Evaluation of Strategies to Reduce Equine Strongyle Infective Larvae on Pasture and Study of Larval Migration and Overwintering in a Nordic Climate
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Faecal Egg Counts and Larval Cultures
2.2. Grass Sampling and Harvesting of Strongyle Larvae
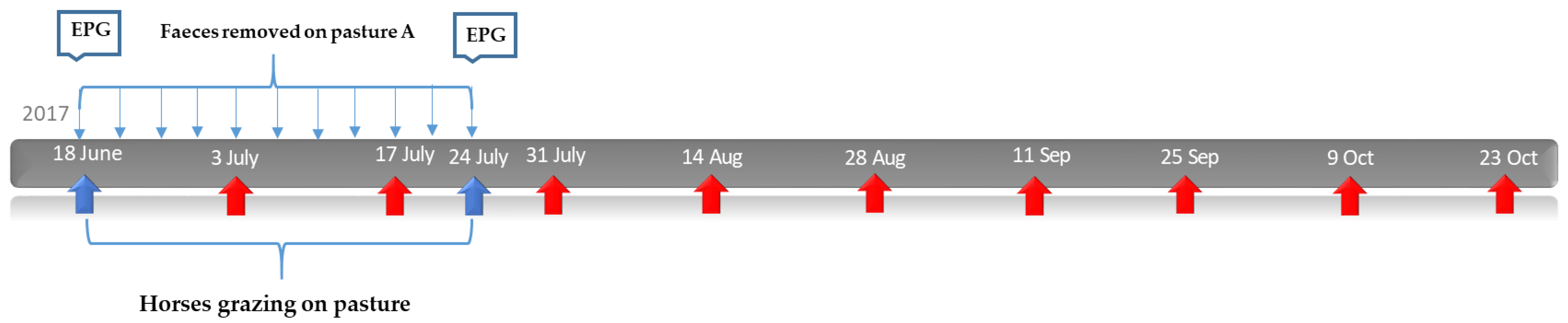
2.3. Study Design: Effect of Faecal Removal on Parasite Larvae on Pasture
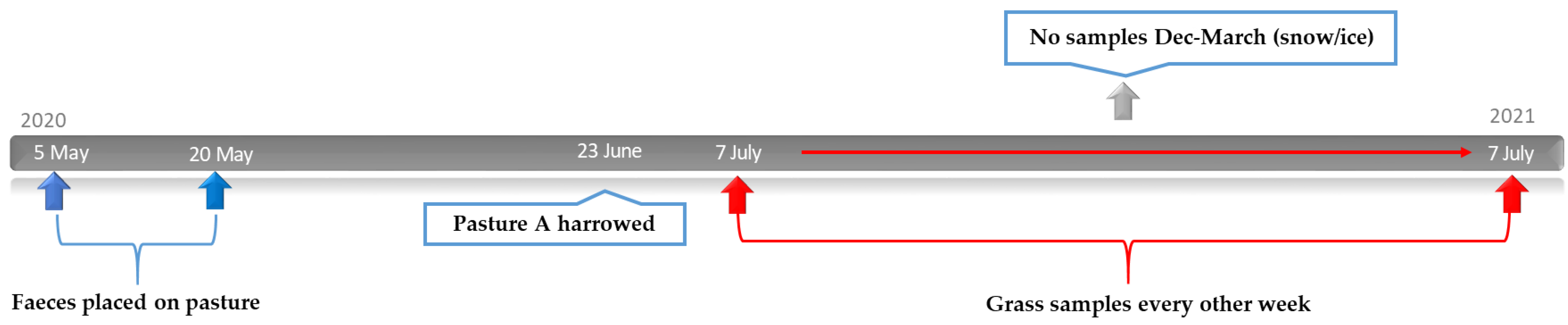
2.4. Study Design: Effect of Harrowing on Parasite Larvae on Pasture
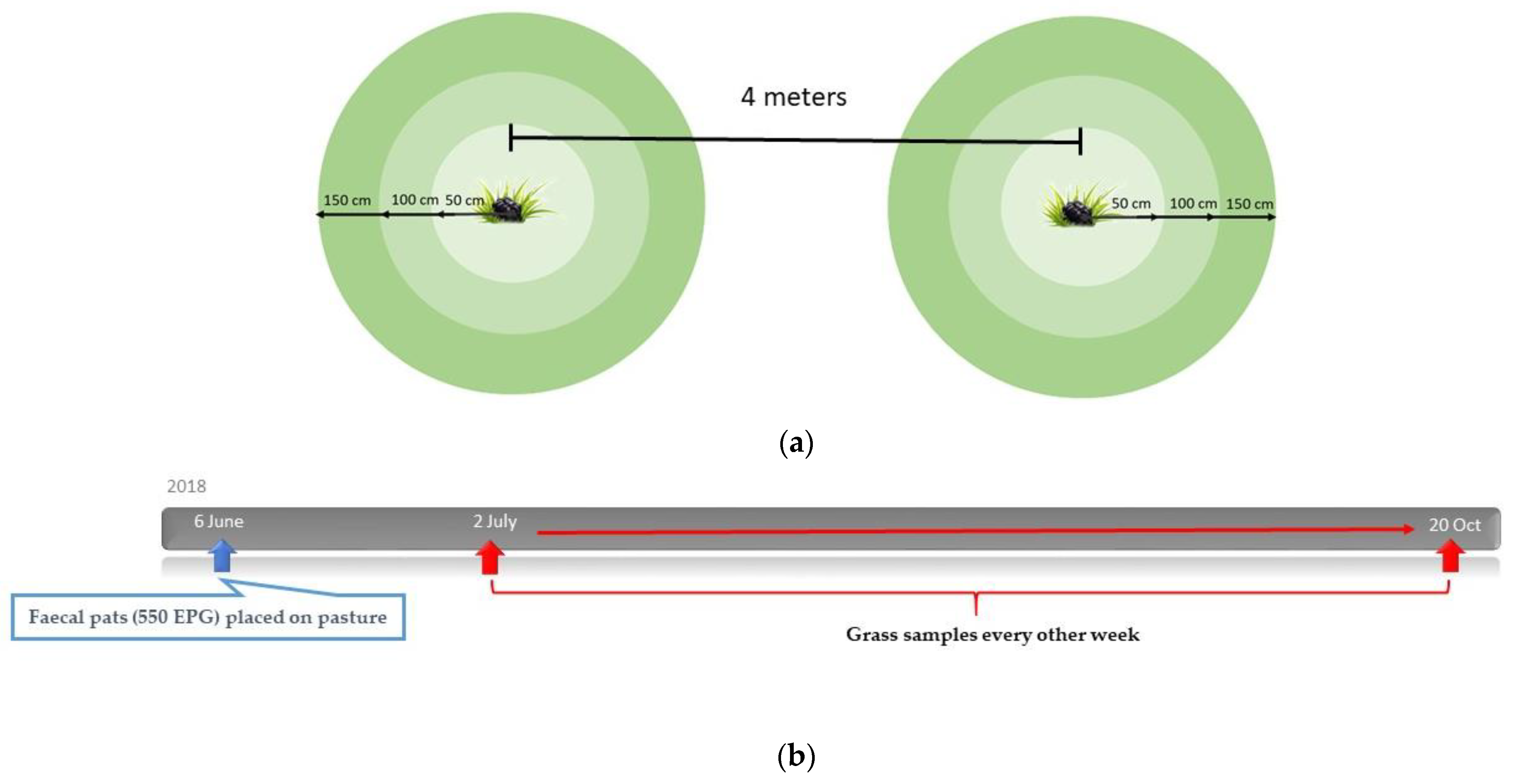
2.5. Study Design: Migration of Larvae
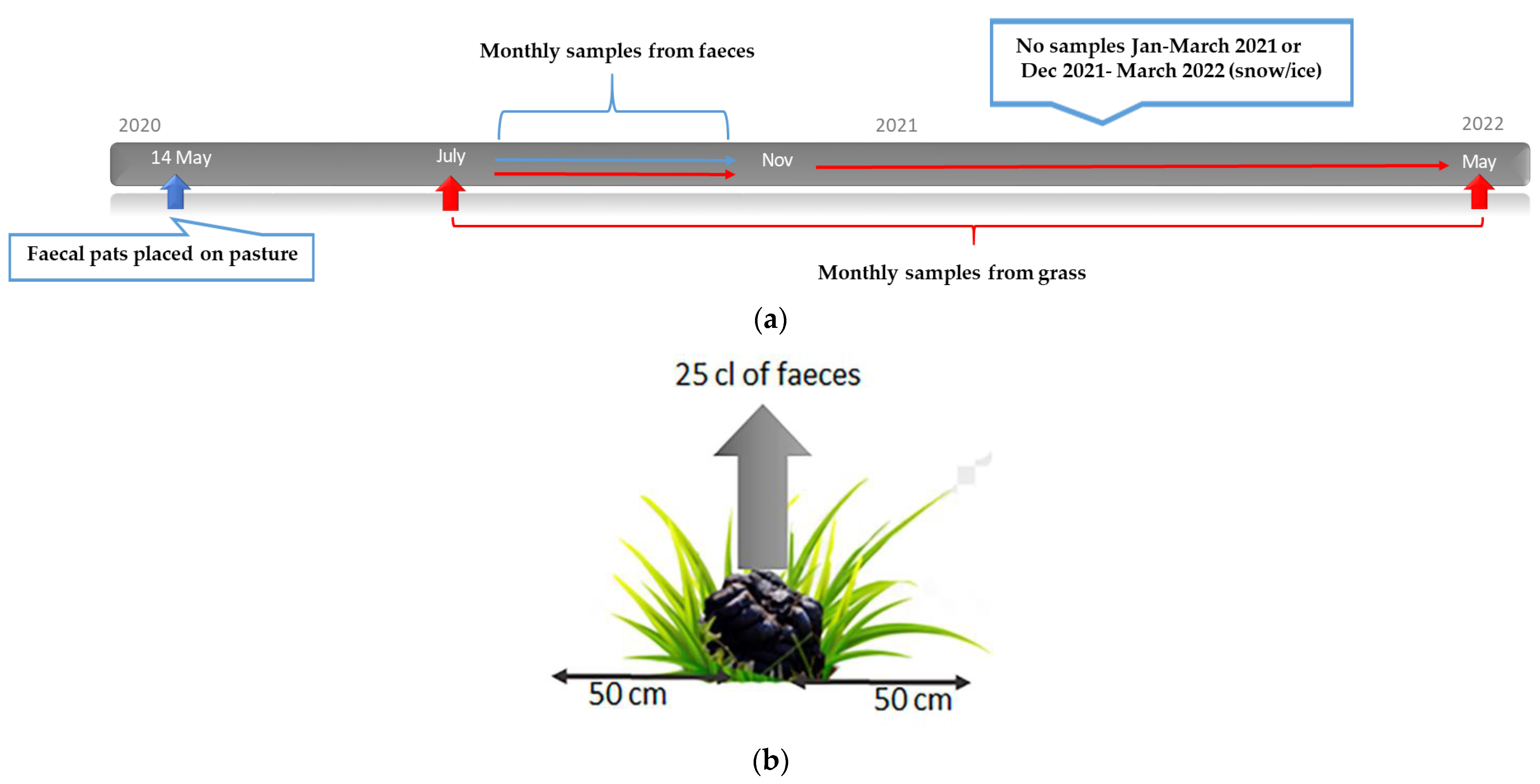
2.6. Study Design: Survival and Overwintering of Larvae
2.7. Collection of Weather Data
2.8. Statistical Analysis
3. Results
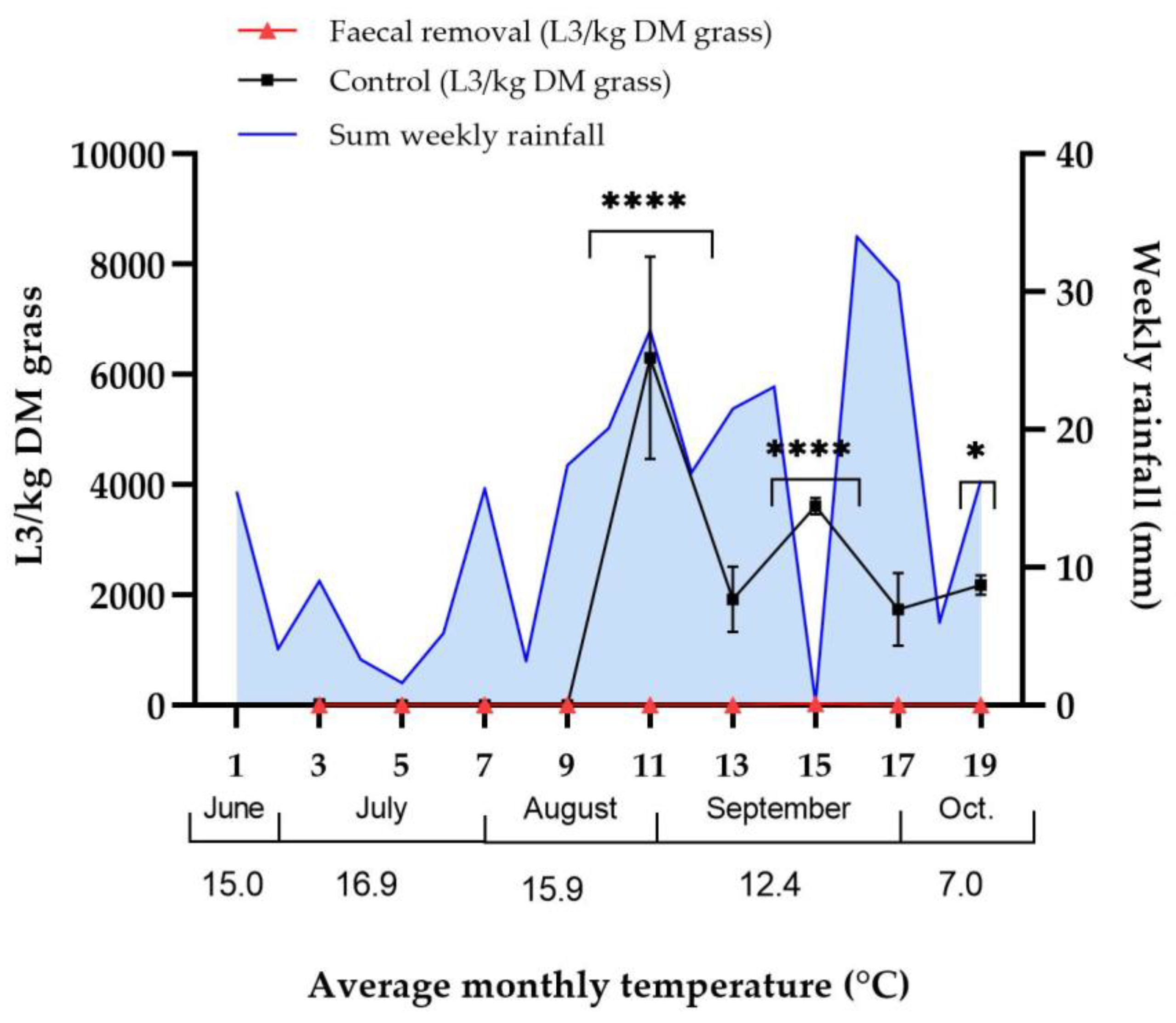
3.1. Effect of Faecal Removal on Strongyle Larvae on Pasture
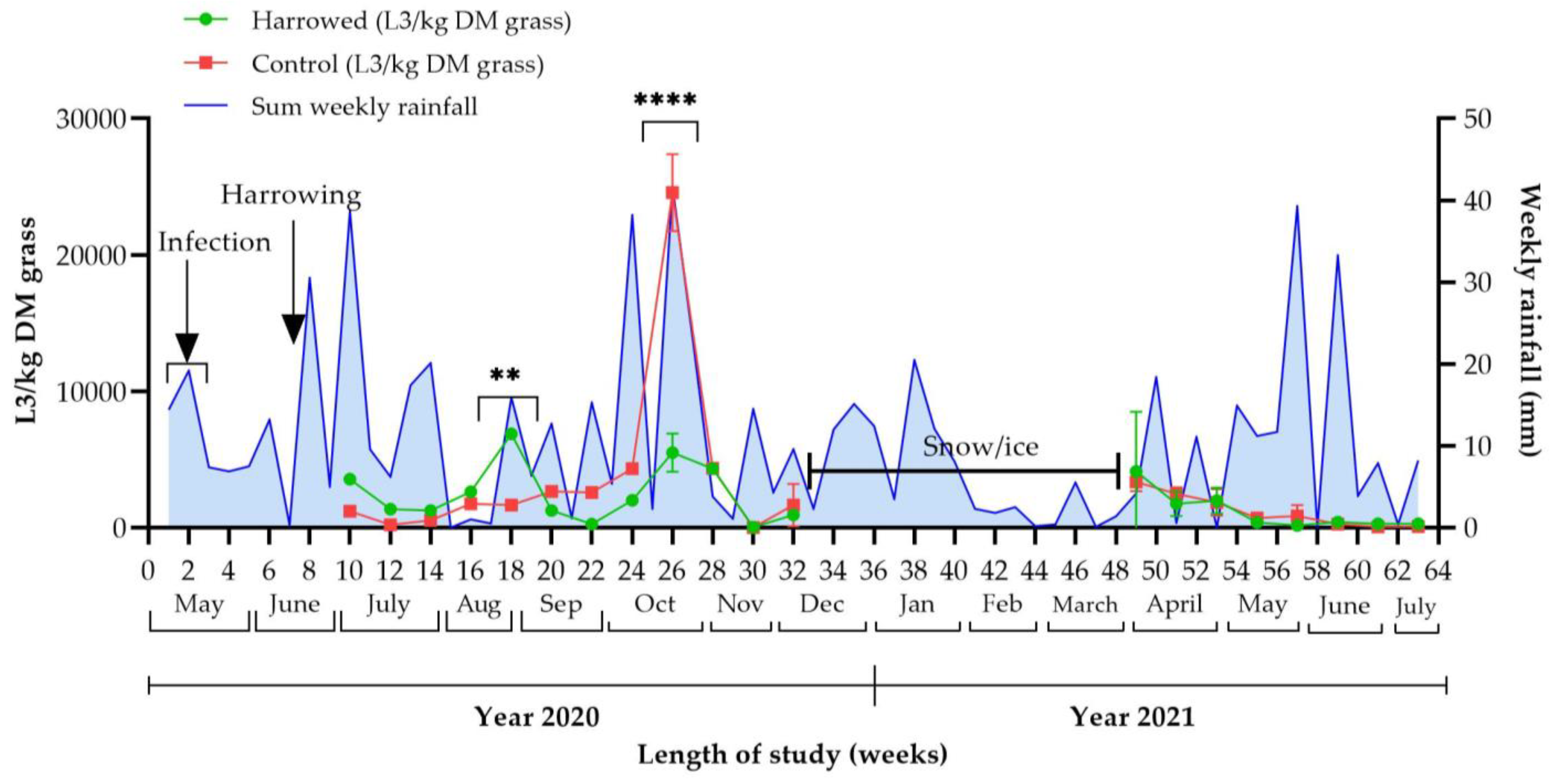
3.2. Effect of Harrowing on Strongyle Larvae on Pasture
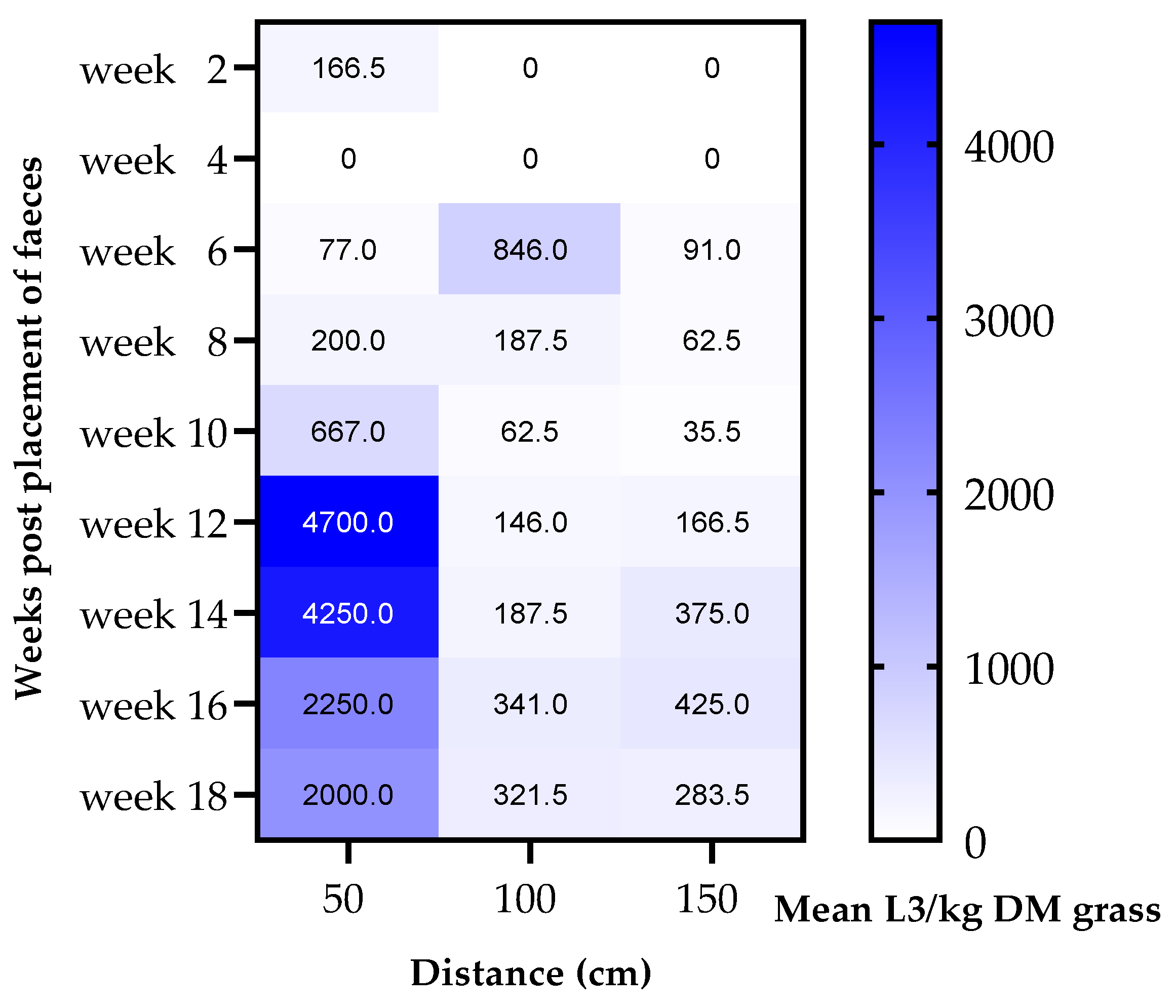
3.3. Migration of Strongyle Larvae
3.4. Survival and Overwintering of Small and Large Strongyle Larvae
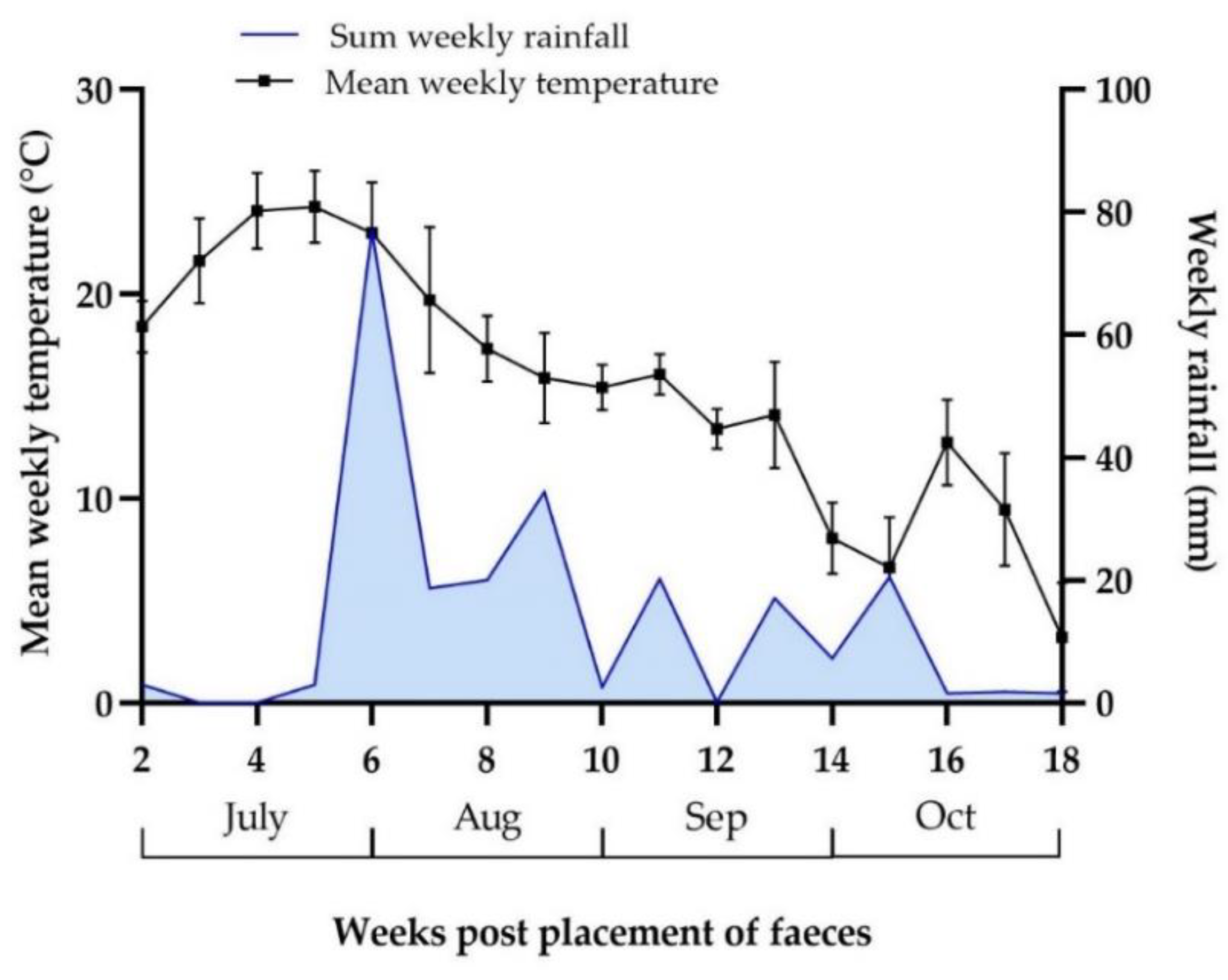
3.4.1. Weather Data
3.4.2. Faecal Samples
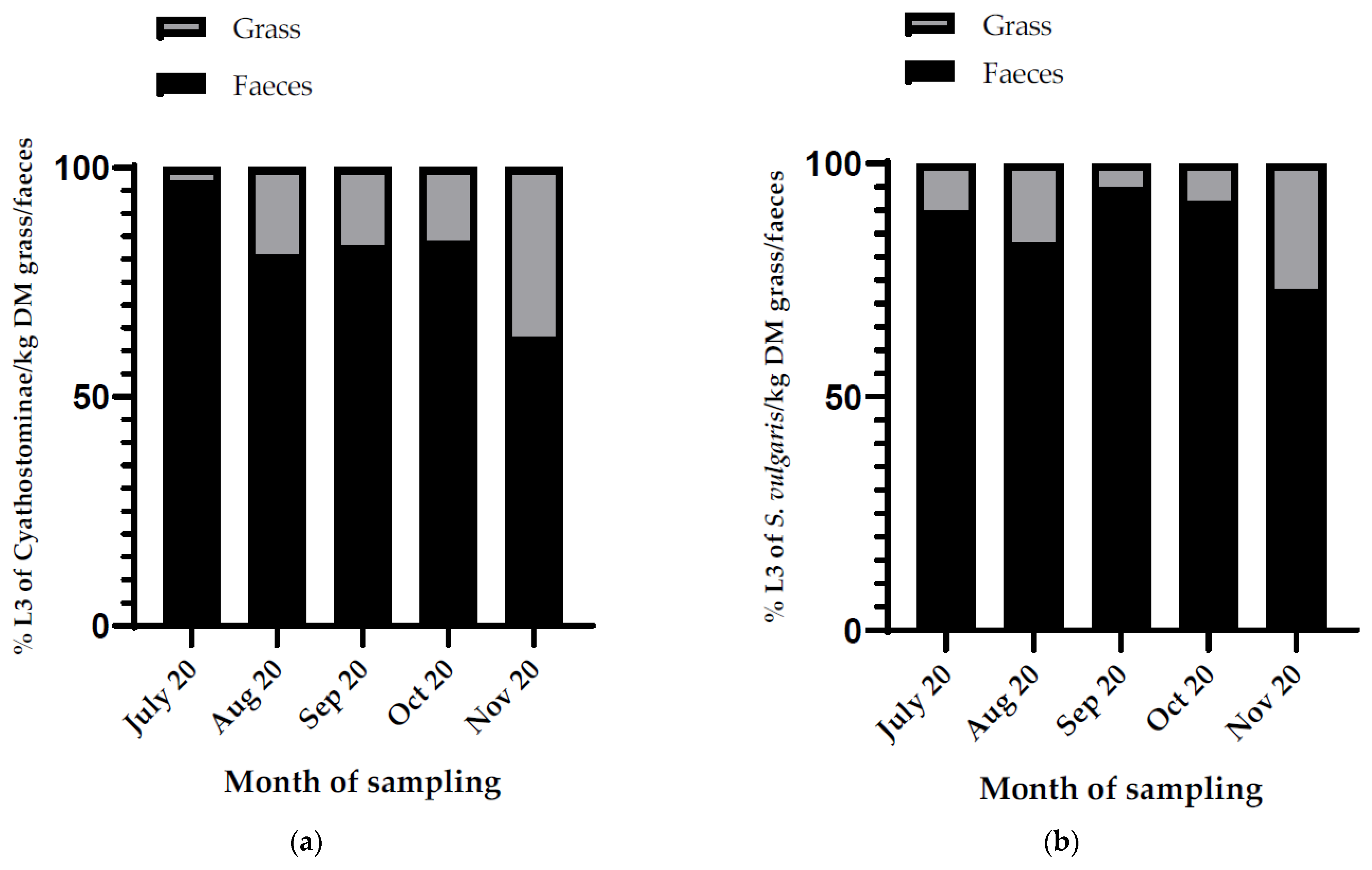
3.4.3. Cyathostominae in Grass Samples
3.4.4. S. vulgaris in Grass Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reinemeyer, C.R.; Nielsen, M.K. Parasitism and colic. Vet. Clin. N. Am. Equine Pract. 2009, 25, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Curtis, L.; Burford, J.H.; England, G.C.W.; Freeman, S.L. Risk factors for acute abdominal pain (colic) in the adult horse: A scoping review of risk factors, and a systematic review of the effect of management-related changes. PLoS ONE 2019, 14, e0219307. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, R.M. Drug resistance in nematodes of veterinary importance: A status report. Trends Parasitol. 