Use of Infrared Thermography to Assess Body Temperature as a Physiological Stress Indicator in Horses during Ridden and Lunging Sessions
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Studied Population
2.2. Personality Assessment
2.3. Ridden Behavior Analysis
2.4. Workload Assessment
2.5. Statistical Analysis
2.5.1. Personality Assessment
2.5.2. Workload Assessment
3. Results
3.1. Personality Assessment
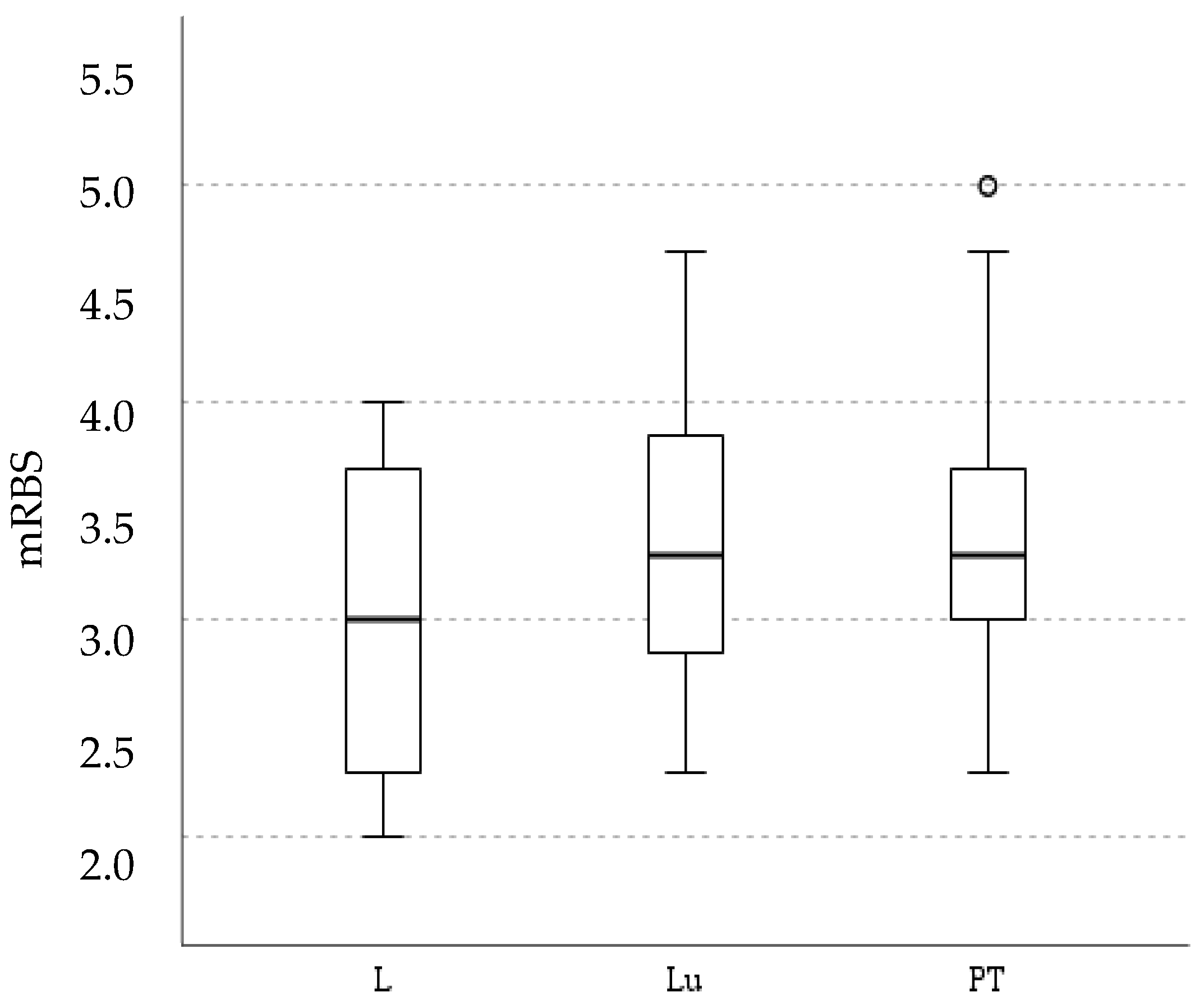
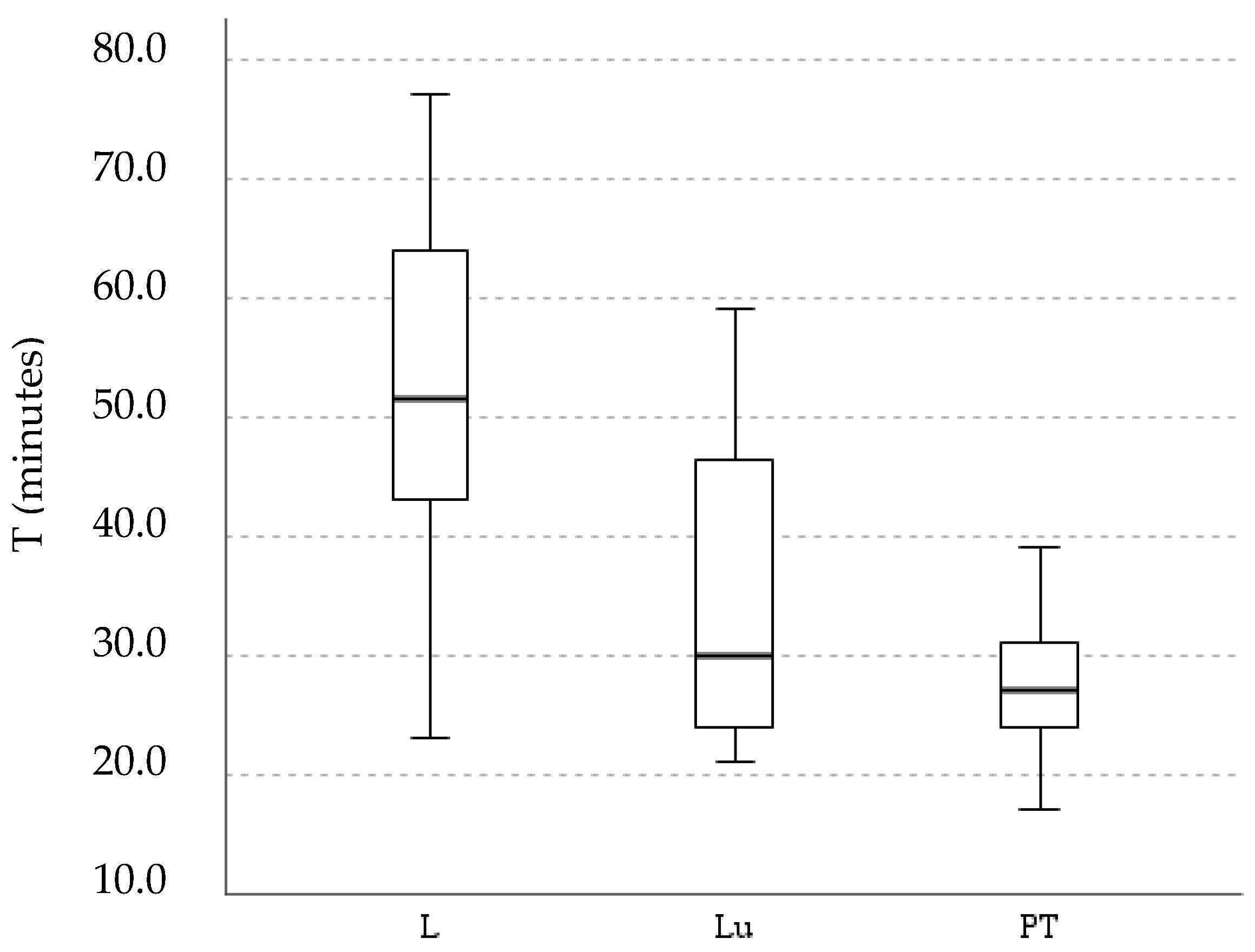
3.2. Workload Assessment
4. Discussion
4.1. Personality Assessment
4.2. Workload Assessment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Score | 1 | 2 | 3 | |
|---|---|---|---|---|
| Markers | ||||
| Position of the ears | Relaxed, might follow the stimulus. | With movement to and from the stimulus. | Fixed on the stimulus or facing back. | |
| Position of the eyes | Relaxed, might look at and follow the stimulus. | Attentive to the stimulus. | Widened, reduced blinking frequency. | |
| Position and movement of the head | Held low, with little erratic movement (head shaking). | Held at the height of the stimulus. | Held high, shaking from side to side and/or up and down. | |
| Breathing | Relaxed, inaudible. | Audible, with small alterations, might blow. | Audible, with alterations and snorts. | |
| Interruption of normal activity | Interacts with other horses, feeding is not interrupted. | Ignores other horses, chewing is interrupted. | Ignores other horses and feeding is interrupted altogether. | |
| Proximity to and interaction with the stimulus | Approaches and explores with the lips. | Approaches without interacting. | Does not approach or interact. | |
