Relationship between Reticulorumen Parameters Measured in Real Time and Methane Emission and Heat Stress Risk in Dairy Cows
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.1.1. Measurements
2.1.2. Classification of Animals
2.2. Measurement of Reticulorumen pH, Temperature, and Walking Activity
2.3. Measurement of CH4
2.4. Data Analysis and Statistics
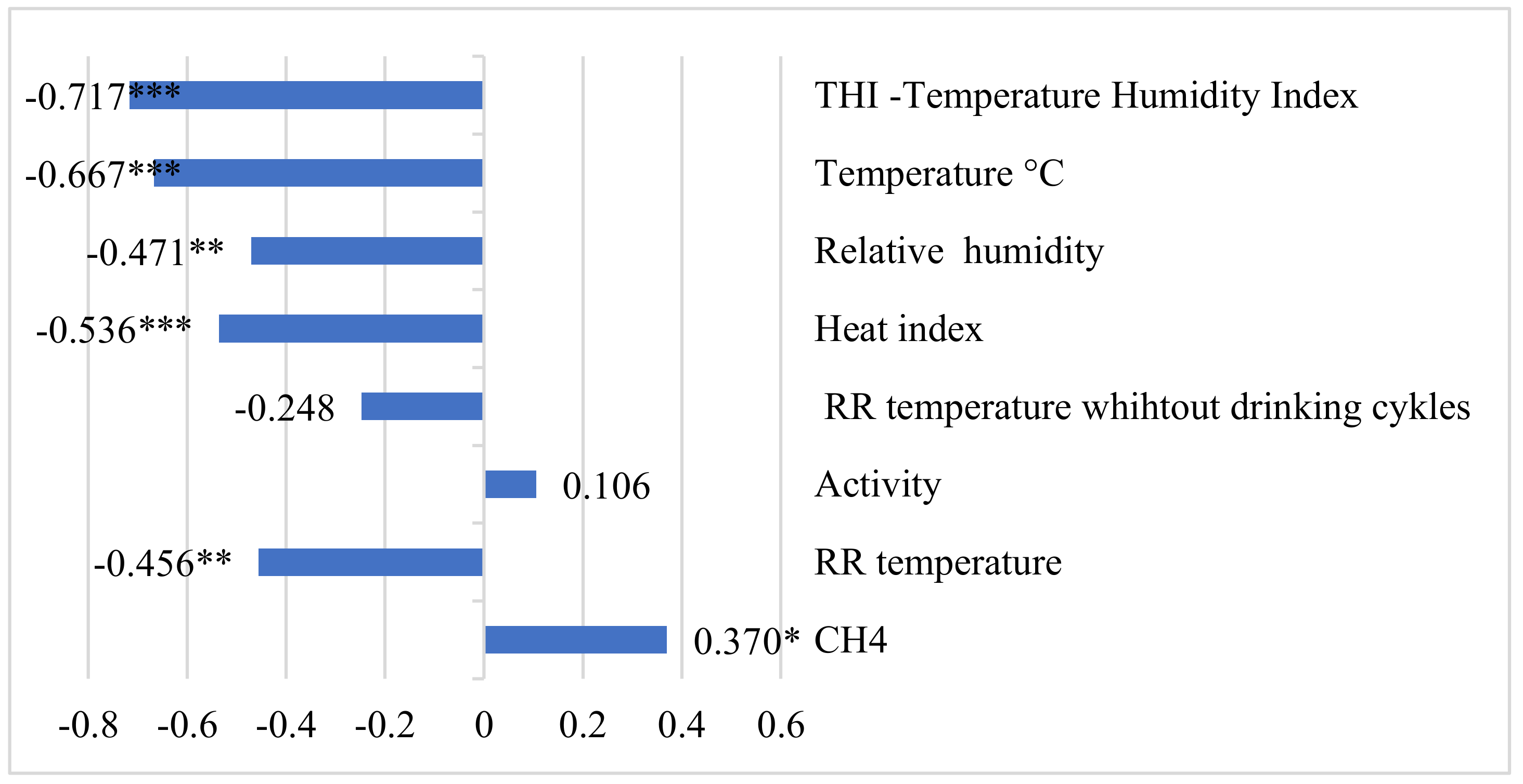
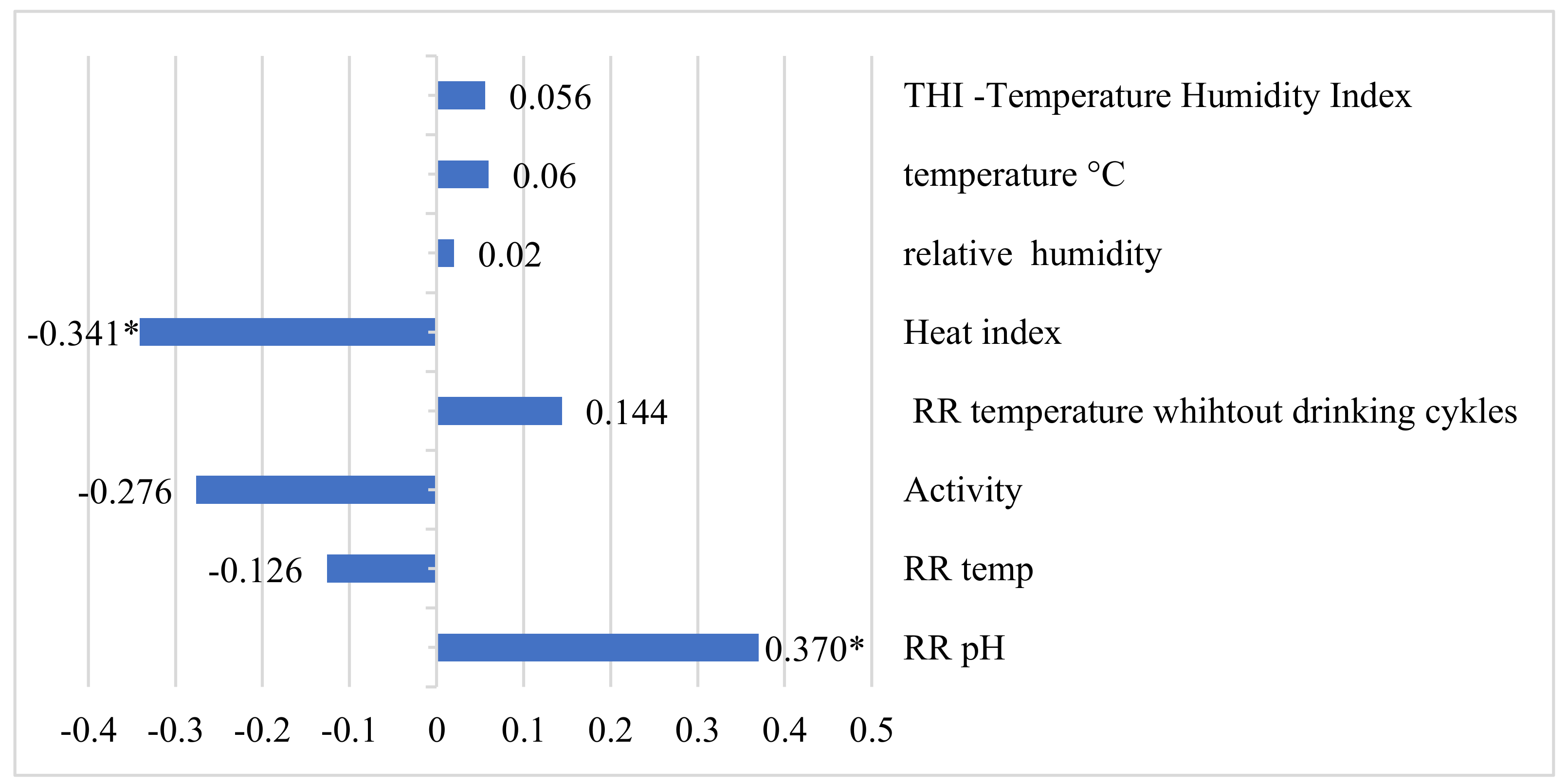
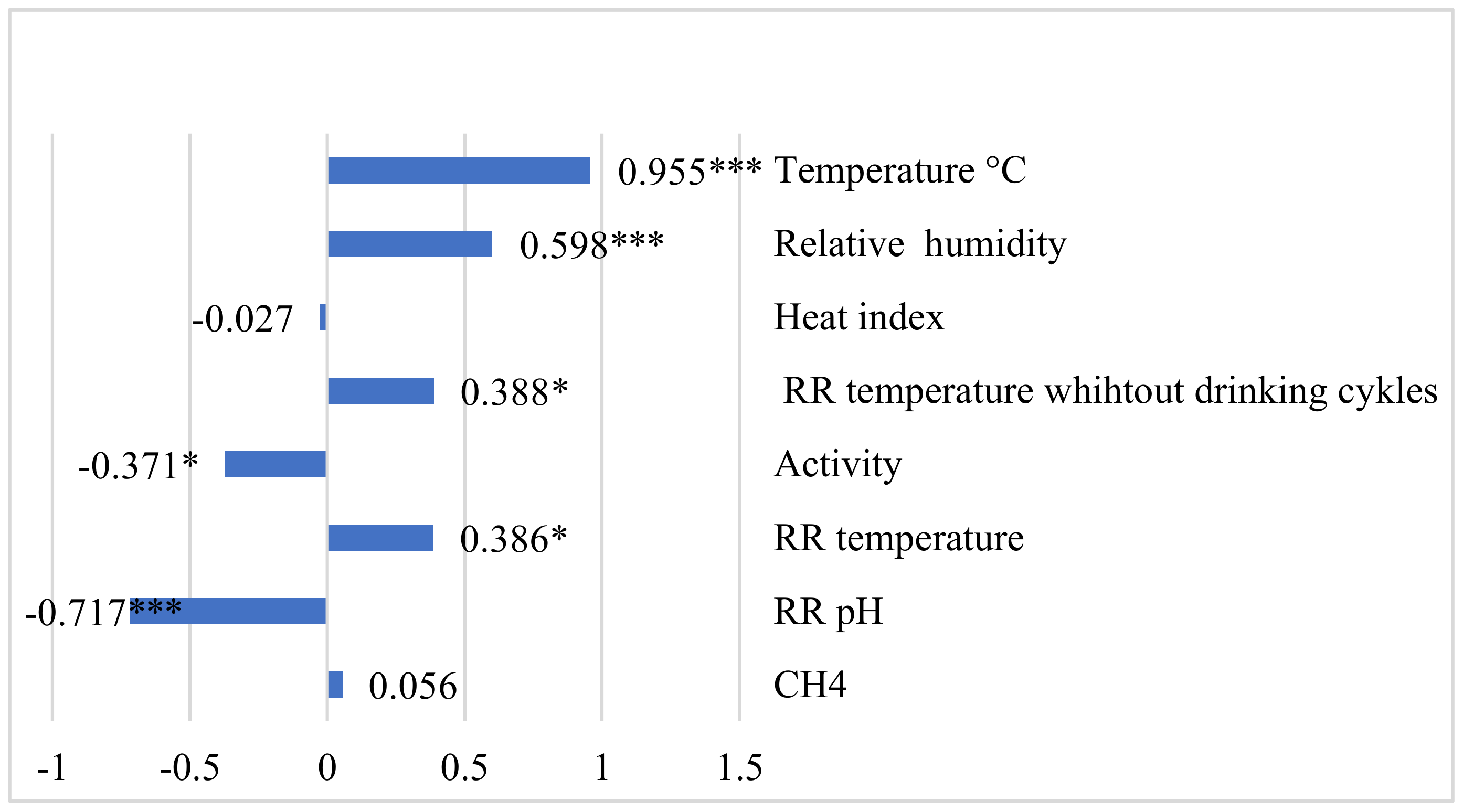
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hristov, A.N.; Kebreab, E.; Niu, M.; Oh, J.; Bannink, A.; Bayat, A.R.; Boland, T.M.; Brito, A.F.; Casper, D.P.; Crompton, L.A.; et al. Symposium Review: Uncertainties in Enteric Methane Inventories, Measurement Techniques, and Prediction Models. J. Dairy Sci. 2018, 101, 6655–6674. [Google Scholar] [CrossRef] [PubMed]
- Opio, C.; Gerber, P.; Mottet, A.; Falcucci, A.; Tempio, G.; MacLeod, M.; Vellinga, T.; Henderson, B.; Steinfeld, H. Greenhouse Gas Emissions from Ruminant Supply Chains—A Global Life Cycle Assessment; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013. [Google Scholar]
- Bekele, W.; Guinguina, A.; Zegeye, A.; Simachew, A.; Ramin, M. Contemporary Methods of Measuring and Estimating Methane Emission from Ruminants. Methane 2022, 1, 82–95. [Google Scholar] [CrossRef]
- Gerber, P.J.; Steinfeld, H.; Henderson, B.; Mottet, A.; Opio, C.; Dijkman, J.; Falcucci, A.; Tempio, G. Tackling Climate Change through Livestock: A Global Assessment of Emissions and Mitigation Opportunities; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013. [Google Scholar]
- Kristiansen, S.; Painter, J.; Shea, M. Animal Agriculture and Climate Change in the US and UK Elite Media: Volume, Responsibilities, Causes and Solutions. Environ. Commun. 2021, 15, 153–172. [Google Scholar] [CrossRef] [PubMed]
