian red deer Cervus elaphus hispanicus in related to mineral profile of diet. Acta Theriol. 2009, 54, 235–242. [Google Scholar] [CrossRef]
- Underwood, E.J.; Suttle, N.F. The Mineral Nutrition of Livestock; CABI North American Office: Boston, MA, USA, 1999. [Google Scholar]
- Gomez, S.; Garcia, A.; Landete-Castillejos, T.; Gallego, L.; Pantelica, A.; Straticiuc, M. Potential of the Bucharest 3 MV Tandetron™ for IBA studies of deer antler mineralization. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2016, 371, 413–418. [Google Scholar] [CrossRef][Green Version]
- Gomez, S.; Garcia, A.J.; Luna, S.; Kierdorf, U.; Kierdorf, H.; Gallego, L.; Landete-Castillejos, T. Labeling studies on cortical bone formation in the antlers of red deer (Cervus elaphus). Bone 2013, 52, 506–515. [Google Scholar] [CrossRef]
- Rafferty, K.; Davies, K.M.; Heaney, R.P. Potassium intake and the calcium economy. J. Am. Coll. Nutr. 2005, 24, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Olguin, C.A.; Landete-Castillejos, T.; Ceacero, F.; García, A.J.; Gallego, L. Effects of Feed Supplementation on Mineral Composition, Mechanical Properties and Structure in Femurs of Iberian Red Deer Hinds (Cervus elaphus hispanicus). PLoS ONE 2013, 8, e65461. [Google Scholar] [CrossRef] [PubMed]
- Tajchman, K.; Bogdaszewski, M.; Kowalczuk-Vasilev, E. Effects of supplementation with different levels of calcium and phosphorus on mineral content of first antler, bone, muscle, and liver of farmed fallow deer (Dama dama). Can. J. Anim. Sci. 2020, 100, 1. [Google Scholar] [CrossRef]
- Pathak, N.N.; Pattanaik, A.K.; Patra, R.C.; Arora, B.M. Mineral composition of antlers of three deer species reared in captivity. Small Rumin. Res. 2001, 42, 61–65. [Google Scholar] [CrossRef]
- Shin, C.S.; Cho, H.Y. Bone remodeling and mineralization. J. Korean Endocr. Soc. 2005, 20, 543–555. [Google Scholar] [CrossRef][Green Version]
- Weaver, C.M.; Gallant, M.H. Nutrition. In Basic and Applied Bone Biology; Burr, D.B., Allen, M.R., Eds.; Academic Press: Waltham, MA, USA, 2014; Chapter 14; pp. 283–297. [Google Scholar]
- Grace, N.D.; Castillo-Alcala, F.; Wilson, P.R. Amounts and distribution of mineral elements associated with live weight gains of grazing red deer (Cervus elaphus). N. Z. J. Agric. Res. 2008, 51, 439–449. [Google Scholar] [CrossRef]
- Köhrle, J.; Contempre, B.; Dumont, J.E.; Jakob, F. Selenium, the thyroid, and the endocrine system. Endocr. Rev. 2005, 26, 944–984. [Google Scholar] [CrossRef]
- Flueck, W.T.; Smith-Flueck, J.M. Age-independent osteopathology in skel- etons of a south American cervid, the Patagonian huemul (Hippocamelus bisulcus). J. Wildl. Dis. 2008, 44, 636–648. [Google Scholar] [CrossRef]
