Effect of Hay Steaming on the Estimated Precaecal Digestibility of Crude Protein and Selected Amino Acids in Horses
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sensory Analyses
2.3. Analytical Methods and Calculations
2.4. Statistical Analysis
3. Results
3.1. Sensory Analyses
3.2. Proximate Nutrients
3.3. Maillard Reaction Products
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kamphues, J.; Wolf, P.; Coenen, M.; Eder, K.; Iben, C.; Kienzle, E.; Liesegang, A.; Männer, K.; Zebeli, Q.; Zentek, J. Supplemente Zur Tierernährung für Studium und Praxis, 12th ed.; Schaper, M.H., Ed.; Schlütersche: Hanover, Germany, 2014; p. 183. [Google Scholar]
- Séguin, V.; Lemauviel-Lavenant, S.; Garon, D.; Bouchart, V.; Gallard, Y.; Blanchet, B.; Diquelou, S.; Personeni, E.; Gauduchon, P.; Ourry, A. Effect of Agricultural and Environmental Factors on the Hay Characteristics Involved in Equine Respiratory Disease. Agric. Ecosyst. Environ. 2010, 135, 206–215. [Google Scholar] [CrossRef]
- Intemann, S.; Reckels, B.; Schubert, D.; Wolf, P.; Kamphues, J.; Visscher, C. The Hygienic Status of Different Forage Types for Horses—A Retrospective Study on Influencing Factors and Associations with Anamnestic Reports. Vet. Sci. 2022, 9, 226. [Google Scholar] [CrossRef] [PubMed]
- Routschek, A.; Schmidt, J.; Kreienkamp, F. Impact of Climate Change on Soil Erosion—A High-Resolution Projection on Catchment Scale until 2100 in Saxony/Germany. CATENA 2014, 121, 99–109. [Google Scholar] [CrossRef]
- Olesen, J.E.; Børgesen, C.D.; Elsgaard, L.; Palosuo, T.; Rötter, R.P.; Skjelvåg, A.O.; Peltonen-Sainio, P.; Börjesson, T.; Trnka, M.; Ewert, F. Changes in Time of Sowing, Flowering and Maturity of Cereals in Europe under Climate Change. Food Addit. Contam. Part A 2012, 29, 1527–1542. [Google Scholar] [CrossRef] [PubMed]
- Bridge, P.; Spooner, B. Soil Fungi: Diversity and Detection. In Interactions in the Root Environment: An Integrated Approach; Powlson, D.S., Bateman, G.L., Davies, K.G., Gaunt, J.L., Hirsch, P.R., Eds.; Springer: Dordrecht, The Netherlands, 2002; pp. 147–154. ISBN 978-94-010-3925-3. [Google Scholar]
- Ivester, K.M.; Couëtil, L.L.; Moore, G.E. An Observational Study of Environmental Exposures, Airway Cytology, and Performance in Racing Thoroughbreds. J. Vet. Intern. Med. 2018, 32, 1754–1762. [Google Scholar] [CrossRef]
- Bullone, M.; Lavoie, J.-P. The Equine Asthma Model of Airway Remodeling: From a Veterinary to a Human Perspective. Cell Tissue Res. 2020, 380, 223–236. [Google Scholar] [CrossRef]
- Couëtil, L.L.; Cardwell, J.M.; Gerber, V.; Lavoie, J.-P.; Léguillette, R.; Richard, E.A. Inflammatory Airway Disease of Horses—Revised Consensus Statement. J. Vet. Intern. Med. 2016, 30, 503–515. [Google Scholar] [CrossRef]
- Couetil, L.; Cardwell, J.M.; Leguillette, R.; Mazan, M.; Richard, E.; Bienzle, D.; Bullone, M.; Gerber, V.; Ivester, K.; Lavoie, J.-P.; et al. Equine Asthma: Current Understanding and Future Directions. Front. Vet. Sci. 2020, 7, 450. [Google Scholar] [CrossRef]
- Bond, S.; Léguillette, R.; Richard, E.A.; Couetil, L.; Lavoie, J.-P.; Martin, J.G.; Pirie, R.S. Equine Asthma: Integrative Biologic Relevance of a Recently Proposed Nomenclature. J. Vet. Intern. Med. 2018, 32, 2088–2098. [Google Scholar] [CrossRef]
- Rossi, H. Equine Lower Airway Inflammation—Insights into Asthma, Diagnostics, and Anaesthesia; University of Helsinki: Helsinki, Finland, 2020; p. 97. Available online: https://helda.helsinki.fi/handle/10138/310496 (accessed on 12 October 2022).