2004, 20, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.K.; Reinemeyer, C.R.; Donecker, J.M.; Leathwick, D.M.; Marchiondo, A.A.; Kaplan, R.M. Anthelmintic resistance in equine parasites--current evidence and knowledge gaps. Vet. Parasitol. 2014, 204, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.K.; Banahan, M.; Kaplan, R.M. Importation of macrocyclic lactone resistant cyathostomins on a US thoroughbred farm. Int. J. Parasitol. Drugs Drug Resist. 2020, 14, 99–104. [Google Scholar] [CrossRef]
- Duncan, J.L.; Love, S. Preliminary observations on an alternative strategy for the control of horse strongyles. Equine Vet. J. 1991, 23, 226–228. [Google Scholar] [CrossRef]
- Gomez, H.H.; Georgi, J.R. Equine helminth infections: Control by selective chemotherapy. Equine Vet. J. 1991, 23, 198–200. [Google Scholar] [CrossRef]
- Roelfstra, L.; Quartier, M.; Pfister, K. Preliminary Data from Six Years of Selective Anthelmintic Treatment on Five Horse Farms in France and Switzerland. Animals 2020, 10, 2395. [Google Scholar] [CrossRef]
- Geurden, T.; de Keersmaecker, F.; de Keersmaecker, S.; Claerebout, E.; Leathwick, D.M.; Nielsen, M.K.; Sauermann, C.W. Three-year study to evaluate an anthelmintic treatment regimen with reduced treatment frequency in horses on two study sites in Belgium. Vet. Parasitol. 2021, 298, 109538. [Google Scholar] [CrossRef]
- Elghryani, N.; Duggan, V.; Relf, V.; de Waal, T. Questionnaire survey on helminth control practices in horse farms in Ireland. Parasitology 2019, 146, 873–882. [Google Scholar] [CrossRef]
- Hedberg-Alm, Y.; Penell, J.; Riihimaki, M.; Osterman-Lind, E.; Nielsen, M.K.; Tydén, E. Parasite Occurrence and Parasite Management in Swedish Horses Presenting with Gastrointestinal Disease-A Case-Control Study. Animals 2020, 10, 638. [Google Scholar] [CrossRef] [PubMed]