- Liu, H.; Lu, Q.; Huang, K. Selenium suppressed hydrogen peroxideinduced vascular smooth muscle cells calcifcation through inhibiting oxidative stress and ERK activation. J. Cell. Biochem. 2010, 111, 1556–1564. [Google Scholar] [CrossRef]
- Liu, H.; Bian, W.; Liu, S.; Huang, K. Selenium protects bone marrow stromal cells against hydrogen peroxide-induced inhibition of osteoblastic differentiation by suppressing oxidative stress and ERK signaling pathway. Biol. Trace Elem. Res. 2012, 150, 441–450. [Google Scholar] [CrossRef]
- Tripathi, D.; Mani, V.; Pal, R.P. Effect of Vanadium Supplementation on Production Performance, Nutrient Utilization, Plasma Mineral Concentration, and Mineral Balance in Lactating Goats. Biol. Trace Elem. Res. 2019, 188, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Lashkari, S.; Habibian, M.; Jensen, S.K. A Review on the Role of Chromium Supplementation in Ruminant Nutrition-Effects on Productive Perfomance, Blood Metabolites, Antioxidant Status, and Immunocompetence. Biol. Trace Elem. Res. 2018, 186, 305–321. [Google Scholar] [CrossRef] [PubMed]
| Analyzed Parameters | Group I (N = 8) | Group II (N = 6) | Group III (N = 7) | Group IV (N = 10) | |||||
|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | M | SD | ||
| Age | year | 2.00 | 0.000 | 3.00 | 0.000 | 4.71 | 0.488 | 6.90 | 0.875 |
| Body mass | kg | 54.40 | 3.454 | 63.33 | 4.226 | 83.71 | 15.692 | 93.90 | 10.743 |
| Antler mass | kg | 0.02 | 0.004 | 0.29 | 0.045 | 0.70 | 0.111 | 0.76 | 0.136 |
| Analyzed Parameters | Group I Position 1 | Group II | Group III | Group IV | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Position 1 | Position 2 | Position 3 | Position 1 | Position 2 | Position 3 | Position 1 | Position 2 | Position 3 | |||||||||||||
| M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | ||
| Macroelements | |||||||||||||||||||||
| Ca | g/kg | 236.18 | 25.593 | 254.01 | 17.631 | 239.81 | 13.995 | 222.68 | 9.218 | 215.68 | 46.228 | 192.66 | 224.00 | 173.14 | 26.242 | 190.85 | 7.927 | 177.97 | 5.481 | 164.46 | 9.234 |
| P | 122.44 | 7.740 | 114.47 | 5.495 | 106.13 | 6.186 | 99.13 | 8.024 | 89.07 | 12.920 | 84.77 | 12.041 | 77.08 | 10.620 | 88.50 | 3.257 | 82.77 | 3.318 | 76.19 | 3.743 | |
| Mg | 4.88 | 0.582 | 4.95 | 0.425 | 4.59 | 0.416 | 4.22 | 0.539 | 4.49 | 0.674 | 4.09 | 0.397 | 3.61 | 0.410 | 4.26 | 0.315 | 3.99 | 0.285 | 3.70 | 0.310 | |
| K | 0.71 | 0.094 | 0.62 | 0.094 | 0.87 | 0.218 | 1.15 | 0.191 | 0.45 | 0.129 | 0.51 | 0.123 | 0.53 | 0.135 | 0.53 | 0.136 | 0.54 | 0.144 | 0.56 | 0.167 | |
| Na | 6.17 | 0.655 | 6.51 | 0.673 | 5.76 | 0.601 | 5.03 | 0.735 | 6.18 | 1.255 | 5.58 | 0.597 | 5.01 | 0.678 | 5.72 | 0.377 | 5.45 | 0.362 | 5.09 | 0.356 | |
| Ca:P | 1.94 | 0.262 | 2.23 | 0.223 | 2.27 | 0.212 | 2.26 | 0.204 | 2.42 | 0.371 | 2.28 | 0.102 | 2.25 | 0.103 | 2.16 | 0.091 | 2.15 | 0.063 | 2.16 | 0.119 | |