- Davis, K.U. Investigation of the Pathophysiology and a Novel Therapeutic Target of Equine Asthma Syndrome; North Carolina State University: Raleigh, NC, USA, 2020; ISBN 9798664735918. [Google Scholar]
- White, S.J.; Moore-Colyer, M.; Marti, E.; Hannant, D.; Gerber, V.; Coüetil, L.; Richard, E.A.; Alcocer, M. Antigen Array for Serological Diagnosis and Novel Allergen Identification in Severe Equine Asthma. Sci. Rep. 2019, 9, 15170. [Google Scholar] [CrossRef]
- Lehmann, B.; Merle, R.; Klier, J.; Gehlen, H. Besitzerbefragung zur chronisch-obstruktiven Bronchitis bei Warmblütern unter Verwendung eines Online-Fragebogens. Pferdeheilkunde 2016, 32, 357–366. [Google Scholar] [CrossRef]
- Moore-Colyer, M.J.S.; Taylor, J.L.E.; James, R. The Effect of Steaming and Soaking on the Respirable Particle, Bacteria, Mould, and Nutrient Content in Hay for Horses. J. Equine Vet. Sci. 2016, 39, 62–68. [Google Scholar] [CrossRef]
- Glatter, M.; Bochnia, M.; Wensch-Dorendorf, M.; Greef, J.M.; Zeyner, A. Feed Intake Parameters of Horses Fed Soaked or Steamed Hay and Hygienic Quality of Hay Stored Following Treatment. Animals 2021, 11, 2729. [Google Scholar] [CrossRef] [PubMed]
- Moore-Colyer, M.J.S.; Fillery, B.G. The Effect of Three Different Treatments on the Respirable Particle Content, Total Viable Count and Mould Concentrations in Hay for Horses. In Forages and Grazing in Horse Nutrition; Springer: Wageningen, The Netherlands, 2012; pp. 101–106. [Google Scholar]
- Gerber, V.; Lindberg, Å.; Berney, C.; Robinson, N.E. Airway Mucus in Recurrent Airway Obstruction–Short-Term Response to Environmental Challenge. J. Vet. Intern. Med. 2004, 18, 92–97. [Google Scholar] [CrossRef] [PubMed]
- VDLUFA. Die Chemische Untersuchung von Futtermitteln Methodenbuch, 3rd ed.; VDLUFA-Verlag: Darmstadt, Germany, 2012. [Google Scholar]
- Bochnia, M.; Pietsch, C.; Wensch-Dorendorf, M.; Greef, M.; Zeyner, A. Effect of Hay Soaking Duration on Metabolizable Energy, Total and Prececal Digestible Crude Protein and Amino Acids, Non-Starch Carbohydrates, Macronutrients and Trace Elements. J. Equine Vet. Sci. 2021, 101, 103452. [Google Scholar] [CrossRef]
- Licitra, G.; Hernandez, T.M.; Van Soest, P.J. Standardization of Procedures for Nitrogen Fractionation of Ruminant Feeds. Anim. Feed Sci. Technol. 1996, 57, 347–358. [Google Scholar] [CrossRef]
- Association of German Agriculture and Research Institutes. The chemical control of feed (supplementary deliveries). In Handbook of the Agricultural Experimental and Research Methodology (VDLUFA-Methodenbuch); Association of German Agriculture and Research Institutes: Darmstadt, Germany, 2016; Volume III. [Google Scholar]
- Kienzle, E.; Zeyner, A. The Development of a Metabolizable Energy System for Horses: ME System for Horses. J. Anim. Physiol. Anim. Nutr. 2010, 94, e231–e240. [Google Scholar] [CrossRef]
- Gesellschaft Für Ernährungsphysiologie. Empfehlungen Zur Energie-Und Nährstoffversorgung von Pferden. In Energie-Und Nährstoffbedarf Landwirtschaftlicher Nutztiere; DLG-Verlag: Frankfurt, Germany, 2014; Volume 11. [Google Scholar]
- Zeyner, A.; Kirchhof, S.; Susenbeth, A.; Südekum, K.-H.; Kienzle, E. A New Protein Evaluation System for Horse Feed from Literature Data. J. Nutr. Sci. 2015, 4, e4. [Google Scholar] [CrossRef]
- Fontaine, J.; Bech-Andersen, S.; Butikofer, U.; de Froidmont-Gortz, I. Determination of Tryptophan in Feed by HPLC-Development of an Optimal Hydrolysis and Extraction Procedure by the EU Commission DG XII in Three International Collaborative Studies. Agribiol. Res. (Ger.) 1998, 51, 97–108. [Google Scholar]
- Krause, R.; Knoll, K.; Henle, T. Studies on the Formation of Furosine and Pyridosine during Acid Hydrolysis of Different Amadori Products of Lysine. Eur. Food Res. Technol. 2003, 216, 277–283. [Google Scholar] [CrossRef]
- Henle, T.; Walter, H.; Krause, I.; Klostermeyer, H. Efficient Determination of Individual Maillard Compounds in Heat-Treated Milk Products by Amino Acid Analysis. Int. Dairy J. 1991, 1, 125–135. [Google Scholar] [CrossRef]
- Förster, A. Quantitative Studien Zu Vorkommen Und Metabolischem Transit Alimentärer Maillard-Reaktions-Produkte. 2006. Available online: https://nbn-resolving.org/urn:nbn:de:swb:14-1168006117145-39700 (accessed on 25 August 2022).
