Tannic Acid and Tea Prevents the Accumulation of Lead and Cadmium in the Lungs, Heart and Brain of Adolescent Male Wistar Rats—Possible Therapeutic Option
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
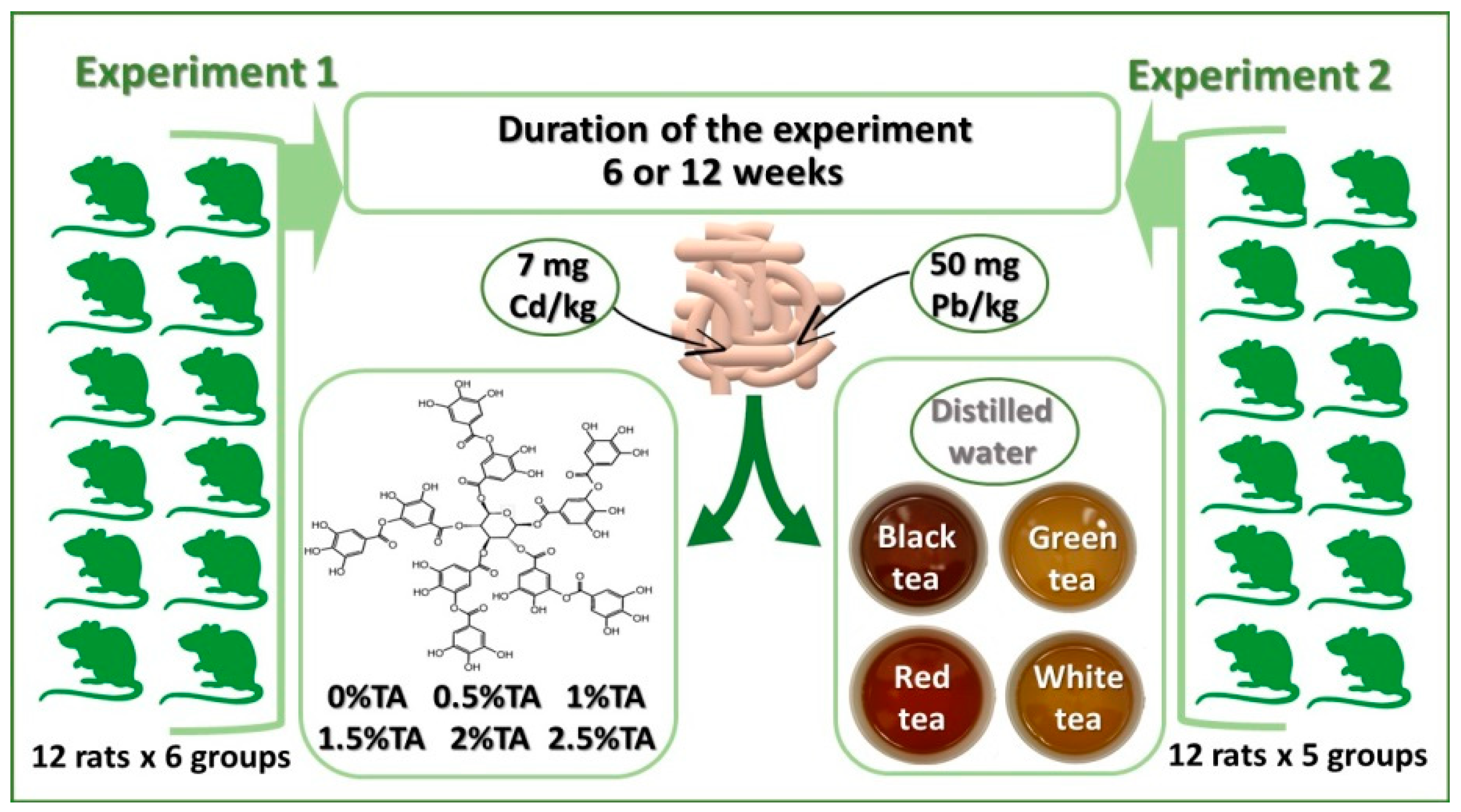
2.1. Animals and Treatments
2.1.1. Experiment 1
2.1.2. Experiment 2
2.2. Chemical Analyses
2.2.1. Determination of the Content of Pb and Cd in the Lungs, Brains and Hearts
2.2.2. Determination of Antioxidant Enzymes Activity
2.2.3. Determination of the Content of TA in Tea Infusions
2.2.4. Chemical Reagents
2.3. Calculations and Statistical Analysis
3. Results
3.1. Experiment 1
3.1.1. Drinking Fluids and Feed Consumption and Body Weight Gain
3.1.2. The Relative Weight of Organs
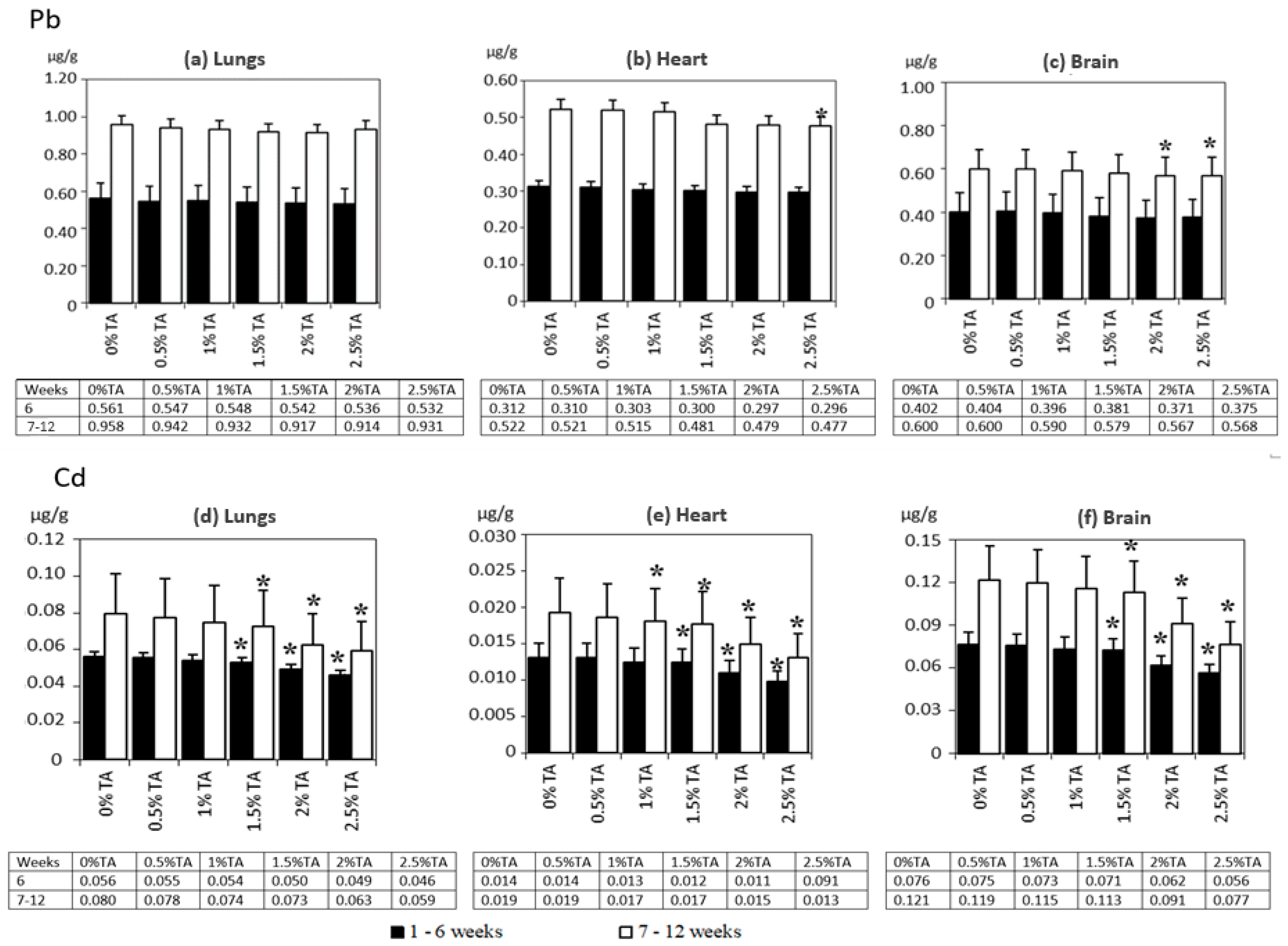
3.1.3. The Distribution of Pb and Cd in Rats’ Lungs, Hearts and Brains
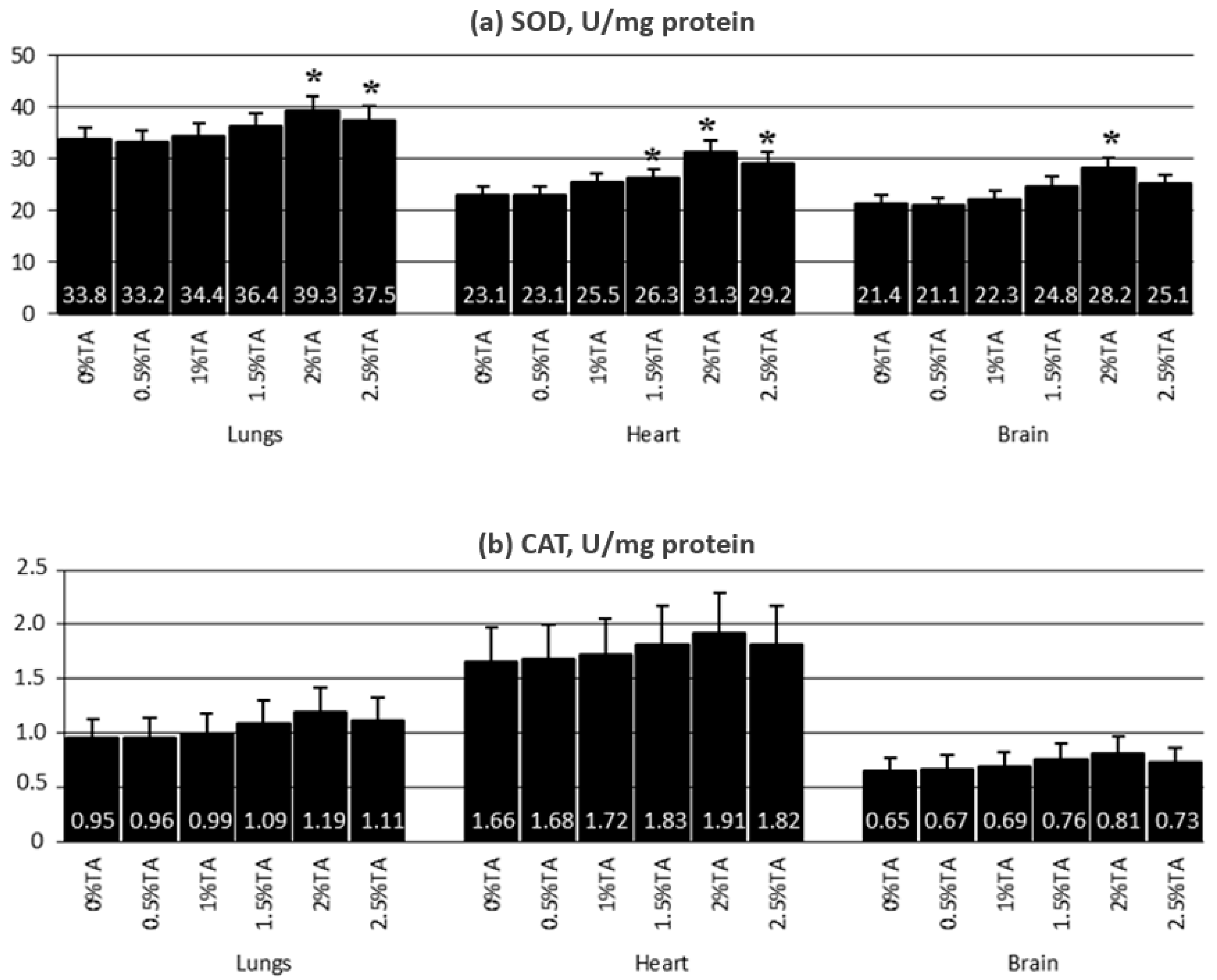
3.1.4. Activity of Antioxidant Enzymes in the Lungs, Hearts and Brains
3.1.5. The Effect of TA on the Lungs, Heart and Brain of Adolescent Rats—Conclusions from Experiment 1
3.2. Experiment 2
3.2.1. Drinking Fluids and Feed Consumption and Body Weight Gain
3.2.2. The Relative Weight of Organs
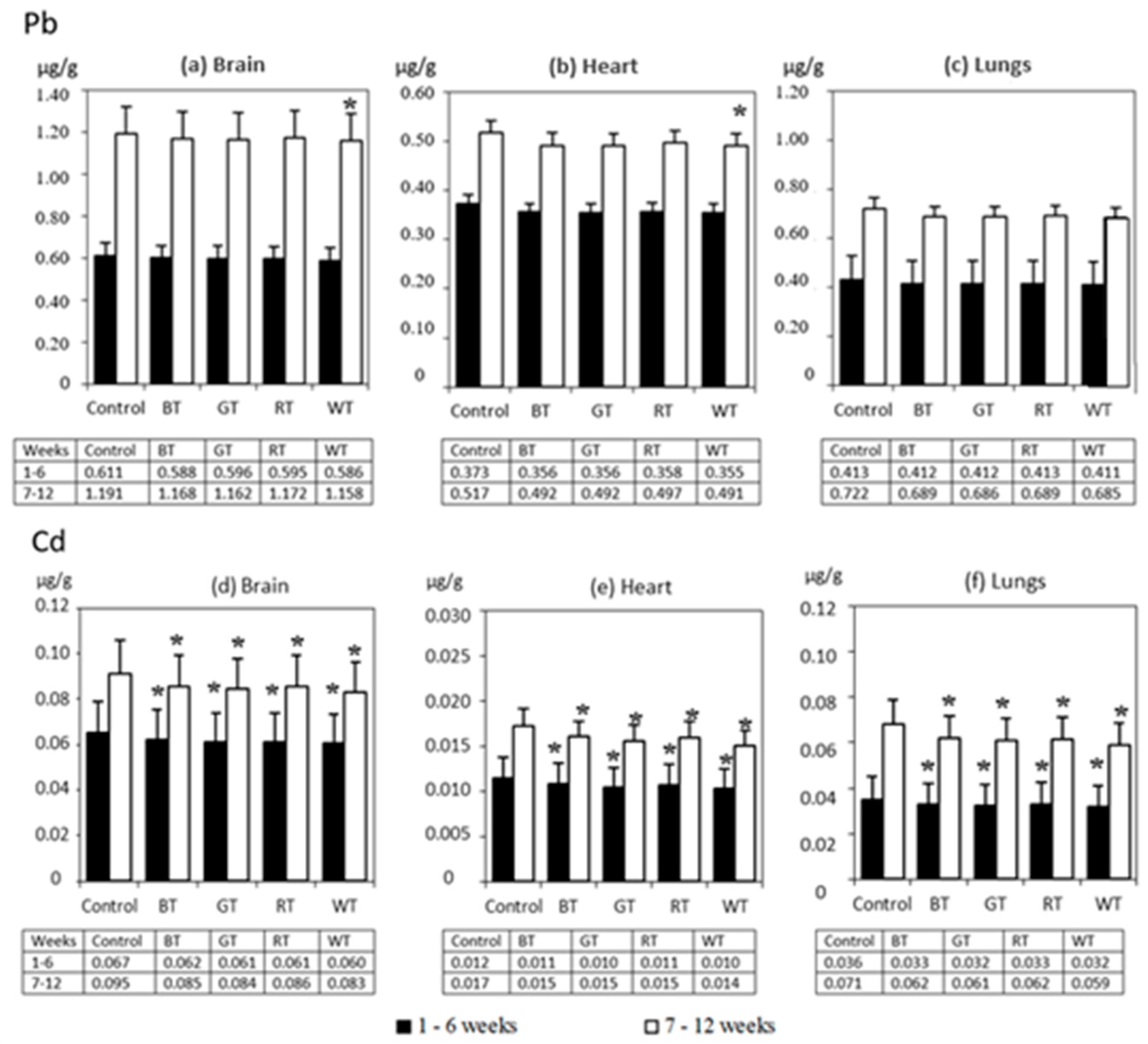
3.2.3. The Distribution of Pb and Cd in Rats’ Lungs, Hearts and Brains
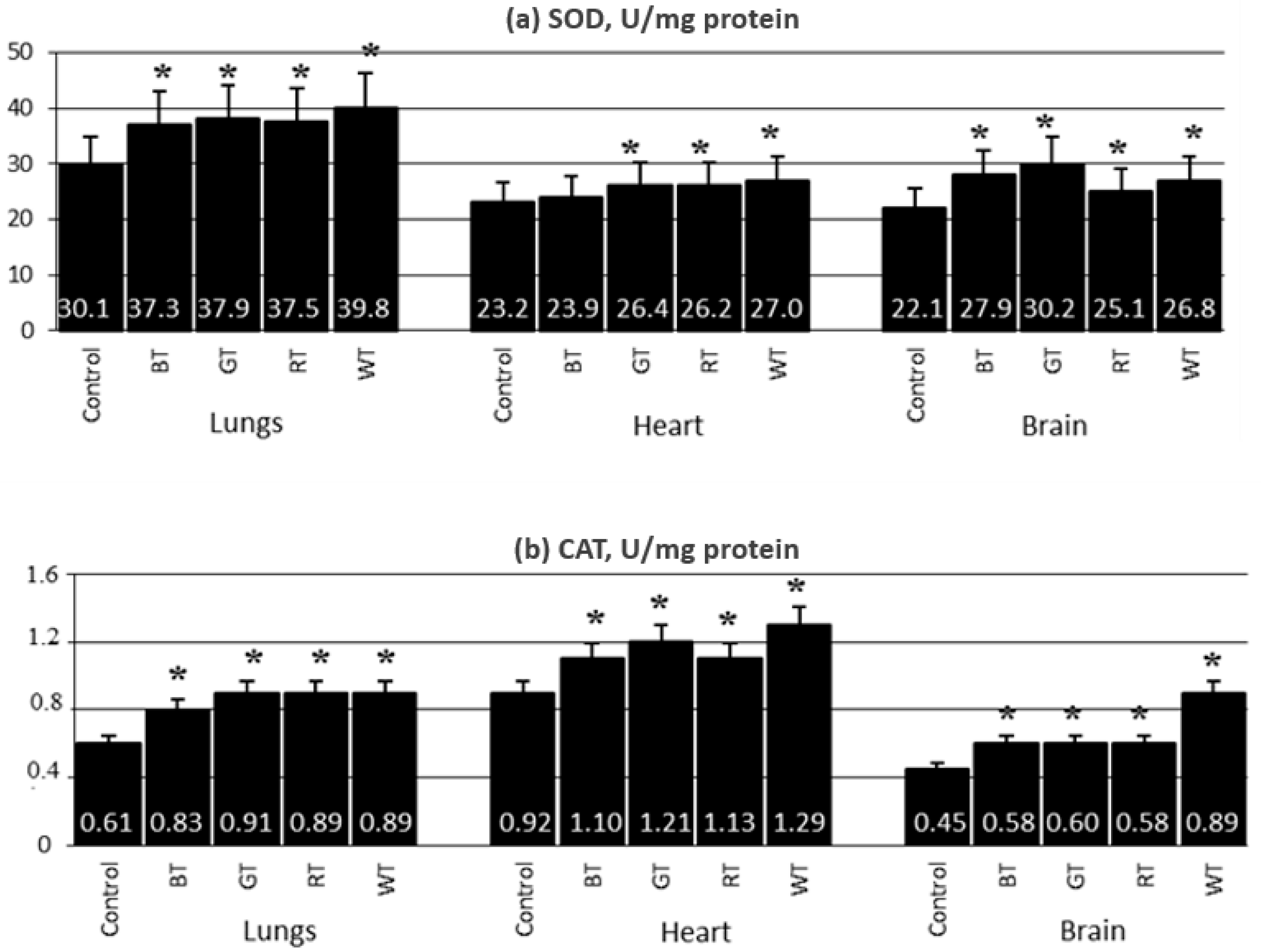
3.2.4. Content of Antioxidant Enzymes in the Lungs, Hearts and Brains
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- EFSA. Lead dietary exposure in the European population. EFSA J. 2012, 10, 2831–2890. [Google Scholar]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The effects of cadmium toxicity. Int. J. Environ. Health Res. 2020, 17, 3782. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Panel on Contaminants in the Food Chain: Scientific opinion on lead in food. EFSA J. 2010, 8, 1423–1570. [Google Scholar]
- EFSA. Cadmium dietary exposure in the European population. EFSA J. 2012, 10, 2551–2588. [Google Scholar] [CrossRef]
- Kaczmarek-Wdowiak, B.; Andrzejak, R.; Skoczyńska, A.; Młynek, V. The effect of chronic exposure to lead and cadmium on lipid peroxidation in rat’s brain. Med. Pract. 2004, 55, 403–410. [Google Scholar]
- Winiarska-Mieczan, A.; Kwiecień, M. The effect of exposure to Cd and Pb in the form of a drinking water or feed on the accumulation and distribution of these metals in the organs of growing Wistar rats. Biol. Trace Elem. Res. 2016, 2, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Higazy, A.; Hashem, M.; ElShafei, A.; Shaker, N.; Hady, M.A. Development of anti-microbial jute fabrics via in situ formation of cellulose-tannic acid-metal ion complex. Carbohydr. Polym. 2010, 79, 890–897. [Google Scholar] [CrossRef]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic mechanisms of five heavy metals: Mercury, lead, chromium, cadmium, and arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef] [PubMed]
- Callegaro, M.G.; Milbradt, B.G.; Alves, E.; Diettrich, T.; Kemerich, D.M.; Hausen, B.S.; Duarte, F.A.; Flores, E.M.; Dressler, V.L.; Emanuelli, T. Effect of wheat bran and flaxseed on cadmium effects and retention in rats. Hum. Exp. Toxicol. 2011, 30, 981–991. [Google Scholar] [CrossRef]
- Ismail, A.; Riaz, M.; Akhtar, S.; Goodwill, J.E.; Sun, J. Heavy metals in milk: Global prevalence and health risk assessment. Toxin Rev. 2019, 38, 1–12. [Google Scholar] [CrossRef]