| Trace elements | |||||||||||||||||||||
| Li | mg/kg | 0.18 | 0.038 | 0.13 | 0.031 | 0.12 | 0.030 | 0.11 | 0.026 | 0.16 | 0.044 | 0.16 | 0.064 | 0.15 | 0.048 | 0.253 | 0.123 | 0.20 | 0.067 | 0.19 | 0.097 |
| V | 0.05 | 0.031 | 0.03 | 0.012 | 0.05 | 0.034 | 0.08 | 0.031 | 0.03 | 0.021 | 0.01 | 0.009 | 0.02 | 0.011 | 0.03 | 0.029 | 0.01 | 0.010 | 0.05 | 0.076 | |
| Cr | 0.08 | 0.042 | 0.14 | 0.099 | 1.57 | 2.992 | 0.35 | 0.258 | 0.28 | 0.430 | 0.15 | 0.175 | 0.25 | 0.442 | 0.13 | 0.178 | 0.15 | 0.160 | 0.35 | 0.335 | |
| Mn | 2.75 | 1.126 | 3.88 | 0.853 | 9.32 | 8.472 | 9.13 | 3.576 | 4.25 | 2.037 | 2.73 | 0.700 | 2.93 | 1.358 | 2.64 | 0.840 | 2.76 | 0.984 | 3.83 | 1.637 | |
| Co | 0.13 | 0.058 | 0.14 | 0.035 | 0.30 | 0.259 | 0.16 | 0.067 | 0.21 | 0.284 | 0.13 | 0.085 | 0.07 | 0.026 | 0.13 | 0.180 | 0.12 | 0.074 | 0.19 | 0.204 | |
| Cu | 0.37 | 0.241 | 0.56 | 0.085 | 1.19 | 0.741 | 1.34 | 0.932 | 0.54 | 0.558 | 0.41 | 0.122 | 0.46 | 0.176 | 0.43 | 0.370 | 0.44 | 0.301 | 0.68 | 0.516 | |
| Zn | 59.27 | 11.314 | 58.82 | 8.432 | 61.71 | 11.416 | 64.81 | 8.158 | 47.85 | 12.948 | 43.18 | 5.502 | 41.96 | 5.404 | 40.93 | 5.158 | 42.54 | 8.581 | 44.11 | 5.225 | |
| Se | 0.05 | 0.031 | 0.05 | 0.011 | 0.07 | 0.025 | 0.10 | 0.025 | 0.54 | 0.851 | 0.37 | 0.460 | 0.89 | 1.508 | 0.75 | 1.499 | 0.72 | 1.396 | 0.55 | 1.121 | |
| Mo | 0.03 | 0.011 | 0.02 | 0.009 | 0.03 | 0.033 | 0.05 | 0.051 | 0.06 | 0.039 | 0.05 | 0.039 | 0.04 | 0.031 | 0.04 | 0.028 | 0.03 | 0.028 | 0.06 | 0.041 | |
| Analyzed Parameters | Group II | Group III | Group IV | Kruskal–Wallis H Test (3, N = 23) | p | Correlation Coefficient between Groups | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | |||||
| Macroelements | ||||||||||
| Ca | g/kg | 238.84 | 10.301 | 193.83 | 28.943 | 177.76 | 5.635 | 11.398 | 0.003 * | II–IV, p = 0.002 * |
| P | 106.58 | 5.273 | 83.64 | 11.741 | 82.49 | 2.572 | 10.843 | 0.004 * | II–III, p = 0.012 * II–IV, p = 0.008 * | |
| Mg | 4.59 | 0.426 | 4.07 | 0.399 | 3.98 | 0.271 | 7.081 | 0.029 * | II–IV, p = 0.041 * | |
| K | 0.88 | 0.155 | 0.50 | 0.118 | 0.55 | 0.103 | 12.929 | 0.002 * | II–III, p = 0.001 * II–IV, p = 0.018 * | |
| Na | 5.77 | 0.627 | 5.59 | 0.741 | 5.43 | 0.332 | 0.838 | 0.657 | - | |
| Ca:P | 2.24 | 0.177 | 2.32 | 0.133 | 2.16 | 0.065 | - | - | - | |
| Trace elements | ||||||||||
| Li | mg/kg | 0.12 | 0.027 | 0.16 | 0.050 | 0.22 | 0.063 | 10.771 | 0.004 * | II–III, p = 0.003 * |
| V | 0.06 | 0.015 | 0.02 | 0.012 | 0.03 | 0.027 | 7.584 | 0.023 * | II–III, p = 0.035 * | |
| Cr | 0.69 | 1.048 | 0.23 | 0.185 | 0.21 | 0.103 | 2.378 | 0.305 | - | |
| Mn | 7.45 | 3.381 | 3.31 | 1.062 | 3.08 | 0.576 | 12.797 | 0.002 * | II–III, p = 0.010 * II–IV, p = 0.002 * | |
| Co | 0.20 | 0.074 | 0.14 | 0.112 | 0.15 | 0.087 | 2.875 | 0.237 | - | |