- Tubiello, F.N.; Salvatore, M.; Rossi, S.; Ferrara, A.; Fitton, N.; Smith, P. The FAOSTAT Database of Greenhouse Gas Emissions from Agriculture. Environ. Res. Lett. 2013, 8, 015009. [Google Scholar] [CrossRef]
- Bačėninaitė, D.; Džermeikaitė, K.; Antanaitis, R. Global Warming and Dairy Cattle: How to Control and Reduce Methane Emission. Animals 2022, 12, 2687. [Google Scholar] [CrossRef]
- Faverdin, P.; Guyomard, H.; Puillet, L.; Forslund, A. Animal Board Invited Review: Specialising and Intensifying Cattle Production for Better Efficiency and Less Global Warming: Contrasting Results for Milk and Meat Co-Production at Different Scales. Animal 2022, 16, 100431. [Google Scholar] [CrossRef]
- Cantor, M.C.; Costa, J.H.C.; Bewley, J.M. Impact of Observed and Controlled Water Intake on Reticulorumen Temperature in Lactating Dairy Cattle. Animals 2018, 8, 194. [Google Scholar] [CrossRef] [PubMed]
- AlZahal, O.; AlZahal, H.; Steele, M.A.; Van Schaik, M.; Kyriazakis, I.; Duffield, T.F.; McBride, B.W. The Use of a Radiotelemetric Ruminal Bolus to Detect Body Temperature Changes in Lactating Dairy Cattle. J. Dairy Sci. 2011, 94, 3568–3574. [Google Scholar] [CrossRef]
- Antanaitis, R.; Zilaitis, V.; Juozaitiene, V.; Stoskus, R.; Televicius, M. Changes in Reticulorumen Content Temperature and PH According to Time of Day and Yearly Seasons. Pol. J. Vet. Sci. 2016, 19, 771–776. [Google Scholar] [CrossRef]
- Šematoviča, I.; Eihvalde, I.; Kairisa, D. Reticulo-Ruminal PH and Temperature Relationship between Dairy Cow Productivity and Milk Composition. Agron. Res. 2017, 15, 576–584. [Google Scholar]
- Van Kessel, J.A.S.; Russell, J.B. The Effect of PH on Ruminal Methanogenesis. FEMS Microbiol. Ecol. 1996, 20, 205–210. [Google Scholar] [CrossRef]
- Aschenbach, J.R.; Penner, G.B.; Stumpff, F.; Gäbel, G. RUMINANT NUTRITION SYMPOSIUM: Role of Fermentation Acid Absorption in the Regulation of Ruminal PH12. J. Anim. Sci. 2011, 89, 1092–1107. [Google Scholar] [CrossRef] [PubMed]