- Mattisson, K.; Tekavec, E.; Lundh, T.; Stroh, E. Cadmium and lead levels in blood and arsenic levels in urine among schoolchildren living in contaminated Glassworks areas, Sweden. Int. J. Environ. Health Res. 2020, 17, 7382. [Google Scholar] [CrossRef]
- Winiarska-Mieczan, A.; Baranowska-Wójcik, E.; Kwiecień, M.; Grela, E.R.; Szwajgier, D.; Kwiatkowska, K.; Kiczorowska, B. The role of dietary antioxidants in the pathogenesis of neurodegenerative diseases and their impact on cerebral oxidoreductive balance. Nutrients 2020, 12, 435. [Google Scholar] [CrossRef] [PubMed]
- Nazima, B.; Manoharan, V.; Prabu, S.M. Cadmium toxicity: Oxidative stress and organ dysfunction. Res. Rev. J. Toxicol. 2014, 4, 1–18. [Google Scholar]
- Fowler, B.A.; Whittaker, M.H.; Lipsky, M.; Wang, G.; Chen, X.Q. Oxidative stress induced by lead, cadmium and arsenic mixtures: 30-day, 90-day, and 180-day drinking water studies in rats: An overview. BioMetals 2004, 17, 567–568. [Google Scholar] [CrossRef] [PubMed]
- Winiarska-Mieczan, A. Protective effect of tea against lead and cadmium-induced oxidative stress—A review. Biometals 2018, 31, 909–926. [Google Scholar] [CrossRef] [PubMed]
- Branca, J.J.V.; Fiorillo, C.; Carrino, D.; Paternostro, F.; Taddei, N.; Gulisano, M.; Pacini, A.; Becatti, M. Cadmium-induced oxidative stress: Focus on the central nervous system. Antioxidants 2020, 9, 492. [Google Scholar] [CrossRef]
- Jedlińska-Krakowska, M. Influence of cadmium, lead and vitamin E and C on the levels of selected oxidative and biochemical indices in rat males. Bromat. Chem. Toksykol. 2010, 4, 551–557. [Google Scholar]
- Karoui-Kharrat, D.; Kaddour, H.; Hamdi, Y.; Mokni, M.; Amri, M.; Mezghani, S. Response of antioxidant enzymes to cadmium-induced cytotoxicity in rat cerebellar granule neurons. Open Life Sci. 2017, 12, 113–119. [Google Scholar] [CrossRef]
- Winiarska-Mieczan, A.; Tomaszewska, E.; Jachimowicz, K. Antioxidant, anti-inflammatory, and immunomodulatory properties of tea—The positive impact of tea consumption on patients with autoimmune diabetes. Nutrients 2021, 13, 3972. [Google Scholar] [CrossRef]
- Olcha, P.; Winiarska-Mieczan, A.; Kwiecień, M.; Nowakowski, Ł.; Miturski, A.; Semczuk, A.; Kiczorowska, B.; Gałczyński, K. Antioxidative, anti-inflammatory, anti-obesogenic, and antidiabetic properties of tea polyphenols—The positive impact of regular tea consumption as an element of prophylaxis and pharmacotherapy support in endometrial cancer. Int. J. Mol. Sci. 2022, 23, 6703. [Google Scholar] [CrossRef]
- Sahiti, H.; Bislimi, K.; Bajgora, A.; Rexhepi, A.; Dalo, E. Metal accumulation and effect of Vitamin C and E in accumulated heavy metals in different tissues in common carp (Cyprinus carpio) treated with heavy metals. Pol. J. Environ. Stud. 2020, 29, 799–805. [Google Scholar] [CrossRef]
- Sahiti, H.; Bislimi, K.; Bajgora, A.; Rexhepi, A.; Dalo, E. Protective effect of Vitamin C against oxidative stress in common carp (Cyprinus carpio) induced by heavy metals. Int. J. Agri. Biosci. 2018, 7, 71–75. [Google Scholar]
- Zoidis, E.; Pappas, A.C.; Al-Waeli, A.; Georgiou, C.A.; Danezis, G.P.; Demiris, N.; Zervas, G.; Fegeros, K. Effects of selenium and zinc supplementation on cadmium toxicity in broilers. Turkish J. Vet. Anim. Sci. 2020, 44, 331–336. [Google Scholar] [CrossRef]