| Cu | 1.03 | 0.448 | 0.47 | 0.256 | 0.52 | 0.173 | 10.006 | 0.006 * | II–III, p = 0.008 * II–IV, p = 0.032 * | |
| Zn | 61.78 | 8.415 | 44.33 | 7.432 | 42.53 | 4.984 | 11.536 | 0.003 * | II–III, p = 0.026 * II–IV, p = 0.003 * | |
| Se | 0.07 | 0.017 | 0.61 | 0.919 | 0.68 | 1.262 | 0.447 | 0.799 | - | |
| Mo | 0.04 | 0.025 | 0.05 | 0.025 | 0.05 | 0.029 | 0.814 | 0.666 | - | |
| Measurements | ||||||||||
| Age | year | 3.00 | 0.000 | 4.71 | 0.488 | 6.90 | 0.875 | 19.946 | <0.001 * | II–IV, p < 0.001 * III–IV, p = 0.033 * |
| Body mass | kg | 63.33 | 4.226 | 83.71 | 15.692 | 93.90 | 10.743 | 13.005 | 0.002 * | II–IV, p = 0.001 * |
| Antler mass | kg | 0.29 | 0.045 | 0.70 | 0.111 | 0.76 | 0.136 | 13.170 | 0.001 * | II–III, p = 0.019 * II–IV, p = 0.001 * |
| Analyzed Parameters | Position 1 | Position 2 | Position 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Kruskal–Wallis H Test (3, N = 31) | p | Correlation Coefficient between Groups | Kruskal–Wallis H Test (3, N = 23) | p | Correlation Coefficient between Groups | Kruskal–Wallis H Test (3, N = 23) | p | Correlation Coefficient between Groups | |
| Macroelements | |||||||||
| Ca | 13.732 | 0.003 * | I–IV, p = 0.036 II–IV, p = 0.004 | 13.356 | 0.001 * | II–IV, p < 0.001 | 10.888 | 0.004 * | II–III, p = 0.022 II–IV, p = 0.005 |
| p | 21.587 | 0.0001 * | I–III, p < 0.001 I–IV, p < 0.001 | 10.378 | 0.005 * | II–III, p = 0.001 II–IV, p = 0.010 | 11.294 | 0.003 * | II–III, p = 0.012 II–IV, p = 0.006 |
| Mg | 7.377 | 0.061 | - | 6.855 | 0.032 * | II–IV, p = 0.034 | 4.987 | 0.083 | - |
| K | 11.938 | 0.007 * | I–III, p = 0.006 | 9.273 | 0.009 * | II–III, p = 0.014 II–IV, p = 0.028 | 12.015 | 0.002 * | II–III, p = 0.004 II–IV, p = 0.009 |
| Na | 4.401 | 0.221 | - | 0.628 | 0.730 | - | 1.007 | 0.605 | - |
| Trace elements | |||||||||
| Li | 11.241 | 0.011 * | II–IV, p = 0.006 | 7.795 | 0.020 * | II–IV, p = 0.016 | 8.212 | 0.016 * | II–IV, p = 0.012 |
| V | 8.753 | 0.033 * | I–IV, p = 0.025 | 8.389 | 0.015 * | II–III, p = 0.046 II–IV, p = 0.023 | 7.789 | 0.020 * | II–III, p = 0.025 |
| Cr | 3.146 | 0.369 | - | 2.401 | 0.301 | - | 2.634 | 0.268 | - |
| Mn | 8.417 | 0.038 | - | 11.311 | 0.003 * | II–III, p = 0.015 II–IV, p = 0.005 | 11.416 | 0.003 * | II–III, p = 0.003 II–IV, p = 0.031 |
| Co | 4.063 | 0.255 | - | 4.952 | 0.084 | - | 5.106 | 0.078 | - |
| Cu | 6.877 | 0.076 | - | 8.704 | 0.013 * | II–IV, p = 0.015 | 5.712 | 0.058 | - |
| Zn | 14.317 | 0.003 * | I–IV, p = 0.009 II–IV, p = 0.010 | 10.928 | 0.004 * | II–III, p = 0.024 II–IV, p = 0.004 | 12.882 | 0.002 * | II–III, p = 0.003 II–IV, p = 0.005 |
| Se | 6.268 | 0.099 | - | 0.594 | 0.742 | - | 1.282 | 0.527 | - |
| Mo | 6.681 | 0.083 | - | 0.764 | 0.683 | - | 1.238 | 0.539 | - |
| Measurements | |||||||||