- Schwarzenbolz, U.; Hofmann, T.; Sparmann, N.; Henle, T. Free Maillard Reaction Products in Milk Reflect Nutritional Intake of Glycated Proteins and Can Be Used to Distinguish “Organic” and “Conventionally” Produced Milk. J. Agric. Food Chem. 2016, 64, 5071–5078. [Google Scholar] [CrossRef] [PubMed]
- Kuchler, M.; Zeyner, A.; Susenbeth, A.; Kienzle, E. The Effect of Crude Protein Content of the Diet on Renal Energy Losses in Horses. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1494–1500. [Google Scholar] [CrossRef] [PubMed]
- Rodehutscord, M.; Dieckmann, A. Comparative Studies with Three-Week-Old Chickens, Turkeys, Ducks, and Quails on the Response in Phosphorus Utilization to a Supplementation of Monobasic Calcium Phosphate1. Poult. Sci. 2005, 84, 1252–1260. [Google Scholar] [CrossRef]
- Bachmann, M.; Glatter, M.; Bochnia, M.; Greef, J.M.; Breves, G.; Zeyner, A. Degradation of Monosaccharides, Disaccharides, and Fructans in the Stomach of Horses Adapted to a Prebiotic Dose of Fructooligosaccharides and Inulin. J. Equine Vet. Sci. 2021, 105, 103731. [Google Scholar] [CrossRef]
- Pavis, N.; Chatterton, N.J.; Harrison, P.A.; Baumgartner, S.; Praznik, W.; Boucaud, J.; Prud’homme, M.P. Structure of Fructans in Roots and Leaf Tissues of Lolium Perenne. New Phytol. 2001, 150, 83–95. [Google Scholar] [CrossRef]
- Erbersdobler, H.F.; Somoza, V. Forty Years of Furosine—Forty Years of Using Maillard Reaction Products as Indicators of the Nutritional Quality of Foods. Mol. Nutr. Food Res. 2007, 51, 423–430. [Google Scholar] [CrossRef]
- Sarrafchi, A.; Blokhuis, H.J. Equine Stereotypic Behaviors: Causation, Occurrence, and Prevention. J. Vet. Behav. 2013, 8, 386–394. [Google Scholar] [CrossRef]