- Horses and Horse Establishments in 2016. Available online: https://www.scb.se/contentassets/3a26a20c92ee42c993081cc209972f56/jo0107_2016m06_sm_jo24sm1701.pdf (accessed on 15 April 2022).
- Monrad, J.; Bjørn, H.; Craven, J.; Perman, M.; Eiersted, L. Parasitologisk diagnostik i stordyrspraksis: Kvantitativ gødningsundersøgelse med henblik på involdsorm hos heste. Dan. Vet. 1999, 82, 118–119. [Google Scholar]
- Coles, G.C.; Bauer, C.; Borgsteede, F.H.; Geerts, S.; Klei, T.R.; Taylor, M.A.; Waller, P.J. World Association for the Advancement of Veterinary Parasitology (W.A.A.V.P.) methods for the detection of anthelmintic resistance in nematodes of veterinary importance. Vet. Parasitol. 1992, 44, 35–44. [Google Scholar] [CrossRef]
- Bellaw, L.J.; Nielsen, K.M. Evaluation of Baermann apparatus sedimentation time on recovery of Strongylus vulgaris and S. edentatus third stage larvae from equine coprocultures. Vet. Parasitol. 2015, 211, 99–101. [Google Scholar] [CrossRef]
- Henriksen, S.A.; Korsholm, H. A method for culture and recovery of gastrointestinal strongyle larvae. Scand. J. Vet. Sci. 1983, 35, 429–430. [Google Scholar]
- Thienpont, D.; Rochette, F.; Vanparijs, O.F.J. Diagnosing Helminthiasis through Coprological Examination, 2nd ed.; Janssen Research Foundation: Beerse, Belgium, 1986; p. 205. [Google Scholar]
- Taylor, E.L. Technique for the estimation of pasture infestation by strongyloid larvae. Parasitology 1939, 31, 473–478. [Google Scholar] [CrossRef]
- Herd, R.P. Epidemiology and control of equine strongylosis at Newmarket. Equine Vet. J. 1986, 18, 447–452. [Google Scholar] [CrossRef]
- Corbett, C.J.; Love, S.; Moore, A.; Burden, F.A.; Matthews, J.B.; Denwood, M.J. The effectiveness of faecal removal methods of pasture management to control the cyathostomin burden of donkeys. Parasit. Vectors 2014, 7, 48. [Google Scholar] [CrossRef]