- Hünerberg, M.; McGinn, S.M.; Beauchemin, K.A.; Entz, T.; Okine, E.K.; Harstad, O.M.; McAllister, T.A. Impact of Ruminal PH on Enteric Methane Emissions1. J. Anim. Sci. 2015, 93, 1760–1766. [Google Scholar] [CrossRef] [PubMed]
- Bewley, J.M.; Grott, M.W.; Einstein, M.E.; Schutz, M.M. Impact of Intake Water Temperatures on Reticular Temperatures of Lactating Dairy Cows. J. Dairy Sci. 2008, 91, 3880–3887. [Google Scholar] [CrossRef] [PubMed]
- Grossi, S.; Rossi, L.; Dell’Anno, M.; Biffani, S.; Sgoifo Rossi, C.A. Effects of Heated Drinking Water on the Growth Performance and Rumen Functionality of Fattening Charolaise Beef Cattle in Winter. Animals 2021, 11, 2218. [Google Scholar] [CrossRef]
- Levit, H.; Pinto, S.; Amon, T.; Gershon, E.; Kleinjan-Elazary, A.; Bloch, V.; Ben Meir, Y.A.; Portnik, Y.; Jacoby, S.; Arnin, A.; et al. Dynamic Cooling Strategy Based on Individual Animal Response Mitigated Heat Stress in Dairy Cows. Animal 2021, 15, 100093. [Google Scholar] [CrossRef]
- Islam, A.; Lomax, S.; Doughty, A.; Islam, M.R.; Jay, O.; Thomson, P.; Clark, C. Automated Monitoring of Cattle Heat Stress and Its Mitigation. Front. Anim. Sci. 2021, 2, 737213. [Google Scholar] [CrossRef]
- Antanaitis, R.; Juozaitienė, V.; Televičius, M.; Malašauskienė, D.; Urbutis, M.; Baumgartner, W. Relation of Subclinical Ketosis of Dairy Cows with Locomotion Behaviour and Ambient Temperature. Animals 2020, 10, 2311. [Google Scholar] [CrossRef]
- Grešáková, Ľ.; Holodová, M.; Szumacher-Strabel, M.; Huang, H.; Ślósarz, P.; Wojtczak, J.; Sowińska, N.; Cieślak, A. Mineral Status and Enteric Methane Production in Dairy Cows during Different Stages of Lactation. BMC Vet. Res. 2021, 17, 287. [Google Scholar] [CrossRef]
- Antanaitis, R.; Juozaitienė, V.; Malašauskienė, D.; Televičius, M. Inline Reticulorumen PH as an Indicator of Cows Reproduction and Health Status. Sensors 2020, 20, 1022. [Google Scholar] [CrossRef]