| Age | 28.863 | <0.001 * | I–III, p = 0.025 I–IV, p < 0.001 II–IV, p = 0.008 | 19.947 | <0.001 * | II–IV, p < 0.001 III–IV, p = 0.033 | 19.947 | <0.001 * | II–IV, p < 0.001 III–IV, p = 0.033 |
| Body mass | 24.496 | <0.001 * | I–III, p = 0.003 I–IV, p < 0.001 II–IV, p = 0.045 | 13.005 | 0.002 * | II–IV, p = 0.001 | 13.005 | 0.002 * | II –IV, p = 0.001 |
| Antler mass | 24.686 | <0.001 * | I–III, p = 0.001 I–IV, p < 0.001 | 13.170 | 0.001 * | II–III, p = 0.019 II–IV, p = 0.001 | 13.170 | 0.001 * | II–III, p = 0.019 II–IV, p = 0.001 |
| Analyzed Parameters N = 23 | Age | Body Mass | Antler Mass | |||
|---|---|---|---|---|---|---|
| R | p | R | p | R | p | |
| Macroelements | ||||||
| Ca | −0.621 | 0.002 * | −0.582 | 0.003 * | −0.554 | 0.006 * |
| P | −0.571 | 0.004 * | −0.524 | 0.010 * | −0.515 | 0.012 * |
| Mg | −0.481 | 0.019 * | −0.477 | 0.021 * | −0.438 | 0.036 * |
| K | −0.481 | 0.019 * | −0.579 | 0.003 * | −0.734 | <0.001 * |
| Na | −0.181 | 0.408 | −0.325 | 0.129 | −0.317 | 0.139 |
| Trace elements | ||||||
| Li | 0.681 | <0.001 * | 0.608 | 0.002 * | 0.470 | 0.024 * |
| V | −0.541 | 0.007 * | −0.177 | 0.416 | −0.412 | 0.050 |
| Cr | −0.322 | 0.134 | −0.135 | 0.536 | −0.362 | 0.088 |
| Mn | −0.695 | <0.001 * | −0.414 | 0.049 * | −0.661 | <0.001 * |
| Co | −0.391 | 0.064 | −0.109 | 0.618 | −0.314 | 0.143 |
| Cu | −0.535 | 0.008 * | −0.426 | 0.042 * | −0.569 | 0.004 * |
| Zn | −0.652 | <0.001 * | −0.705 | <0.001 * | −0.720 | <0.001 * |
| Se | −0.028 | 0.897 | 0.175 | 0.423 | 0.229 | 0.291 |
| Mo | −0.067 | 0.762 | 0.008 | 0.969 | −0.077 | 0.726 |
| Measurements | ||||||
| Age animals | - | - | 0.663 | <0.001 * | 0.665 | <0.001 * |
| Body mass | 0.663 | <0.001 * | - | - | ||
| Antler mass | 0.665 | <0.001 * | 0.727 | <0.001 * | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tajchman, K.; Ukalska-Jaruga, A.; Ceacero, F.; Pecio, M.; Steiner-Bogdaszewska, Ż. Concentration of Macroelements and Trace Elements in Farmed Fallow Deer Antlers Depending on Age. Animals 2022, 12, 3409. https://doi.org/10.3390/ani12233409
Tajchman K, Ukalska-Jaruga A, Ceacero F, Pecio M, Steiner-Bogdaszewska Ż. Concentration of Macroelements and Trace Elements in Farmed Fallow Deer Antlers Depending on Age. Animals. 2022; 12(23):3409. https://doi.org/10.3390/ani12233409
Chicago/Turabian StyleTajchman, Katarzyna, Aleksandra Ukalska-Jaruga, Francisco Ceacero, Monika Pecio, and Żaneta Steiner-Bogdaszewska. 2022. "Concentration of Macroelements and Trace Elements in Farmed Fallow Deer Antlers Depending on Age" Animals 12, no. 23: 3409. https://doi.org/10.3390/ani12233409
APA StyleTajchman, K., Ukalska-Jaruga, A., Ceacero, F., Pecio, M., & Steiner-Bogdaszewska, Ż. (2022). Concentration of Macroelements and Trace Elements in Farmed Fallow Deer Antlers Depending on Age. Animals, 12(23), 3409. https://doi.org/10.3390/ani12233409