- Tzelos, T.; Barbeito, J.S.; Nielsen, M.K.; Morgan, E.R.; Hodgkinson, J.E.; Matthews, J.B. Strongyle egg reappearance period after moxidectin treatment and its relationship with management factors in UK equine populations. Vet. Parasitol. 2017, 237, 70–76. [Google Scholar] [CrossRef]
- Lloyd, S.; Smith, J.; Connan, R.M.; Hatcher, M.A.; Hedges, T.R.; Humphrey, D.J.; Jones, A.C. Parasite control methods used by horse owners: Factors predisposing to the development of anthelmintic resistance in nematodes. Vet. Rec. 2000, 146, 487–492. [Google Scholar] [CrossRef]
- Hillyer, M.H.; Taylor, F.G.; French, N.P. A cross-sectional study of colic in horses on thoroughbred training premises in the British Isles in 1997. Equine Vet. J. 2001, 33, 380–385. [Google Scholar] [CrossRef]
- Comer, K.C.; Hillyer, M.H.; Coles, G.C. Anthelmintic use and resistance on thoroughbred training yards in the UK. Vet. Rec. 2006, 158, 596–598. [Google Scholar] [CrossRef]
- Papini, R.A.; de Bernart, F.M.; Sgorbini, M. A Questionnaire Survey on Intestinal Worm Control Practices in Horses in Italy. J. Equine Vet. Sci. 2015, 35, 70–75. [Google Scholar] [CrossRef]
- Nielsen, M.K.; Branan, M.A.; Wiedenheft, A.M.; Digianantonio, R.; Garber, L.P.; Kopral, C.A.; Phillippi-Taylor, A.M.; Traub-Dargatz, J.L. Parasite control strategies used by equine owners in the United States: A national survey. Vet. Parasitol. 2018, 250, 45–51. [Google Scholar] [CrossRef]
- Lind, E.O.; Rautalinko, E.; Uggla, A.; Waller, P.J.; Morrison, D.A.; Hoglund, J. Parasite control practices on Swedish horse farms. Acta Vet. Scand. 2007, 49, 25. [Google Scholar] [CrossRef]
- Lindfors, R. Preventive Measures against Horse Strongyles and Their Ability to Replace Anthelmintics. Master’s Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 10 May 2019. [Google Scholar]