- Moretti, R.; Biffani, S.; Chessa, S.; Bozzi, R. Heat Stress Effects on Holstein Dairy Cows’ Rumination. Animal 2017, 11, 2320–2325. [Google Scholar] [CrossRef]
- Antanaitis, R.; Juozaitienė, V.; Urbonavičius, G.; Malašauskienė, D.; Televičius, M.; Urbutis, M.; Džermeikaitė, K.; Baumgartner, W. Identification of Risk Factors for Lameness Detection with Help of Biosensors. Agriculture 2021, 11, 610. [Google Scholar] [CrossRef]
- Yang, H.; Heirbaut, S.; Jeyanathan, J.; Jing, X.P.; De Neve, N.; Vandaele, L.; Fievez, V. Subacute Ruminal Acidosis Phenotypes in Periparturient Dairy Cows Differ in Ruminal and Salivary Bacteria and in the in Vitro Fermentative Activity of Their Ruminal Microbiota. J. Dairy Sci. 2022, 105, 3969–3987. [Google Scholar] [CrossRef]
- Chagunda, M.G.; Larsen, T.; Bjerring, M.; Ingvartsen, K.L. L-Lactate Dehydrogenase and N-Acetyl-β-D-Glucosaminidase Activities in Bovine Milk as Indicators of Non-Specific Mastitis. J. Dairy Res. 2006, 73, 431–440. [Google Scholar] [CrossRef]
- Sorg, D. Measuring Livestock CH4 Emissions with the Laser Methane Detector: A Review. Methane 2022, 1, 38–57. [Google Scholar] [CrossRef]
- Pickering, N.K.; Chagunda, M.G.G.; Banos, G.; Mrode, R.; McEwan, J.C.; Wall, E. Genetic Parameters for Predicted Methane Production and Laser Methane Detector Measurements1. J. Anim. Sci. 2015, 93, 11–20. [Google Scholar] [CrossRef]
- Reintke, J.; Brügemann, K.; Yin, T.; Engel, P.; Wagner, H.; Wehrend, A.; König, S. Assessment of Methane Emission Traits in Ewes Using a Laser Methane Detector: Genetic Parameters and Impact on Lamb Weaning Performance. Arch. Anim. Breed. 2020, 63, 113–123. [Google Scholar] [CrossRef]
- Vranken, H.; Suenkel, M.; Hargreaves, P.; Chew, L.; Towers, E. Reduction of Enteric Methane Emission in a Commercial Dairy Farm by a Novel Feed Supplement. Open J. Anim. Sci. 2019, 9, 286–296. [Google Scholar] [CrossRef]