| Erratic movements | Normal movement to change position, explore or interact. | Limits normal movement. | Exaggerated movements, walks in circles to and from the stimulus. | |
| Flight response | Does not react to movement. | Shivers in response to the movement. | Flees from the movement. | |
References
- Visser, E.K.; Van Wijk-Jansen, E.C. Diversity in horse enthusiasts with respect to horse welfare: An explorative study. J. Vet. Behav. 2012, 7, 295–304. [Google Scholar] [CrossRef]
- Waran, N.K.; McGreevy, P.D.; Casey, R.A. Training methods and horse welfare. In The Welfare of Horses, 1st ed.; Waran, N.K., Ed.; Springer: Dordrecht, The Netherlands, 2007; pp. 151–180. ISBN 78-0-306-48215-1. [Google Scholar]
- Lesimple, C. Indicators of horse welfare: State-of-the-art. Animals 2020, 10, 294. [Google Scholar] [CrossRef] [PubMed]
- Arruda, T.Z.; Brass, K.E.; De La Corte, F.D. Thermographic assessment of saddles used on jumping horses. J. Equine Vet. Sci. 2011, 31, 625–629. [Google Scholar] [CrossRef]
- Von Borstel, U.K. Assessing and influencing personality for improvement of animal welfare: A review of equine studies. CAB Rev. 2013, 8, 1–27. [Google Scholar] [CrossRef]
- Lansade, L.; Bouissou, M.F.; Erhard, H.W. Fearfulness in horses: A temperament trait stable across time and situations. Appl. Anim. Behav. Sci. 2008, 115, 182–200. [Google Scholar] [CrossRef]
- Calviello, R.F.; Titto, E.A.; Infante, P.; Leme-dos-Santos, T.M.; Neto, M.C.; Pereira, A.M.; Titto, C.G. Proposal and validation of a scale of composite measure reactivity score to characterize the reactivity in horses during handling. J. Equine Vet. Sci. 2016, 47, 62–70. [Google Scholar] [CrossRef]