- Mfitilodze, M.W.; Hutchinson, G.W. Development and survival of free-living stages of equine strongyles under laboratory conditions. Vet. Parasitol. 1987, 23, 121–133. [Google Scholar] [CrossRef]
- Corning, S. Equine cyathostomins: A review of biology, clinical significance and therapy. Parasit Vectors. 2009, 2 (Suppl. 2), S1. [Google Scholar] [CrossRef]
- Ramsey, Y.H.; Christley, R.M.; Matthews, J.B.; Hodgkinson, J.E.; McGoldrick, J.; Love, S. Seasonal development of Cyathostominae larvae on pasture in a northern temperate region of the United Kingdom. Vet. Parasitol. 2004, 119, 307–318. [Google Scholar] [CrossRef]
- Stratford, C.H.; Lester, H.E.; Morgan, E.R.; Pickles, K.J.; Relf, V.; McGorum, B.C.; Matthews, J.B. A questionnaire study of equine gastrointestinal parasite control in Scotland. Equine Vet. J. 2014, 46, 25–31. [Google Scholar] [CrossRef]
- Slocombe, O. A harrowing experience with strongyles in ponies. Can. Vet. J. 1988, 20, 136–140. [Google Scholar]
- Keating, D.L.; Lehman, J.S.; Burk, S.V. Salivary Cortisol, Equine Characteristics, and Management Factors Associated with Strongyle-Type Egg Shedding of Ohio Horses. J. Equine Vet. Sci. 2021, 101, 103431. [Google Scholar] [CrossRef] [PubMed]
- Swiderski, C.E.; French, D.D. Paradigms for Parasite Control in Adult Horse Populations: A Review. In Proceedings of the AAEP Annual Convention, San Diego, CA, USA, 10 December 2008. [Google Scholar]
- Nielsen, M.K.; Reinemeyer, C.R. Handbook of Equine Parasite Control, 2nd ed.; Wiley-Blackwell: London, UK, 2018; pp. 75–76. [Google Scholar]
- Giedt, E.J.; Hiney, K. Controlling Common Internal Parasites of the Horse. Available online: https://extension.okstate.edu/fact-sheets/controlling-common-internal-parasites-of-the-horse.html (accessed on 10 September 2022).