- Denninger, T.M.; Schwarm, A.; Dohme-Meier, F.; Münger, A.; Bapst, B.; Wegmann, S.; Grandl, F.; Vanlierde, A.; Sorg, D.; Ortmann, S.; et al. Accuracy of Methane Emissions Predicted from Milk Mid-Infrared Spectra and Measured by Laser Methane Detectors in Brown Swiss Dairy Cows. J. Dairy Sci. 2020, 103, 2024–2039. [Google Scholar] [CrossRef]
- Pinto, A.; Yin, T.; Reichenbach, M.; Bhatta, R.; Malik, P.K.; Schlecht, E.; König, S. Enteric Methane Emissions of Dairy Cattle Considering Breed Composition, Pasture Management, Housing Conditions and Feeding Characteristics along a Rural-Urban Gradient in a Rising Megacity. Agriculture 2020, 10, 628. [Google Scholar] [CrossRef]
- Iseki, T. A Portable Remote Methane Detector Using an InGaAsP DFB Laser. Environ. Geol. 2004, 46, 1064–1069. [Google Scholar] [CrossRef]
- Roessler, R.; Schlecht, E. Application of the Laser Methane Detector for Measurements in Freely Grazing Goats: Impact on Animals’ Behaviour and Methane Emissions. Animal 2021, 15, 100070. [Google Scholar] [CrossRef] [PubMed]
- Lana, R.P.; Russell, J.B.; Van Amburgh, M.E. The Role of PH in Regulating Ruminal Methane and Ammonia Production. J. Anim. Sci. 1998, 76, 2190–2196. [Google Scholar] [CrossRef] [PubMed]
- Janssen, P.H. Influence of Hydrogen on Rumen Methane Formation and Fermentation Balances through Microbial Growth Kinetics and Fermentation Thermodynamics. Anim. Feed Sci. Technol. 2010, 160, 1–22. [Google Scholar] [CrossRef]
- Dijkstra, J.; Ellis, J.L.; Kebreab, E.; Strathe, A.B.; López, S.; France, J.; Bannink, A. Ruminal PH Regulation and Nutritional Consequences of Low PH. Anim. Feed Sci. Technol. 2012, 172, 22–33. [Google Scholar] [CrossRef]