- Ellis, A.D.; Stephenson, M.; Preece, M.; Harris, P. A novel approach to systematically compare behavioural patterns between and within groups of horses. Appl. Anim. Behav. Sci. 2014, 161, 60–74. [Google Scholar] [CrossRef]
- Ijichi, C.; Wild, H.; Dai, F.; Bordin, A.; Cameron-Whytock, H.; White, S.J.; Yarnell, K.; Starbuck, G.; Jolivald, A.; Birkbeck, L.; et al. Dually investigated: The effect of a pressure headcollar on the behavior, discomfort and stress of trained horses. Appl. Anim. Behav. Sci. 2020, 232, 105101. [Google Scholar] [CrossRef]
- Yarnell, K.; Hall, C.; Billett, E. An assessment of the aversive nature of an animal management procedure (clipping) using behavioral and physiological measures. Physiol. Behav. 2013, 118, 32–39. [Google Scholar] [CrossRef]
- Brunberg, E.; Gille, S.; Mikko, S.; Lindgren, G.; Keeling, L.J. Icelandic horses with the silver coat colour show altered behavior in a fear reaction test. Appl. Anim. Behav. Sci. 2013, 146, 72–78. [Google Scholar] [CrossRef]
- Squibb, K.; Griffin, K.; Favier, R.; Ijichi, C. Poker face: Discrepancies in behavior and affective states in horses during stressful handling procedures. Appl. Anim. Behav. Sci. 2018, 202, 34–38. [Google Scholar] [CrossRef]
- Rankins, E.M.; Wickens, C.L. A systematic review of equine personality. Appl. Anim. Behav. Sci. 2020, 231, 105076. [Google Scholar] [CrossRef]
- Lönnell, C. Yard Differences in Training, Management and Orthopedic Injury in Showjumping, Riding School, and Thoroughbred Race Horses. Ph.D. Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2012. Available online: https://res.slu.se/id/publ/79039 (accessed on 6 December 2021).