- Parnell, I.W. Notes on the survival of the eggs and free-living larvae of sclerostomes on pasture. Sci. Agric. 1936, 16, 391–397. [Google Scholar]
- Baker, D.W.; Salisbury, G.W.; Britton, J.W. Control of equine strongylosis. Part 1. The effect of natural factors on the development of strongylosis in foals. Cornell Vet. 1939, 29, 297–308. [Google Scholar]
- Langrová, I.; Jankovska, I.; Borovský, M.; Fiala, T. Effect of climatic influences on the migrations of infective larvae of Cyathostominae. Vet. Med. 2003, 48, 18–24. [Google Scholar] [CrossRef]
- Kuzmina, T.A.; Kuzmin, Y.I.; Kharchenko, V.A. Field study on the survival, migration and overwintering of infective larvae of horse strongyles on pasture in central Ukraine. Vet. Parasitol. 2006, 141, 264–272. [Google Scholar] [CrossRef]
- Grønvold, J. A field experiment on rain splash dispersal of infective larvae of Ostertagia ostertagi (Trichostrongylidae) from cow pats to surrounding grass. Acta Vet. Scand. 1987, 28, 459–461. [Google Scholar] [CrossRef]
- Wang, T.; Vineer, H.R.; Redman, E.; Morosetti, A.; Chen, R.; McFarland, C.; Colwell, D.D.; Morgan, E.-R.; Gilleard, J.S. An improved model for the population dynamics of cattle gastrointestinal nematodes on pasture: Parameterisation and field validation for Ostertagia ostertagi and Cooperia oncophora in northern temperate zones. Veterinary Parasitol. 2022, 310. [Google Scholar] [CrossRef]
- Ödberg, F.O.; Francis-Smith, K. A study on eliminative and grazing behaviour -the use of the field by captive horses. Equine Vet. J. 1976, 8, 147–149. [Google Scholar] [CrossRef]
- Medica, D.L.; Hanaway, M.J.; Ralston, S.L.; Sukhdeo, M.V.K. Grazing behavior of horses on pasture: Predisposition to strongyloid infection? J. Equine Vet. Sci. 1996, 16, 421–427. [Google Scholar] [CrossRef]