- Benaouda, M.; Martin, C.; Li, X.; Kebreab, E.; Hristov, A.N.; Yu, Z.; Yáñez-Ruiz, D.R.; Reynolds, C.K.; Crompton, L.A.; Dijkstra, J.; et al. Evaluation of the Performance of Existing Mathematical Models Predicting Enteric Methane Emissions from Ruminants: Animal Categories and Dietary Mitigation Strategies. Anim. Feed Sci. Technol. 2019, 255, 114207. [Google Scholar] [CrossRef]
- Hook, S.E.; Steele, M.A.; Northwood, K.S.; Dijkstra, J.; France, J.; Wright, A.-D.G.; McBride, B.W. Impact of Subacute Ruminal Acidosis (SARA) Adaptation and Recovery on the Density and Diversity of Bacteria in the Rumen of Dairy Cows. FEMS Microbiol. Ecol. 2011, 78, 275–284. [Google Scholar] [CrossRef]
- Pragna, P.; Chauhan, S.S.; Sejian, V.; Leury, B.J.; Dunshea, F.R. Climate Change and Goat Production: Enteric Methane Emission and Its Mitigation. Animals 2018, 8, 235. [Google Scholar] [CrossRef]
- Ekine-Dzivenu, C.C.; Mrode, R.; Oyieng, E.; Komwihangilo, D.; Lyatuu, E.; Msuta, G.; Ojango, J.M.K.; Okeyo, A.M. Evaluating the Impact of Heat Stress as Measured by Temperature-Humidity Index (THI) on Test-Day Milk Yield of Small Holder Dairy Cattle in a Sub-Sahara African Climate. Livest. Sci. 2020, 242, 104314. [Google Scholar] [CrossRef]
- Zhao, S.; Min, L.; Zheng, N.; Wang, J. Effect of Heat Stress on Bacterial Composition and Metabolism in the Rumen of Lactating Dairy Cows. Animals 2019, 9, 925. [Google Scholar] [CrossRef]
- Bernabucci, U.; Lacetera, N.; Baumgard, L.H.; Rhoads, R.P.; Ronchi, B.; Nardone, A. Metabolic and Hormonal Acclimation to Heat Stress in Domesticated Ruminants. Animal 2010, 4, 1167–1183. [Google Scholar] [CrossRef]
- Meneses, J.A.M.; de Sá, O.A.A.L.; Coelho, C.F.; Pereira, R.N.; Batista, E.D.; Ladeira, M.M.; Casagrande, D.R.; Gionbelli, M.P. Effect of Heat Stress on Ingestive, Digestive, Ruminal and Physiological Parameters of Nellore Cattle Feeding Low- or High-Energy Diets. Livest. Sci. 2021, 252, 104676. [Google Scholar] [CrossRef]