- Munsters, C.C.; Van den Broek, J.; Van Weeren, R.; Van Oldruitenborgh-Oosterbaan, M.M. A prospective study on fitness, workload and reasons for premature training ends and temporary training breaks in two groups of riding horses. Prev. Vet. Med. 2013, 108, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Murray, R.C.; Walters, J.M.; Snart, H.; Dyson, S.J.; Parkin, T.D. Identification of risk factors for lameness in dressage horses. Vet. J. 2010, 184, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Potter, S.J.; Bamford, N.J.; Harris, P.A.; Bailey, S.R. Prevalence of obesity and owners’ perceptions of body condition in pleasure horses and ponies in south-eastern Australia. Aust. Vet. J. 2016, 94, 427–432. [Google Scholar] [CrossRef]
- Stephenson, H.M.; Green, M.J.; Freeman, S.L. Prevalence of obesity in a population of horses in the UK. Vet. Rec. 2011, 168, 131. [Google Scholar] [CrossRef]
- Burn, C.C.; Dennison, T.L.; Whay, H.R. Relationships between behaviour and health in working horses, donkeys, and mules in developing countries. Appl. Anim. Behav. Sci. 2010, 126, 109–118. [Google Scholar] [CrossRef]
- Munsters, C.C.; Van Iwaarden, A.; Van Weeren, R.; Van Oldruitenborgh-Oosterbaan, M.M. Exercise testing in warmblood sport horses under field conditions. Vet. J. 2014, 1, 11–19. [Google Scholar] [CrossRef]
- Gramkow, H.L.; Evans, D.L. Correlation of race earnings with velocity at maximal heart rate during a field exercise test in thoroughbred racehorses. Equine Vet. J. Suppl. 2006, 36, 118–122. [Google Scholar] [CrossRef]
- Davie, A.L.; Evans, D.J. Blood lactate responses to submaximal field exercise tests in thoroughbred horses. Vet. J. 2000, 159, 252–258. [Google Scholar] [CrossRef]
- Munsters, C.C.; Van den Broek, J.; Welling, E.; Van Weeren, R.; Van Oldruitenborgh-Oosterbaan, M.M. A prospective study on a cohort of horses and ponies selected for participation in the European Eventing Championship: Reasons for withdrawal and predictive value of fitness tests. BMC Vet. Res. 2013, 9, 182. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Frippiat, T.; van Beckhoven, C.; Moyse, E.; Art, T. Accuracy of a heart rate monitor for calculating heart rate variability parameters in exercising horses. J. Equine Vet. Sci. 2021, 104, 103716. [Google Scholar] [CrossRef]
- Hodgson, D.R. Thermoregulation. In The Athletic Horse, 2nd ed.; Hodgson, D.R., McKeever, K.H., McGowan, C.M., Eds.; Saunders Ltd.: St. Louis, MO, USA, 2014; pp. 108–124. [Google Scholar] [CrossRef]
- Lindinger, M.I. Exercise in the heat: Thermoregulatory limitations to performance in humans and horses. Can. J. Appl. Physiol. 1999, 24, 152–163. [Google Scholar] [CrossRef]