- Moeller, B.A.; McCall, C.A.; Silverman, S.J.; McElhenney, W.H. Estimation of Saliva Production in Crib-Biting and Normal Horses. J. Equine Vet. Sci. 2008, 28, 85–90. [Google Scholar] [CrossRef]
- Merritt, A.M.; Julliand, V. Gastrointestinal Physiology. In Equine Applied and Clinical Nutrition: Health, Welfare and Performance; Elsevier Health Sciences: Amsterdam, The Netherlands, 2013; pp. 3–32. [Google Scholar]
- Earing, J.E.; Hathaway, M.R.; Sheaffer, C.C.; Hetchler, B.P.; Jacobson, L.D.; Paulson, J.C.; Martinson, K.L. Effect of Hay Steaming on Forage Nutritive Values and Dry Matter Intake by Horses1. J. Anim. Sci. 2013, 91, 5813–5820. [Google Scholar] [CrossRef]
- Blackman, M.; Moore-Colyer, M.J.S. Hay for Horses: The Effects of Three Different Wetting Treatments on Dust and Nutrient Content. Anim. Sci. 1998, 66, 745–750. [Google Scholar] [CrossRef]
- Brown, E.; Tracey, S.; Gowers, I. An Investigation to Determine the Palatability of Steamed Hay, Dry Hay and Haylage. Pro. BSAS Innov. Anim. Sci. Necessity Option 2013, 4, 104. [Google Scholar]
- Moore-Colyer, M.J.S.; Payne, V. Palatability and ingestion behaviour of six polo ponies offered a choice of dry, soaked and steamed hay for 1 h on three separate occassions. Adv. Anim. Biosci. 2012, 3, 127. [Google Scholar]
- van Soest, P.J.; Mason, V.C. The Influence of the Maillard Reaction upon the Nutritive Value of Fibrous Feeds. Anim. Feed Sci. Technol. 1991, 32, 45–53. [Google Scholar] [CrossRef]
- Hellwig, M.; Henle, T. Baking, Ageing, Diabetes: A Short History of the Maillard Reaction. Angew. Chem. Int. Ed. 2014, 53, 10316–10329. [Google Scholar] [CrossRef]