- Wu, C.; Li, L.; Jiang, Y.X.; Kim, W.K.; Wu, B.; Liu, G.M.; Wang, J.; Lin, Y.; Zhang, K.Y.; Song, J.P.; et al. Effects of selenium supplementation on the ion homeostasis in the reproductive organs and eggs of laying hens fed with the diet contaminated with cadmium, lead, mercury, and chromium. Front. Vet. Sci. 2022, 9, 902355. [Google Scholar] [CrossRef]
- Drąg-Kozak, E.; Łuszczek-Trojnar, E.; Socha, M.; Bojarski, B. Effects of melatonin on cadmium accumulation and haematological parameters in cadmium intoxicated Prussian carp (Carassius gibelio B.). Ann. Anim. Sci. 2020, 21, 899–923. [Google Scholar] [CrossRef]
- Kobylińska, A.; Posmyk, M.M. Melatonin restricts Pb-induced PCD by enhancing BI-1 expression in tobacco suspension cells. Biometals 2016, 29, 1059–1074. [Google Scholar] [CrossRef] [PubMed]
- Kukongviriyapan, U.; Pannangpetch, P.; Kukongviriyapan, V.; Donpunha, W.; Sompamit, K.; Surawattanawan, P. Curcumin protects against cadmium-induced vascular dysfunction, hypertension and tissue cadmium accumulation in mice. Nutrients 2014, 6, 1194–1208. [Google Scholar] [CrossRef] [PubMed]
- Tubsakul, A.; Sangartit, W.; Pakdeechote, P.; Kukongviriyapan, V.; Apaijit, K.; Kukongviriyapan, U. Curcumin mitigates hypertension, endothelial dysfunction and oxidative stress in rats with chronic exposure to lead and cadmium. Tohoku J. Exp. Med. 2021, 253, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Amal, E.A.; Mona, H.M. Protective effect of some antioxidants on the brain of adult male albino rats, Rattus rattus, exposed to heavy metals. Biosci. Res. 2009, 1, 12–19. [Google Scholar]
- Waheeb, A.I.; Ali, L.H. Garlic extract and honey as potential protective agents against cadmium-induced nephrotoxicity in male rats. Eurasia J. Biosci. 2020, 14, 5019–5026. [Google Scholar]
- Salem, N.A.; Salem, E.A. Protective Antioxidant efficiency of garlic against lead-induced renal and testicular toxicity in adult male rats. J. Heavy Met. Toxicity Dis. 2016, 1, 3. [Google Scholar]
- Vijaya, P.; Kaur, H.; Garg, N.; Sharma, S. Protective and therapeutic effects of garlic and tomato on cadmium-induced neuropathology in mice. JoBAZ 2020, 81, 23. [Google Scholar] [CrossRef]
- Han, C.; Zhu, Y.; Yang, Z.; Fu, S.; Zhang, W.; Liu, C. Protective effect of Polygonatum sibiricum against cadmium-induced testicular injury in mice through inhibiting oxidative stress and mitochondria-mediated apoptosis. J. Ethnopharmacol. 2020, 261, 113060. [Google Scholar] [CrossRef] [PubMed]
- Udefa, A.L.; Amama, E.A.; Archibong, E.A.; Nwangwa, J.N.; Adama, S.; Inyang, V.U.; Inyaka, G.U.; Aju, G.J.; Okpa, S.; Inah, I.O. Antioxidant, anti-inflammatory and anti-apoptotic effects of hydro-ethanolic extract of Cyperus esculentus L. (tigernut) on lead acetate-induced testicular dysfunction in Wistar rats. Biomed. Pharmacother. 2020, 129, 110491. [Google Scholar] [CrossRef]
- Alkhedaide, A.; Alshehri, Z.S.; Sabry, A.; Abdel-Ghaffar, T.; Soliman, M.M.; Attia, H. Protective effect of grape seed extract against cadmium-induced testicular dysfunction. Mol. Med. Rep. 2016, 13, 3101–3109. [Google Scholar] [CrossRef]
- Morsi, A.A.; Shawky, L.M.; El Bana, E.A. The potential gonadoprotective effects of grape seed extract against the histopathological alterations elicited in an animal model of cadmium-induced testicular toxicity. Folia Morphol. 2020, 79, 767–776. [Google Scholar] [CrossRef]