- Hinchcliff, K.W.; Geor, R.J. The horse as an athlete: A physiological overview. In Equine Exercise Physiology, 1st ed.; Hinchcliff, K.W., Geor, R.J., Kaneps, A.J., Eds.; Saunders Ltd.: Philadelphia, PA, USA, 2008; pp. 2–11. [Google Scholar] [CrossRef]
- Eddy, A.L.; Van Hoogmoed, L.M.; Snyder, J.R. The role of thermography in the management of equine lameness. Vet. J. 2001, 162, 172–181. [Google Scholar] [CrossRef] [PubMed]
- McKeever, K.H. Body fluids and electrolytes: Responses to exercise and training. In Equine Exercise Physiology, 1st ed.; Hinchcliff, K.W., Geor, R.J., Kaneps, A.J., Eds.; Saunders Ltd.: Philadelphia, PA, USA, 2008; pp. 328–349. [Google Scholar] [CrossRef]
- Witkowska-Piłaszewicz, O.; Maśko, M.; Domino, M.; Winnicka, A. Infrared thermography correlates with lactate concentration in blood during race training in horses. Animals 2020, 10, 2072. [Google Scholar] [CrossRef]
- Trindade, P.H.; Ferraz, G.C.; Lima, M.L.; Negrão, J.A.; da Costa, M.J. Eye Surface Temperature as a Potential Indicator of Physical Fitness in Ranch Horses. J. Equine Vet. Sci. 2019, 75, 1–8. [Google Scholar] [CrossRef]
- Seabra, J.C.; Dittrich, J.R.; do Vale, M.M.; Janiszewski, J.R.; de Hollanda, R.S. Alteração da temperatura ocular em resposta ao treinamento de corrida em cavalos Puro-Sangue-Inglês no Jockey Club. Arch. Vet. Sci. 2019, 24, 50–59. [Google Scholar] [CrossRef]
- Brownlow, M.A. How do thoroughbred racehorses cope so effectively with the physiological challenge of strenuous exercise in hot and humid conditions? Observations from the racetrack. CVE C. T. 2017, 289, 35–42. [Google Scholar]
- Maśko, M.; Domino, M.; Zdrojkowski, L.; Jasiński, T.; Gajewski, Z. The effect of lunging with three aids on the thermographically determined temperatures of the distal portion of horse limbs. J. Equine Vet. Sci. 2020, 95, 103316. [Google Scholar] [CrossRef] [PubMed]
- Simon, E.L.; Gaughan, E.M.; Epp, T.; Spire, M. Influence of exercise on thermographically determined surface temperatures of thoracic and pelvic limbs in horses. J. Am. Vet. Med. Assoc. 2006, 229, 1940–1944. [Google Scholar] [CrossRef] [PubMed]
- Wilk, I.; Wnuk-Pawlak, E.; Janczarek, I.; Kaczmarek, B.; Dybczyńska, M.; Przetacznik, M. Distribution of superficial body temperature in horses ridden by two riders with varied body weights. Animals 2020, 10, 340. [Google Scholar] [CrossRef]
- Christensen, J.W.; Munk, R.; Hawson, L.; Palme, R.; Larsen, T.; Egenvall, A.; Von Borstel, U.U.; Rørvang, M.V. Rider effects on horses’ conflict behaviour, rein tension, physiological measures and rideability scores. Appl. Anim. Behav. Sci. 2021, 234, 105184. [Google Scholar] [CrossRef]