- Wiame, E.; Delpierre, G.; Collard, F.; Van Schaftingen, E. Identification of a Pathway for the Utilization of the Amadori Product Fructoselysine in Escherichia coli. J. Biol. Chem. 2002, 277, 42523–42529. [Google Scholar] [CrossRef]
- Erbersdobler, H.; Gunsser, I.; Weber, G. Abbau von Fruktoselysin Durch Die Darmflora. Zent. Für Veterinärmedizin Reihe A 1970, 17, 573–575. [Google Scholar] [CrossRef]
- Tuohy, K.M.; Hinton, D.J.S.; Davies, S.J.; Crabbe, M.J.C.; Gibson, G.R.; Ames, J.M. Metabolism of Maillard Reaction Products by the Human Gut Microbiota—Implications for Health. Mol. Nutr. Food Res. 2006, 50, 847–857. [Google Scholar] [CrossRef]
- Thornalley, P.J. Protein and Nucleotide Damage by Glyoxal and Methylglyoxal in Physiological Systems—Role in Ageing and Disease. Drug Metab. Drug Interact 2008, 23, 125–150. [Google Scholar] [CrossRef]
- Longland, A.C.; Barfoot, C.; Harris, P.A. Effect of Period, Water Temperature and Agitation on Loss of Water-Soluble Carbohydrates and Protein from Grass Hay: Implications for Equine Feeding Management. Vet. Rec. 2014, 174, 68. [Google Scholar] [CrossRef]
- Longland, A.C.; Barfoot, C.; Harris, P.A. Effects of Soaking on the Water-Soluble Carbohydrate and Crude Protein Content of Hay. Vet. Rec. 2011, 168, 618. [Google Scholar] [CrossRef] [PubMed]
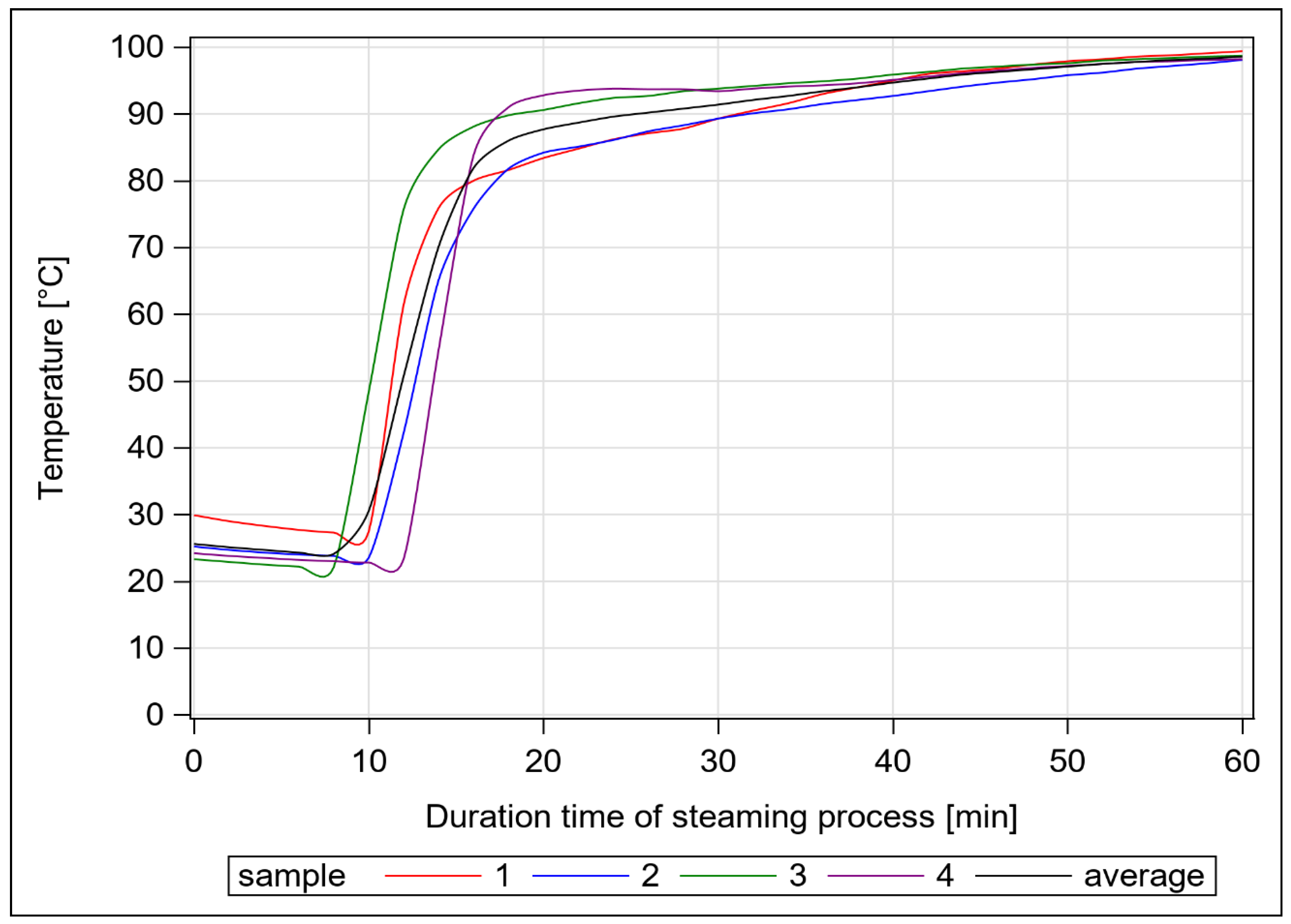


| Hay Sample | Forage Value | Points | Hygienic Status | Points |
|---|---|---|---|---|
| Hay 1 | satisfying (dusty) | 13 | flawless | 0 |
| Hay 2 | very low (straw-like, dusty) | 3 | clear deficits (musty, sandy) | −8 |
| Hay 3 | satisfying | 15 | clear deficits (rodent droppings) | −7 |