- Onopiuk, B.M.; Dąbrowska, Z.N.; Rogalska, J.; Brzóska, M.M.; Dąbrowski, A.; Bijowski, K.; Onopiuk, P.; Mroczko, B.; Orywal, K.; Dąbrowska, E. The beneficial impact of the black chokeberry extract against the oxidative stress in the sublingual salivary gland of rats intoxicated with cadmium. Oxid. Med. Cell. Longev. 2021, 2021, 6622245. [Google Scholar] [CrossRef]
- Ibrahim, N.K. Possible protective effect of kombucha tea ferment on cadmium chloride induced liver and kidney damage in irradiated rats. Int. J. Biol. Life. Sci. 2013, 9, 7–12. [Google Scholar]
- Bagherpour, H.; Karimpour Malekshah, A.; Talebpour Amiri, F.; Azadbakht, M. Protective effect of green tea extract on the deltamethrin-induced toxicity in mice testis: An experimental study. Int. J. Reprod. Biomed. 2018, 17, 337–348. [Google Scholar] [CrossRef]
- Parasuraman, S.; Beng, J.Y.K.; Hui, L.C.; Qin, B.N.Y. Effect of epigallocatechin gallate on cadmium chloride-induced oxidative stress in female Sprague Dawley rats. Free Radic. Antioxid. 2020, 10, 29–34. [Google Scholar] [CrossRef]
- Engelhardt, U.H. Tea chemistry—What do and what don’t we know?—A micro review. Food Res. Int. 2020, 132, 109120. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Chen, H.; Chen, L.; Yu, S.Y.; Yang, H.; Zeng, Y.; Gu, D.; Ng, T.P. Type of tea consumption and depressive symptoms in Chinese older adults. BMC Geriatr. 2021, 21, 331. [Google Scholar] [CrossRef] [PubMed]
- Savolainen, H. Tannin content of tea and coffee. J. Appl. Toxicol. 1992, 12, 191–192. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Kim, H.W.; Lee, S.H.; Kim, Y.J.; Asamenew, G.; Choi, J.; Lee, J.W.; Jung, H.A.; Yoo, S.M.; Kim, J.B. Characterization of catechins, theaflavins, and flavonols by leaf processing step in green and black teas (Camellia sinensis) using UPLC-DAD-QToF/MS. Eur. Food Res. Technol. 2019, 245, 997–1010. [Google Scholar] [CrossRef]
- Zujko, M.E.; Witkowska, A.M. Antioxidant potential and polyphenol content of beverages, chocolates, nuts, and seeds. Int. J. Food Prop. 2014, 17, 86–92. [Google Scholar] [CrossRef]
- El-Sayed, I.H.; Lotfy, M.; El-Khawaga, O.A.Y.; Nasif, W.A.; El-Shahat, M. Prominent free radicals scavenging activity of tannic acid in lead-induced oxidative stress in experimental mice. Toxicol. Ind. Health 2006, 4, 157–163. [Google Scholar] [CrossRef]
- Leenen, R.; Roodenburg, A.J.; Tijburg, L.B.; Wiseman, S.A. A single dose of tea with or without milk increases plasma antioxidant activity in humans. Eur. J. Clin. Nutr. 2000, 54, 87–92. [Google Scholar] [CrossRef]
- Serafini, M.; Ghiselli, A.; Ferro-Luzzi, A. In vivo antioxidant effect of green and black tea in man. Eur. J. Clin. Nutr. 1996, 50, 28–32. [Google Scholar]
- Szulińska, M.; Stępień, M.; Kręgielska-Narożna, M.; Suliburska, J.; Skrypnik, D.; Bąk-Sosnowska, M.; Kujawska-Łuczak, M.; Grzymisławska, M.; Bogdański, P. Effects of green tea supplementation on inflammation markers, antioxidant status and blood pressure in NaCl-induced hypertensive rat model. Food Nutr. Res. 2017, 61, 1295525. [Google Scholar] [CrossRef]
- Pastoriza, S.; Mesías, M.; Cabrera, C.; Rufián-Henares, J.A. Healthy properties of green and white teas: An update. Food Funct. 2017, 8, 2650–2662. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C.; Jiménez-Escrig, A.; Saura-Calixto, F. Study of low-density lipoprotein oxidizability indexes to measure the antioxidant activity of dietary polyphenols. Nutr. Res. 2000, 20, 941–953. [Google Scholar] [CrossRef]