- Correia Sales, G.F.; Carvalho, B.F.; Schwan, R.F.; de Figueiredo Vilela, L.; Moreno Meneses, J.A.; Gionbelli, M.P.; da Silva Ávila, L.C. Heat Stress Influence the Microbiota and Organic Acids Concentration in Beef Cattle Rumen. J. Therm. Biol. 2021, 97, 102897. [Google Scholar] [CrossRef]
- Castro-Costa, A.; Salama, A.A.K.; Moll, X.; Aguiló, J.; Caja, G. Using Wireless Rumen Sensors for Evaluating the Effects of Diet and Ambient Temperature in Nonlactating Dairy Goats. J. Dairy Sci. 2015, 98, 4646–4658. [Google Scholar] [CrossRef]
| Nutrients | Composition |
| Dry matter (DM) | 49% |
| Neutral detergent fiber | 28 (% of DM) |
| Acid detergent fiber | 20 (% of DM) |
| Crude protein | 16 (% of DM) |
| Non-fiber carbohydrates Net energy for lactation | 39 (% of DM) 1.80 Mcal/kg DM |
| Parameter | Units | Interval of Measurements |
|---|---|---|
| Reticulorumen pH | Value | April–August 2022 |
| Reticulorumen temperature | °C | April–August 2022 |
| Reticulorumen temperature without drinking cycles | °C | April–August 2022 |
| Ambient temperature | °C | April–August 2022 |
| Ambient relative humidity | % | April–August 2022 |
| Cow activity | Steps/hour | April–August 2022 |
| Heat index | - | April–August 2022 |
| Temperature–humidity index | - | April–August 2022 |
| Methane emission | ppm | Estimated from multiple breath measurements with laser methane detector (LMD) HESAI HS4000 from April to August 2022 (at the same time: 09:00, 10:00, 11:00 a.m.). LMD recordings were 3–5 min |
| Ph Class | CH4 (ppm) | RR Temperature (°C) | Cow Activity (Steps/Hour) | RR Temperature without Drinking Cycles (°C) | Heat Index | Relative Humidity % | Ambient Temperature (°C) | THI |
|---|---|---|---|---|---|---|---|---|
| 1. pH < 6.22 | 187.64 ± 20.33 ** b | 38.59 ± 0.16 ** b | 6.52 ± 0.83 | 39.46 ± 0.03 | 2.08 ± 0.60 | 70.95 ± 2.81 ** b | 17.82 ± 0.17 * b | 63.16 ± 0.32 ** b |