| Hay 4 | moderate (straw-like) | 6 | small deficits | −2 |
| Hay 5 | satisfying | 10 | small deficits | −5 |
| Hay 6 | satisfying | 11 | small deficits | −5 |
| Item | Unit | Treatment | |||
|---|---|---|---|---|---|
| Native | Steamed | SE | p-Value | ||
| DM | g/kg | 961 | 888 | ±3.4 | <0.001 |
| CA | g/kg DM | 62 | 61 | ±3.7 | 0.551 |
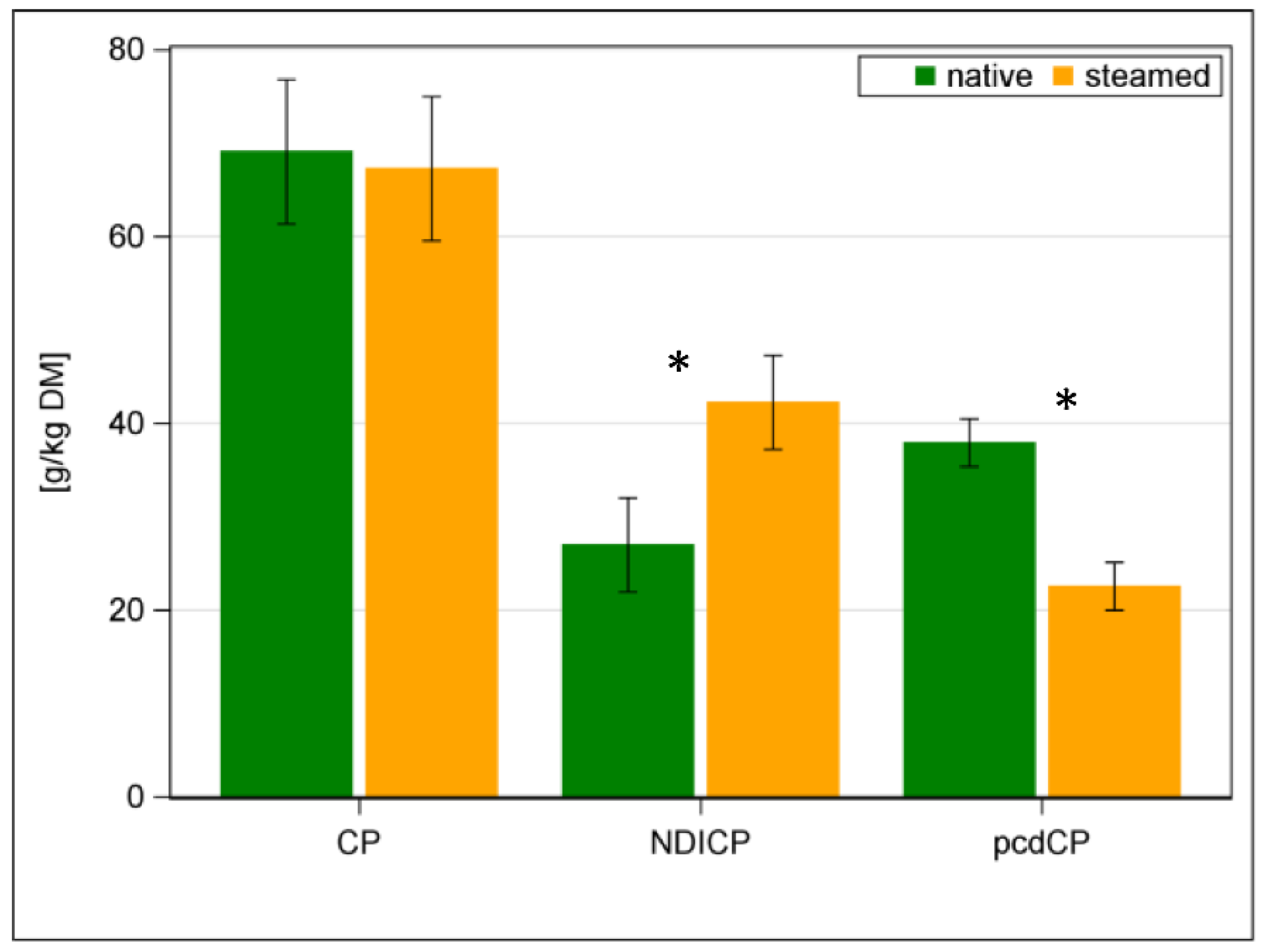
| CP | g/kg DM | 69 | 67 | ±7.7 | 0.29 |
| CL | g/kg DM | 8 | 9 | ±0.8 | 0.352 |
| CF | g/kg DM | 330 | 334 | ±15.2 | 0.411 |
| aNDFom | g/kg DM | 673 | 695 | ±18.5 | <0.001 |
| ADFom | g/kg DM | 371 | 372 | ±14.9 | 0.869 |
| ADL | g/kg DM | 49 | 52 | ±3.2 | 0.003 |
| NDICP | g/kg DM | 27 | 42 | ±5.0 | <0.001 |
| NDSCP | g/kg DM | 42 | 25 | ±2.8 | <0.001 |
| pcdCP a | g/kg DM | 38 | 23 | ±2.6 | <0.001 |
| piCP | g/kg DM | 27 | 31 | ±3.4 | <0.001 |
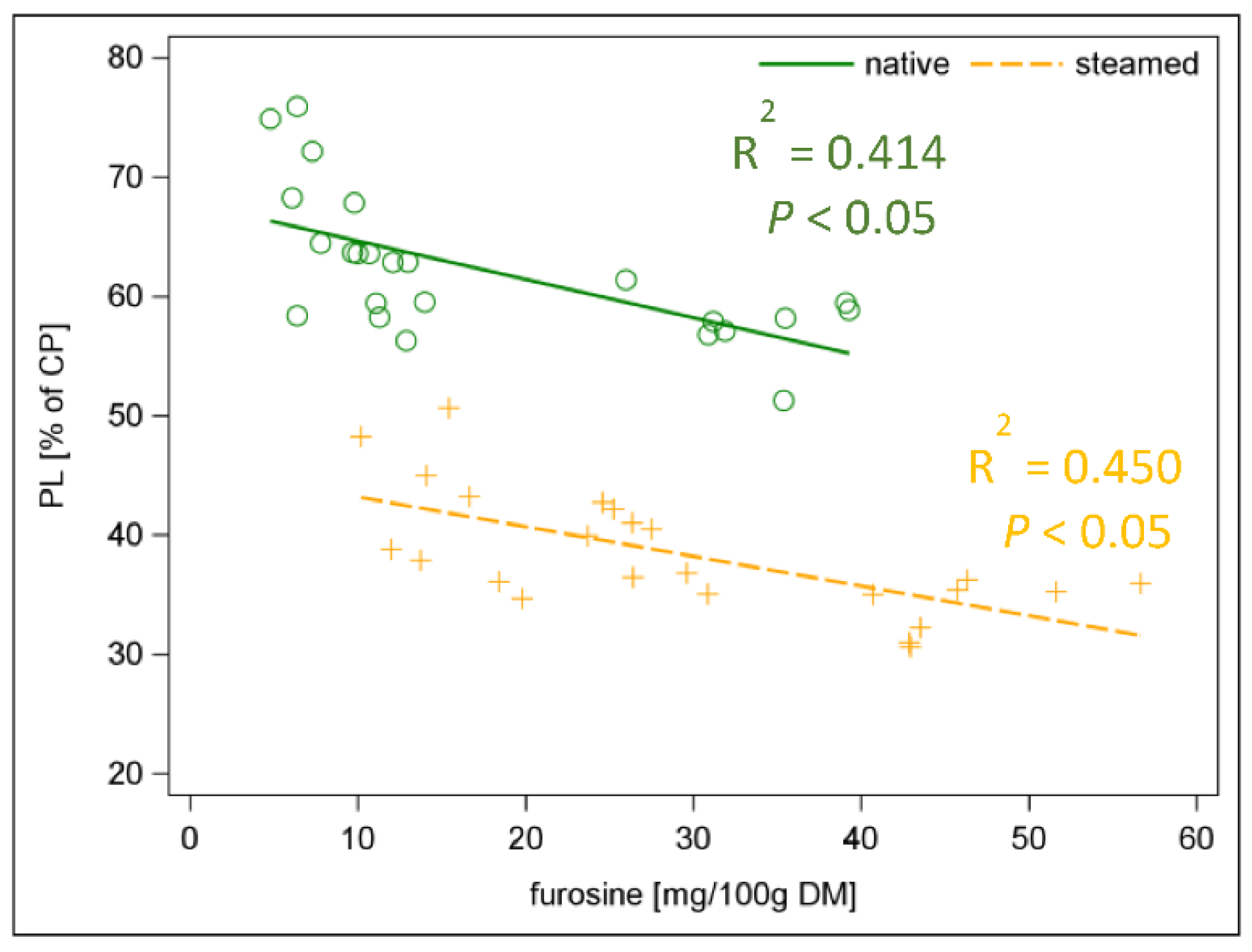
| pcD of CP a | % of CP | 56 | 35 | ±2.0 | <0.001 |
| PS a | % of CP | 62 | 38 | ±2.3 | <0.001 |
| piCP | % of CP | 40 | 46 | ±1.3 | <0.001 |
| glucose | g/kg DM | 13 | 11 | ±1.7 | 0.001 |
| fructose | g/kg DM | 25 | 25 | ±3.2 | 0.838 |
| sucrose | g/kg DM | 9 | 11 | ±1.9 | 0.009 |
| fructans | g/kg DM | 65 | 67 | ±9.3 | 0.22 |
| total WSC | g/kg DM | 113 | 113 | ±12.7 | 0.709 |
| CML | mg/100 g DM | 5.1 | 11.3 | ±1.61 | <0.001 |
| furosine | mg/100 g DM | 17.6 | 29.4 | ±5.46 | <0.001 |
| P | g/kg DM | 1.84 | 1.83 | ±0.139 | 0.878 |
| Ca | g/kg DM | 3.99 | 3.66 | ±0.317 | 0.011 |
| K | g/kg DM | 13.51 | 13.68 | ±2.214 | 0.634 |
| Na | g/kg DM | 0.09 | 0.07 | ±0.044 | 0.108 |
| Mg | g/kg DM | 1.37 | 1.31 | ±0.126 | 0.034 |