- Pekdemir, T.; Tokunaga, S.; Ishigami, Y.; Kyung-Jin, H. Removal of cadmium or lead from polluted water by biological amphiphiles. J. Surf. Deterg. 2000, 3, 43–46. [Google Scholar] [CrossRef]
- Winiarska-Mieczan, A. Protective effect of tannic acid on the brain of adult rats exposed to cadmium and lead. Environ. Toxicol. Pharmacol. 2013, 36, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Misra, H.P.; Fridowich, I. The role of superoxide anion in the autoxidation of Epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef]
- Sinha, A.K. Colorimetric assay of catalase. Anal. Biochem. 1972, 47, 389–394. [Google Scholar] [CrossRef]
- Peaslee, M.H.; Einhellig, F.A. Protective effect of tannic acid in mice receiving dietary lead. Experientia 1977, 33, 1206. [Google Scholar] [CrossRef]
- Truong, V.L.; Jeong, W.S. Cellular defensive mechanisms of tea polyphenols: Structure-activity relationship. Int. J. Mol. Sci. 2021, 22, 9109. [Google Scholar] [CrossRef]
- Cerbin-Koczorowska, M.; Waszyk-Nowaczyk, M.; Bakun, P.; Goslinski, T.; Koczorowski, T. Current view on green tea catechins formulations, their interactions with selected drugs, and prospective applications for various health conditions. Appl. Sci. 2021, 11, 4905. [Google Scholar] [CrossRef]
- Falla, N.M.; Demasi, S.; Caser, M.; Scariot, V. Phytochemical profile and antioxidant properties of Italian green tea, a new high quality niche product. Horticulturae 2021, 7, 91. [Google Scholar] [CrossRef]
- Quesille-Villalobos, A.M.; Torricoa, J.S.; Ranilla, L.G. Phenolic compounds, antioxidant capacity, and in vitro α-amylase inhibitory potential of tea infusions (Camellia sinensis) commercialized in Chile. CYTA-J. Food 2013, 11, 60–67. [Google Scholar] [CrossRef]
- Gülçin, I.; Huyut, Z.; Elmastaş, M.; Aboul-Enein, H.Y. Radical scavenging and antioxidant activity of tannic acid. Arabian J. Chem. 2010, 3, 43–53. [Google Scholar] [CrossRef]
- Oliveira, C.S.; Nogara, P.A.; Lima, L.S.; Galiciolli, M.E.; Souza, J.V.; Aschner, M.; Rocha, J.B. Toxic metals that interact with thiol groups and alteration in insect behavior. Curr. Opin. Insect. Sci. 2022, 52, 100923. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.G.; Ahn, R.M.; Hwang, S.H. The effects of tannic acid to the cadmium on mouse. J. Food Hyg. Saf. 1998, 13, 87–93. [Google Scholar]
- Winiarska-Mieczan, A.; Krusiński, R.; Kwiecień, M. Tannic acid influence on lead and cadmium accumulation in the hearts and lungs of rats. Adv. Clin. Exp. Med. 2013, 22, 615–620. [Google Scholar] [PubMed]
- Tomaszewska, E.; Dobrowolski, P.; Winiarska-Mieczan, A.; Kwiecień, M.; Tomczyk, A.; Muszyński, S. The effect of tannic acid on the bone tissue of adult male Wistar rats exposed to cadmium and lead. Exp. Toxicol. Pathol. 2016, 69, 131–141. [Google Scholar] [CrossRef]
- Winiarska-Mieczan, A. The potential protective effect of green, black, red and white tea infusions against adverse effect of cadmium and lead during chronic exposure—A rat model study. Regulat. Toxicol. Pharmacol. 2015, 73, 521–529. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Dobrowolski, P.; Winiarska-Mieczan, A.; Kwiecień, M.; Tomczyk, A.; Muszyński, S.; Radzki, R. Alteration in bone geometric and mechanical properties, histomorphometrical parameters of trabecular bone, articular cartilage and growth plate in adolescent rats after chronic co-exposure to cadmium and lead in the case of supplementation with green, black, red and white tea. Environ. Toxicol. Pharmacol. 2016, 46, 36–44. [Google Scholar] [PubMed]