- Nielsen, M.K.; Kaplan, R.M.; Thamsborg, S.M.; Monrad, J.; Olsen, S.N. Climatic influences on development and survival of free-living stages of equine strongyles: Implications for worm control strategies and managing anthelmintic resistance. Vet. J. 2007, 174, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Dimander, S.O.; Hoglund, J.; Waller, P.J. The origin and overwintering survival of the free living stages of cattle parasites in Sweden. Acta Vet. Scand. 1999, 40, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Avramenko, R.W.; Redman, E.M.; Wit, J.; Gilleard, J.S.; Colwell, D.D. High levels of third-stage larvae (L3) overwinter survival for multiple cattle gastrointestinal nematode species on western Canadian pastures as revealed by ITS2 rDNA metabarcoding. Parasites Vectors 2020, 13, 458. [Google Scholar] [CrossRef]
- Hasslinger, M.A. Effect of various temperatures on eggs and larvae of equine Strongyloidea under laboratory conditions and the behavior of these exogenous stages in the pasture. Berl. Munch Tierarztl. Wochenschr. 1981, 94, 1–5. (In German) [Google Scholar] [PubMed]
- Ogbourne, C.P.; Duncan, J.L. Strongylus Vulgaris in the Horse: Its Biology and Veterinary Importance, 2nd ed.; Commonwealth Agricultural Bureaux: Slough, UK, 1985; pp. 1–68. [Google Scholar]
- Kaspar, A.; Pfister, K.; Nielsen, M.K.; Silaghi, C.; Fink, H.; Scheuerle, M.C. Detection of Strongylus vulgaris in equine faecal samples by real-time PCR and larval culture—Method comparison and occurrence assessment. BMC Vet. Res. 2017, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Duncan, J.L.; Pirie, H.M. The pathogenesis of single experimental infections with Strongylus vulgaris in foals. Res. Vet. Sci. 1975, 18, 82–93. [Google Scholar] [CrossRef]
- Pihl, T.H.; Nielsen, M.K.; Olsen, S.N.; Leifsson, P.S.; Jacobsen, S. Nonstrangulating intestinal infarctions associated with Strongylus vulgaris: Clinical presentation and treatment outcomes of 30 horses (2008–2016). Equine Vet. J. 2018, 50, 474–480. [Google Scholar] [CrossRef]