| Zn | mg/kg DM | 38.65 | 39.37 | ±13.159 | 0.493 |
| Mn | mg/kg DM | 114.06 | 120.53 | ±45.260 | 0.06 |
| Cu | mg/kg DM | 4.54 | 4.58 | ±0.572 | 0.766 |
| Fe | mg/kg DM | 142.73 | 180.1 | ±34.178 | 0.061 |
| ME b | MJ/kg DM | 6.9 | 6.9 | ±0.3 | 0.648 |
| ME mod. c | MJ/kg DM | 6.8 | 6.8 | ±0.3 | 0.599 |
| Treatment of Hay | ||||||||
|---|---|---|---|---|---|---|---|---|
| Native | Steamed | SE | p-Value | |||||
| g/kg DM | g/16 g N | g/kg DM | g/16 g N | g/kg DM | g/16 g N | g/kg DM | g/16 g N | |
| Essential amino acids | ||||||||
| Histidine | 1.1 | 1.6 | 1 | 1.4 | ±0.12 | ±0.06 | <0.001 | <0.001 |
| Isoleucine | 2.9 | 4.1 | 2.8 | 4.1 | ±0.34 | ±0.10 | 0.367 | 0.393 |
| Leucine | 5.2 | 7.6 | 5.1 | 7.4 | ±0.61 | ±0.17 | 0.189 | 0.229 |
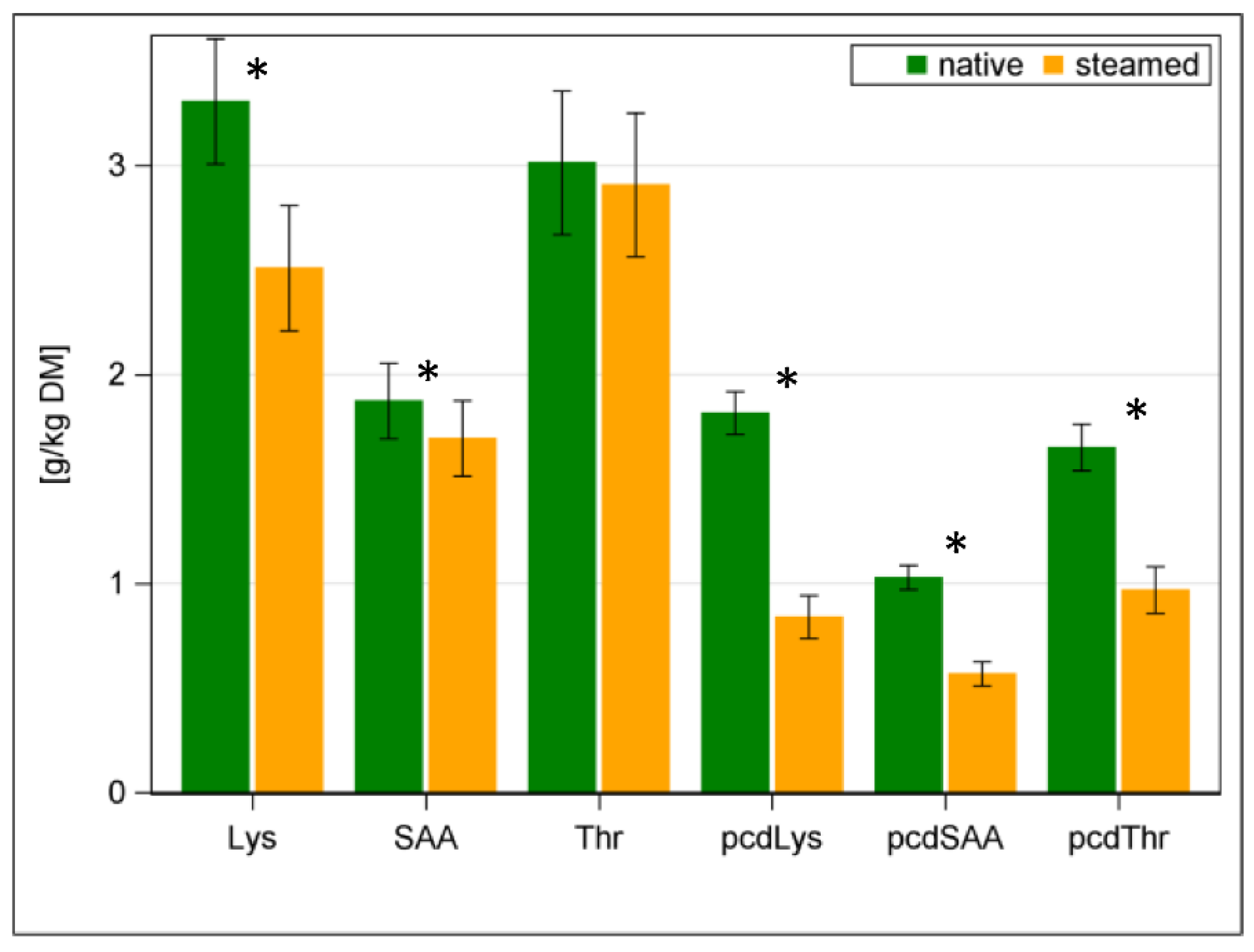
| Lysine | 3.3 | 4.8 | 2.5 | 3.7 | ±0.30 | ±0.10 | <0.001 | <0.001 |
| Methionine | 1.2 | 1.7 | 1.1 | 1.6 | ±0.13 | ±0.04 | 0.003 | 0.011 |
| Phenylalanine | 3.2 | 4.6 | 3.1 | 4.5 | ±0.41 | ±0.14 | 0.133 | 0.085 |
| Threonine | 3 | 4.4 | 2.9 | 4.3 | ±0.34 | ±0.08 | 0.155 | 0.176 |
| Tryptophan | 0.9 | 1.3 | 0.9 | 1.3 | ±0.12 | ±0.05 | 0.047 | 0.007 |
| Valine | 3.6 | 5.3 | 3.5 | 5.2 | ±0.43 | ±0.10 | 0.253 | 0.263 |
| Non-essential amino acids | ||||||||
| Alanine | 4.3 | 6.2 | 4.1 | 6 | ±0.51 | ±0.14 | 0.133 | 0.147 |
| Arginine | 3.5 | 5 | 3.2 | 4.6 | ±0.38 | ±0.09 | <0.001 | <0.001 |
| Aspartic acid | 6.1 | 8.7 | 5.8 | 8.4 | ±0.74 | ±0.20 | 0.091 | 0.034 |
| Cysteine | 0.7 | 1.1 | 0.6 | 0.9 | ±0.05 | ±0.05 | <0.001 | <0.001 |
| Glutamic acid | 7.1 | 10.2 | 6.8 | 9.9 | ±0.82 | ±0.17 | 0.112 | 0.09 |
| Glycine | 3.6 | 5.2 | 3.5 | 5.1 | ±0.41 | ±0.09 | 0.228 | 0.307 |