- O’Keeffe, L.M.; Taylor, G.; Huxley, R.R.; Mitchell, P.; Woodward, M.; Peters, S.A.E. Smoking as a risk factor for lung cancer in women and men: A systematic review and meta-analysis. BMJ Open 2018, 8, e021611. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, Y.; Ohishi, T.; Nakamura, Y.; Fukutomi, R.; Miyoshi, N. Anti-cancer effects of dietary polyphenols via ROS-mediated pathway with their modulation of microRNAs. Molecules 2022, 27, 3816. [Google Scholar] [CrossRef]
- Maleki Dana, P.; Sadoughi, F.; Asemi, Z.; Yousefi, B. The role of polyphenols in overcoming cancer drug resistance: A comprehensive review. Cell Mol. Biol. Lett. 2022, 27, 1. [Google Scholar] [CrossRef]
- Poli, V.; Madduru, R.; Aparna, Y.; Kandukuri, V.; Motireddy, S.R. Amelioration of cadmium-induced oxidative damage in wistar rats by Vitamin C, zinc and N-acetylcysteine. Med. Sci. 2022, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Lou, W.; Chen, Y.; Ma, H.; Liang, G.; Liu, B. Antioxidant and α-amylase inhibitory activities of tannic acid. J. Food Sci. Technol. 2018, 55, 3640–3646. [Google Scholar] [CrossRef] [PubMed]
- Guliasar, T.; Aydogdu, N.; Cakina, S.; Sipahi, T.; Kaymak, K.; Sener, S. Trace elements in rat model of cadmium toxicity: The effect of taurine, melatonin and N-acetylcysteine. Trakya Univ. Tip. Fak. Derg. 2010, 27, 23–27. [Google Scholar]
- Khalaf, A.A.; Moselhy, W.A.; Abdel-Hamed, M.I. The protective effect of green tea extract on lead induced oxidative and DNA damage on rat brain. Neurotoxicology 2012, 33, 280–289. [Google Scholar] [CrossRef]
- Wei, H.; Meng, Z. Protective effect of epigallocatechin-3-gallate against lead-induced oxidative damage. Hum. Exp. Toxicol. 2011, 30, 1521–1528. [Google Scholar] [CrossRef] [PubMed]
- Areba, G.O.; Khalid, R.; Ngure, R.M.; Maloba, F.; Nyaga, N.; Moseti, K.O.; Ngotho, M.; Wanyoko, J.K.; Karori, S.M.; Wachira, F.N. Neuroprotective effects of tea against cadmium toxicity. Bioact. Compd. Health Dis. 2019, 2, 230–246. [Google Scholar]
| Pb | Cd | |
|---|---|---|
| Wave length, nm | 217.0 | 228.8 |
| Lamp current, mA | 10 | 4 |
| Spectral band pass, nm | 1 | 0.5 |
| LOD, mg/kg | 0.011 | 0.001 |
| LOQ, mg/kg | 0.03 | 0.004 |
| Pure gas | Argon | Argon |
| Background correction | Zeeman | Zeeman |
| Mean recovery rate | 95% | 96% |
| The deviation of duplicate measurement | 5.2% | 5.0% |
| Treatment | Mean Weekly Fluids Consumption, mL | Mean Weekly TA Consumption, g | Mean Weekly Feed Consumption, g | Total Intake of Pb, mg/kg BW | Total Intake of Cd, mg/kg BW | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1–6 Weeks | 7–12 Weeks | 1–6 Weeks | 7–12 Weeks | 1–6 Weeks | 7–12 Weeks | 1–6 Weeks | 7–12 Weeks | 1–6 Weeks | 7–12 Weeks | |
| Experiment 1 | ||||||||||
| Control | 125.7 b | 158.2 b | 0.00 a | 0.00 a | 125.7 | 158.2 | 163.2 b | 141.0 b | 22.85 | 19.74 ab |
| 0.5% TA | 128.3 b | 162.2 b | 0.64 b | 0.81 b | 128.3 | 162.2 | 157.1 ab | 143.4 b | 22.00 | 20.07 b |