| 2. pH 6.22–6.42 | 348.64 ± 56.50 ** a | 37.51 ± 0.36 ** a | 6.30 ± 0.54 | 39.39 ± 0.02 | 1.60 ± 0.21 | 56.08 ± 4.91 ** a | 17.12 ± 0.11 * a | 61.60 ±0.03 ** a |
| Date | ||||
|---|---|---|---|---|
| Hours | 13/05 (A) | 08/06 (B) | 15/06 (C) | 22/06 (D) |
| 1. 9 (a) | 166.28 ± 3.00 *c; **D | 177.62 ± 9.51 | 197.46 ± 24.48 | 282.56 ± 6.86 **A |
| 2. 10 (b) | 237.05 ± 2.10 **c | 210.24 ± 4.38 | 261.01 ± 9.47 | 210.13 ± 4.08 |
| 3. 11 (c) | 91.49 ± 6.34 **b; *a***B, **C, *D | 215.98 ± 2.29 ***A | 221.99 ± 7.17 **A | 221.37 ± 5.03 *A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antanaitis, R.; Anskienė, L.; Rapaliutė, E.; Bilskis, R.; Džermeikaitė, K.; Bačėninaitė, D.; Juškienė, V.; Juška, R.; Meškinytė, E. Relationship between Reticulorumen Parameters Measured in Real Time and Methane Emission and Heat Stress Risk in Dairy Cows. Animals 2022, 12, 3257. https://doi.org/10.3390/ani12233257
Antanaitis R, Anskienė L, Rapaliutė E, Bilskis R, Džermeikaitė K, Bačėninaitė D, Juškienė V, Juška R, Meškinytė E. Relationship between Reticulorumen Parameters Measured in Real Time and Methane Emission and Heat Stress Risk in Dairy Cows. Animals. 2022; 12(23):3257. https://doi.org/10.3390/ani12233257
Chicago/Turabian StyleAntanaitis, Ramūnas, Lina Anskienė, Eglė Rapaliutė, Ronaldas Bilskis, Karina Džermeikaitė, Dovilė Bačėninaitė, Violeta Juškienė, Remigijus Juška, and Edita Meškinytė. 2022. "Relationship between Reticulorumen Parameters Measured in Real Time and Methane Emission and Heat Stress Risk in Dairy Cows" Animals 12, no. 23: 3257. https://doi.org/10.3390/ani12233257
APA StyleAntanaitis, R., Anskienė, L., Rapaliutė, E., Bilskis, R., Džermeikaitė, K., Bačėninaitė, D., Juškienė, V., Juška, R., & Meškinytė, E. (2022). Relationship between Reticulorumen Parameters Measured in Real Time and Methane Emission and Heat Stress Risk in Dairy Cows. Animals, 12(23), 3257. https://doi.org/10.3390/ani12233257