- Visser, E.K.; Van Reenen, C.G.; Blokhuis, M.Z.; Morgan, E.K.; Hassmén, P.; Rundgren., T.M.; Blokhuis, H.J. Does horse temperament influence horse–rider cooperation? J. Appl. Anim. Welf. Sci. 2008, 11, 267–284. [Google Scholar] [CrossRef]
- Bartolomé, E.; Sánchez, M.J.; Molina, A.; Schaefer, A.L.; Cervantes, I.; Valera, M. Using eye temperature and heart rate for stress assessment in young horses competing in jumping competitions and its possible influence on sport performance. Animal 2013, 7, 2044–2053. [Google Scholar] [CrossRef]
- Valera, M.; Bartolomé, E.; Sánchez, M.J.; Molina, A.; Cook, N.; Schaefer, A.L. Changes in eye temperature and stress assessment in horses during show jumping competitions. J. Equine Vet. Sci. 2012, 32, 827–830. [Google Scholar] [CrossRef]
- Sánchez, M.J.; Bartolomé, E.; Valera, M. Genetic study of stress assessed with infrared thermography during dressage competitions in the Pura Raza Español horse. Appl. Anim. Behav. Sci. 2016, 174, 58–65. [Google Scholar] [CrossRef]
- McGreevy, P.; Warren-Smith, A.; Guisard, Y. The effect of double bridles and jaw-clamping crank nosebands on temperature of eyes and facial skin of horses. J. Vet. Behav. 2012, 7, 142–148. [Google Scholar] [CrossRef]
- De Bruijn, C.M.; Houterman, W.; Ploeg, M.; Ducro, B.; Boshuizen, B.; Goethals, K.; Verdegaal, E.L.; Delesalle, C. Monitoring training response in young Friesian dressage horses using two different standardised exercise tests (SETs). BMC Vet. Res. 2017, 13, 49. [Google Scholar] [CrossRef]
- Munsters, C.C.; Van den Broek, J.; Van Weeren, R.; Van Oldruitenborgh-Oosterbaan, M.M. Young Friesian horses show familial aggregation in fitness response to a 7-week performance test. Vet. J. 2013, 198, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Clayton, H. Time motion analysis in equestrian sports: The Grand Prix dressage test. In Proceedings of the Annual Convention of American Association of Equine Practitioners, Lexington, KY, USA, 2–5 December 1990. [Google Scholar]
- Williams, R.J.; Chandler, R.E.; Marlin, D.J. Heart rates of horses during competitive dressage. Comp. Exerc. Physiol. 2009, 6, 7–15. [Google Scholar] [CrossRef][Green Version]
- Van Oldruitenborgh-Oosterbaan, M.M.; Wensing, T.; Barneveld, A.; Breukink, H.J. Heart rate, blood biochemistry and performance of horses competing in a 100 km endurance ride. Vet. Rec. 1991, 128, 175–179. [Google Scholar] [CrossRef]
- Morgan, K. Thermoneutral zone and critical temperatures of horses. J. Therm. Biol. 1998, 23, 59–61. [Google Scholar] [CrossRef]