| 1% TA | 124.8 b | 163.7 b | 1.25 c | 1.64 c | 124.8 | 163.7 | 152.2 ab | 149.2 b | 21.31 | 20.89 b |
| 1.5% TA | 131.0 b | 160.5 b | 1.97 d | 2.41 d | 131.0 | 160.5 | 153.6 ab | 145.7 b | 21.51 | 20.40 b |
| 2% TA | 132.8 b | 167.5 b | 2.66 f | 3.35 f | 132.8 | 167.5 | 152.7 ab | 145.7 b | 21.38 | 20.42 b |
| 2.5% TA | 98.50 a | 122.2 a | 2.46 e | 3.06 e | 98.50 | 122.2 | 150.0 a | 126.2 a | 21.00 | 17.66 a |
| Experiment 2 | ||||||||||
| Control | 147.9 ab | 167.5 b | 0.00 a | 0.00 a | 141.0 a | 157.4 c | 200.7 a | 164.0 b | 23.17 ab | 19.28 ab |
| BT | 136.7 a | 152.1 a | 2.74 b | 3.04 b | 148.0 ab | 150.2 b | 221.5 b | 153.0 a | 25.78 b | 17.85 a |
| GT | 139.5 a | 158.4 ab | 2.79 b | 3.17 bc | 147.0 ab | 151.8 b | 228.5 b | 163.3 b | 26.57 b | 20.20 b |
| RT | 155.4 b | 164.8 b | 3.11 c | 3.30 c | 145.0 ab | 140.3 a | 207.2 a | 170.7 bc | 22.74 a | 17.60 a |
| WT | 151.9 b | 163.4 b | 3.04 c | 3.27 c | 153.0 b | 146.7 ab | 214.3 ab | 172.3 c | 25.08 ab | 18.00 a |
| Body Weight, g | Body Weight Gain Throughout the Period of Experience | ||
|---|---|---|---|
| at 6 Week | at 12 Week | ||
| Experiment 1 | |||
| Control | 231.9 b ± 22.1 | 336.6 b ± 17.9 | 105.6 d ± 3.74 |
| 0.5% TA | 245.7 b ± 9.98 | 339.3 b ± 31.5 | 94.37 c ± 3.05 |
| 1% TA | 246.0 b ± 15.4 | 329.1 b ± 25.7 | 83.19 b ± 1.98 |
| 1.5% TA | 255.8 b ± 21.1 | 330.5 b ± 21.3 | 74.74 a ± 5.22 |
| 2% TA | 261.9 b ± 17.6 | 344.8 b ± 9.64 | 83.81 b ± 4.07 |
| 2.5% TA | 197.5 a ± 8.39 | 290.5 a ± 15.6 | 93.50 c ± 2.95 |
| Experiment 2 | |||
| Control | 255.6 b ± 9.05 | 342.6 b ± 8.35 | 132.0 bc ± 7.22 |
| BT | 241.1 ab ± 17.1 | 343.6 b ± 20.2 | 138.0 c ± 18.3 |
| GT | 232.4 a ± 8.23 | 316.4 a ± 19.1 | 105.5 a ± 13.7 |
| RT | 261.8 b ± 21.2 | 334.2 b ± 16.2 | 126.1 b ± 18.9 |
| WT | 256.2 b ± 20.4 | 343.5 b ± 30.4 | 133.6 bc ± 25.4 |
| Relative Weight,% | ||
|---|---|---|
| 1–6 Weeks | 7–12 Weeks | |
| Lungs | ||
| 0% TA | 0.736 a ± 0.04 | 0.714 a ± 0.06 |
| 0.5% TA | 0.707 a ± 0.06 | 0.715 a ± 0.02 |
| 1% TA | 0.729 a ± 0.01 | 0.740 a ± 0.01 |
| 1.5% TA | 0.713 a ± 0.02 | 0.737 a ± 0.02 |
| 2% TA | 0.706 a ± 0.03 | 0.715 a ± 0.05 |
| 2.5% TA | 0.933 b ± 0.08 | 0.838 b ± 0.03 |
| Heart | ||
| 0% TA | 0.445 a ± 0.03 | 0.364 a ± 0.03 |
| 0.5% TA | 0.459 a ± 0.02 | 0.362 a ± 0.03 |
| 1% TA | 0.460 a ± 0.04 | 0.370 a ± 0.01 |
| 1.5% TA | 0.438 a ± 0.01 | 0.369 a ± 0.02 |
| 2% TA | 0.429 a ± 0.01 | 0.355 a ± 0.01 |
| 2.5% TA | 0.569 b ± 0.02 | 0.420 b ± 0.01 |
| Brain | ||
| 0% TA | 0.730 a ± 0.05 | 0.594 a ± 0.03 |
| 0.5% TA | 0.693 a ± 0.01 | 0.619 a ± 0.05 |
| 1% TA | 0.694 a ± 0.05 | 0.627 a ± 0.01 |
| 1.5% TA | 0.667 a ± 0.02 | 0.628 a ± 0.01 |
| 2% TA | 0.653 a ± 0.02 | 0.607 a ± 0.05 |
| 2.5% TA | 0.868 b ± 0.06 | 0.711 b ± 0.02 |
| Pb | Cd | |||
|---|---|---|---|---|
| 1–6 Weeks | 7–12 Weeks | 1–6 Weeks | 7–12 Weeks | |
| Lungs | ||||
| 0.5% TA | −1.37 a | −1.83 a | −1.13 a | −0.06 a |
| 1% TA | −2.26 b | −2.21 b | −4.91 b | −6.24 b *‡ |
| 1.5% TA | −3.23 c | −3.73 c ‡ | −5.53 c * | −7.91 c *‡ |
| 2% TA | −4.08 d | −4.18 d | −15.9 d * | −22.6 d *‡ |
| 2.5% TA | −4.43 d | −4.63 e | −25.7 e * | −31.6 e *‡ |
| Heart | ||||
| 0.5% TA | 0.00 a | 0.00 a | 0.00 a | −3.53 a |
| 1% TA | −2.16 b | −2.93 b | −3.40 b | −3.94 a |
| 1.5% TA | −3.18 c | −4.48 c ‡ | −5.79 c * | −6.34 b *‡ |
| 2% TA | −4.26 d | −4.82 cd ‡ | −12.0 d * | −19.4 c *‡ |