| Proline | 4 | 5.6 | 3.9 | 5.5 | ±0.68 | ±0.41 | 0.622 | 0.525 |
| Serine | 2.9 | 4.2 | 2.8 | 4.1 | ±0.32 | ±0.07 | 0.069 | 0.074 |
| Tyrosine | 2.1 | 3 | 1.9 | 2.8 | ±0.22 | ±0.08 | 0.006 | 0.005 |
| Precaecal digestible AA a | ||||||||
| pcdLys | 1.82 | 0.84 | ±0.103 | <0.001 | ||||
| pcdMet | 0.63 | 0.36 | ±0.043 | <0.001 | ||||
| pcdCys | 0.4 | 0.21 | ±0.018 | <0.001 | ||||
| pcdSAA | 1.03 | 0.57 | ±0.058 | <0.001 | ||||
| pcdThr | 1.65 | 0.97 | ±0.112 | <0.001 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pisch, C.; Wensch-Dorendorf, M.; Schwarzenbolz, U.; Henle, T.; Greef, J.M.; Zeyner, A. Effect of Hay Steaming on the Estimated Precaecal Digestibility of Crude Protein and Selected Amino Acids in Horses. Animals 2022, 12, 3092. https://doi.org/10.3390/ani12223092
Pisch C, Wensch-Dorendorf M, Schwarzenbolz U, Henle T, Greef JM, Zeyner A. Effect of Hay Steaming on the Estimated Precaecal Digestibility of Crude Protein and Selected Amino Acids in Horses. Animals. 2022; 12(22):3092. https://doi.org/10.3390/ani12223092
Chicago/Turabian StylePisch, Caroline, Monika Wensch-Dorendorf, Uwe Schwarzenbolz, Thomas Henle, Jörg Michael Greef, and Annette Zeyner. 2022. "Effect of Hay Steaming on the Estimated Precaecal Digestibility of Crude Protein and Selected Amino Acids in Horses" Animals 12, no. 22: 3092. https://doi.org/10.3390/ani12223092
APA StylePisch, C., Wensch-Dorendorf, M., Schwarzenbolz, U., Henle, T., Greef, J. M., & Zeyner, A. (2022). Effect of Hay Steaming on the Estimated Precaecal Digestibility of Crude Protein and Selected Amino Acids in Horses. Animals, 12(22), 3092. https://doi.org/10.3390/ani12223092