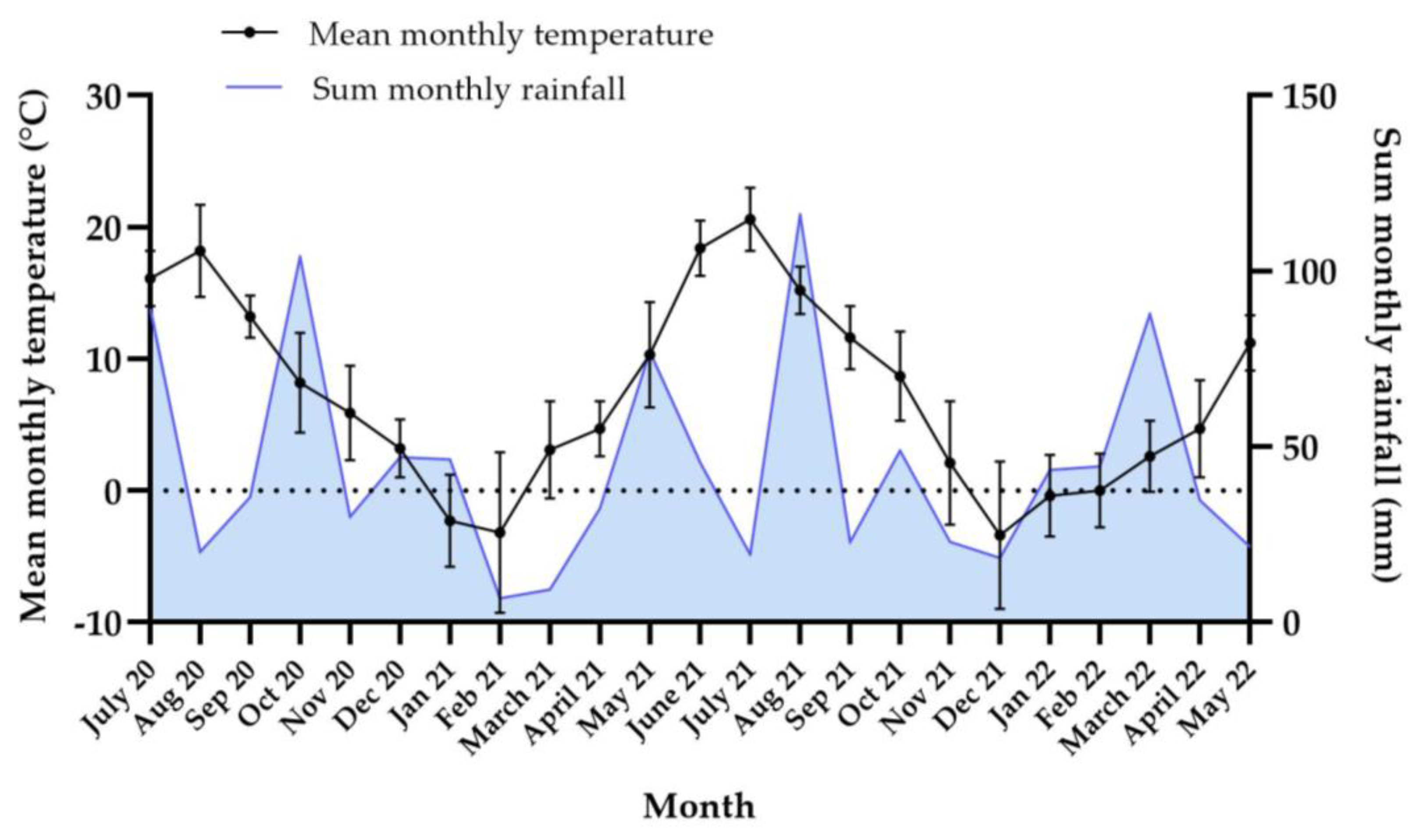

| Year | Jan. | Feb. | March | April | May | June | July | Aug. | Sep. | Oct. | Nov. | Dec. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2020 | --- | --- | --- | --- | 9.2 (3.1) | 18.4 (3.4) | 16.1 (2.1) | 18.2 (3.5) | 13.2 (1.6) | 8.2 (3.8) | 5.9 (3.6) | 3.2 (2.2) |
| 2021 | −2.3 (3.5) | −3.2 (6.1) | 3.1 (3.7) | 4.7 (2.1) | 10.3 (4.0) | 18.4 (2.1) | 20.6 (2.4) | --- | --- | --- | --- | --- |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osterman-Lind, E.; Hedberg Alm, Y.; Hassler, H.; Wilderoth, H.; Thorolfson, H.; Tydén, E. Evaluation of Strategies to Reduce Equine Strongyle Infective Larvae on Pasture and Study of Larval Migration and Overwintering in a Nordic Climate. Animals 2022, 12, 3093. https://doi.org/10.3390/ani12223093
Osterman-Lind E, Hedberg Alm Y, Hassler H, Wilderoth H, Thorolfson H, Tydén E. Evaluation of Strategies to Reduce Equine Strongyle Infective Larvae on Pasture and Study of Larval Migration and Overwintering in a Nordic Climate. Animals. 2022; 12(22):3093. https://doi.org/10.3390/ani12223093
Chicago/Turabian StyleOsterman-Lind, Eva, Ylva Hedberg Alm, Hillevi Hassler, Hanna Wilderoth, Helena Thorolfson, and Eva Tydén. 2022. "Evaluation of Strategies to Reduce Equine Strongyle Infective Larvae on Pasture and Study of Larval Migration and Overwintering in a Nordic Climate" Animals 12, no. 22: 3093. https://doi.org/10.3390/ani12223093
APA StyleOsterman-Lind, E., Hedberg Alm, Y., Hassler, H., Wilderoth, H., Thorolfson, H., & Tydén, E. (2022). Evaluation of Strategies to Reduce Equine Strongyle Infective Larvae on Pasture and Study of Larval Migration and Overwintering in a Nordic Climate. Animals, 12(22), 3093. https://doi.org/10.3390/ani12223093