| 2.5% TA | −4.97 e | −5.18 d * | −17.7 e * | −23.8 d *‡ |
| Brain | ||||
| 0.5% TA | 0.00 a | 0.00 a | −1.48 a | −1.73 a |
| 1% TA | −0.64 b | −0.75 b | −4.14 b | −4.22 b |
| 1.5% TA | −2.72 c | −3.32 c | −5.55 c * | −7.26 c *‡ |
| 2% TA | −3.35 d | −5.38 d *‡ | −19.2 d * | −23.3 d *‡ |
| 2.5% TA | −4.92 e | −5.89 e *‡ | −21.7 d * | −21.9 d *‡ |
| Relative Weight,% | ||
|---|---|---|
| 1–6 Weeks | 7–12 Weeks | |
| Lungs | ||
| Control | 0.670 ± 0.02 | 0.683 ± 0.01 |
| BT | 0.682 ± 0.05 | 0.645 ± 0.03 |
| GT | 0.659 ± 0.01 | 0.672 ± 0.03 |
| RT | 0.662 ± 0.01 | 0.657 ± 0.05 |
| WT | 0.672 ± 0.04 | 0.675 ± 0.02 |
| Heart | ||
| Control | 0.435 ± 0.02 | 0.351 ± 0.03 |
| BT | 0.455 ± 0.02 | 0.338 ± 0.08 |
| GT | 0.432 ± 0.08 | 0.330 ± 0.01 |
| RT | 0.456 ± 0.01 | 0.340 ± 0.01 |
| WT | 0.441 ± 0.05 | 0.345± 0.02 |
| Brain | ||
| Control | 0.670 ± 0.05 | 0.543 ± 0.02 |
| BT | 0.688 ± 0.09 | 0.541 ± 0.01 |
| GT | 0.692 ± 0.01 | 0.563 ± 0.05 |
| RT | 0.672 ± 0.01 | 0.552± 0.02 |
| WT | 0.668 ± 0.02 | 0.544 ± 0.04 |
| Pb | Cd | |||
|---|---|---|---|---|
| 1–6 Weeks | 7–12 Weeks | 1–6 Weeks | 7–12 Weeks | |
| Lungs | ||||
| BT | −1.96 a | −3.32 a ‡ | −15.0 a * | −26.4 a *‡ |
| GT | −2.42 b | −4.18 b ‡ | −16.8 b * | −27.6 b *‡ |
| RT | −2.62 b | −4.10 b ‡ | −16.6 b * | −26.3 a *‡ |
| WT | −3.68 c | −4.22 b ‡ | −17.4 c * | −28.8 c *‡ |
| Heart | ||||
| BT | −4.35 b | −4.78 b ‡ | −15.2 a * | −17.1 a *‡ |
| GT | −4.52 bc | −4.83 b | −18.4 c * | −19.6 b *‡ |
| RT | −4.03 a | −3.91 a ‡ | −16.1 b * | −17.5 a *‡ |
| WT | −4.66 c | −5.01 b *‡ | −19.7 d * | −25.8 c *‡ |
| Brain | ||||
| BT | −4.38 a | −4.64 a | −20.4 a * | −21.7 a *‡ |
| GT | −4.41 a | −4.97 a ‡ | −21.2 b * | −23.2 c *‡ |
| RT | −4.43 a | −4.75 a | −20.9 a * | −22.5 b *‡ |
| WT | −4.66 b | −5.19 b *‡ | −21.7 b * | −23.9 d *‡ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winiarska-Mieczan, A.; Kwiecień, M.; Bąkowski, M.; Krusiński, R.; Jachimowicz-Rogowska, K.; Demkowska-Kutrzepa, M.; Kiczorowska, B.; Krupa, W. Tannic Acid and Tea Prevents the Accumulation of Lead and Cadmium in the Lungs, Heart and Brain of Adolescent Male Wistar Rats—Possible Therapeutic Option. Animals 2022, 12, 2838. https://doi.org/10.3390/ani12202838
Winiarska-Mieczan A, Kwiecień M, Bąkowski M, Krusiński R, Jachimowicz-Rogowska K, Demkowska-Kutrzepa M, Kiczorowska B, Krupa W. Tannic Acid and Tea Prevents the Accumulation of Lead and Cadmium in the Lungs, Heart and Brain of Adolescent Male Wistar Rats—Possible Therapeutic Option. Animals. 2022; 12(20):2838. https://doi.org/10.3390/ani12202838
Chicago/Turabian StyleWiniarska-Mieczan, Anna, Małgorzata Kwiecień, Maciej Bąkowski, Robert Krusiński, Karolina Jachimowicz-Rogowska, Marta Demkowska-Kutrzepa, Bożena Kiczorowska, and Wanda Krupa. 2022. "Tannic Acid and Tea Prevents the Accumulation of Lead and Cadmium in the Lungs, Heart and Brain of Adolescent Male Wistar Rats—Possible Therapeutic Option" Animals 12, no. 20: 2838. https://doi.org/10.3390/ani12202838
APA StyleWiniarska-Mieczan, A., Kwiecień, M., Bąkowski, M., Krusiński, R., Jachimowicz-Rogowska, K., Demkowska-Kutrzepa, M., Kiczorowska, B., & Krupa, W. (2022). Tannic Acid and Tea Prevents the Accumulation of Lead and Cadmium in the Lungs, Heart and Brain of Adolescent Male Wistar Rats—Possible Therapeutic Option. Animals, 12(20), 2838. https://doi.org/10.3390/ani12202838