- Vianna, D.M.; Carrive, P. Changes in cutaneous and body temperature during and after conditioned fear to context in the rat. Eur. J. Neurosci. 2005, 21, 2505–2512. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.; Schaefer, A.L.; Haley, D.B.; Colyn, J.; Cook, N.J.; Stafford., K.J.; Webster, J.R. Infrared thermography as a noninvasive method for detecting fear-related responses of cattle to handling procedures. Anim. Welf. 2008, 17, 387–393. [Google Scholar]
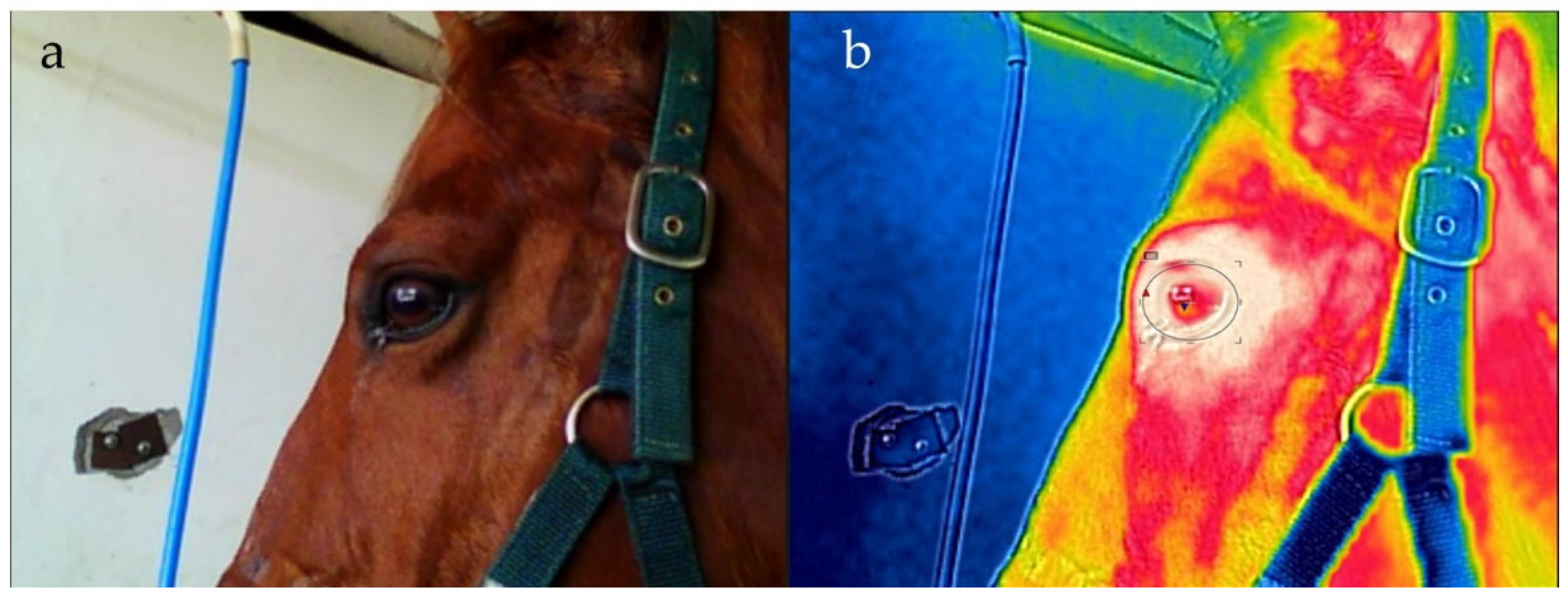
| Reactivity Score | Descriptors |
|---|---|
| 1 | Inspects the object, no reaction to position, ears and eyes turn to the object, returns to normal activity. |
| 2 | Inspects the object, reacts to position, ears and eyes turn to the object, limits normal activity. |
| 3 | Avoids the object, head high and away from object, fixated on the object, walks in circles, interrupts normal activity. |
| Reactivity Score | Descriptors |
|---|---|
| 1 | Slow, requires constant aid to move forward, falls back on trot/canter, falls back on exercises. |
| 2 | Relaxed, requires aids to move forward, falls back on exercises. |
| 3 | Relaxed, good response to aids, moving forward and performing exercises. |
| 4 | Slightly aroused, rushing paces and exercises, jumpy responses to aids. |
| 5 | Agitated, threatens to bolt, light bucks and erratic movements, refuses exercises. |
| 6 | Highly agitated, bolting, rearing or bucking. |
| Mean ± SD | Significance Value | Minimum | Maximum | |||||
|---|---|---|---|---|---|---|---|---|
| Before | After | Z | p-Value | Before | After | Before | After | |
| L | 35.42 ± 1.86 | 35.62 ± 1.90 | Z: −0.004 | p: 0.689 | 31.63 | 31.64 | 38.85 | 38.84 |
| Lu | 34.67 ± 2.05 | 34.83 ± 1.97 | Z: −0.661 | p: 0.508 | 31.07 | 31.20 | 38.40 | 38.35 |
| PT | 35.40 ± 1.90 | 35.43 ± 1.85 | Z: −0.035 | p: 0.972 | 31.60 | 31.65 | 39.12 | 39.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, J.N.; Silva, S.R. Use of Infrared Thermography to Assess Body Temperature as a Physiological Stress Indicator in Horses during Ridden and Lunging Sessions. Animals 2022, 12, 3255. https://doi.org/10.3390/ani12233255
Martins JN, Silva SR. Use of Infrared Thermography to Assess Body Temperature as a Physiological Stress Indicator in Horses during Ridden and Lunging Sessions. Animals. 2022; 12(23):3255. https://doi.org/10.3390/ani12233255
Chicago/Turabian StyleMartins, Joana Noronha, and Severiano R. Silva. 2022. "Use of Infrared Thermography to Assess Body Temperature as a Physiological Stress Indicator in Horses during Ridden and Lunging Sessions" Animals 12, no. 23: 3255. https://doi.org/10.3390/ani12233255
APA StyleMartins, J. N., & Silva, S. R. (2022). Use of Infrared Thermography to Assess Body Temperature as a Physiological Stress Indicator in Horses during Ridden and Lunging Sessions. Animals, 12(23), 3255. https://doi.org/10.3390/ani12233255






