Simple Summary
Ticks pose a threat to humans and animals. However, microbial interactions on ticks are underappreciated. Complex microbial interactions shape microbial communities. There is limited information about microbial interactions on ticks, including Dermacentor silvarum, Ixodes persulcatus, and Haemaphysalis concinna. This study evaluated the microbial community composition of these three species and the microbial interactions based on pairwise interactions. The results reveal that the bacterial richness and microbiota structures of the three tick species are significantly different, and the bacterial richness of all ticks decreased significantly after they became engorged. A substantial expansion of the list of bacterial interactions was observed in ticks.
Abstract
Ticks carry and transmit a variety of pathogens, which are very harmful to humans and animals. To characterize the microbial interactions in ticks, we analysed the microbiota of the hard ticks, Dermacentor silvarum, Ixodes persulcatus, and Haemaphysalis concinna, using 16S rRNA, showing that microbial interactions are underappreciated in terms of shaping arthropod microbiomes. The results show that the bacterial richness and microbiota structures of these three tick species had significant differences. Interestingly, the bacterial richness (Chao1 index) of all ticks decreased significantly after they became engorged. All the operational taxonomic units (OTUs) were assigned to 26 phyla, 67 classes, 159 orders, 279 families, and 627 genera. Microbial interactions in D. silvarum demonstrated more connections than in I. persulcatus and H. concinna. Bacteria with a high abundance were not important families in microbial interactions. Positive interactions of Bacteroidaceae and F_Solibacteraceae Subgroup 3 with other bacterial families were detected in all nine groups of ticks. This study provides an overview of the microbiota structure and interactions of three tick species and improves our understanding of the role of the microbiota in tick physiology and vector capacity, thus being conducive to providing basic data for the prevention of ticks and tick-borne diseases.
1. Introduction
In blood-sucking arthropods, the microbiome can vary widely [1,2,3,4]. Globally, ticks pose a threat to animals and humans. The biological characteristics of most microorganisms and their effects on ticks have not yet been explored and are typically neglected. However, such microorganisms have a variety of harmful, neutral, and beneficial effects on host ticks and play a variety of roles in health, nutrition, adaptation, development, reproduction, defence against environmental stress, and immunity [5]. In addition to pathogenic microorganisms, non-pathogenic microorganisms may be involved in the transmission of tick-borne pathogens, which may impact animal and human health in numerous ways [6]. Despite the growing knowledge about the factors that influence the composition and abundance of the microbiota, there are still many unanswered questions regarding microbiota in ticks. Currently, there is no consensus about which factor is conducive to the characterization of tick microbiota composition, and there is a lack of understanding of the different relationships between tick microbiota [7].
More than 900 valid species of tick are known worldwide, of which 124 species are found in China [8]. The growing number of tick bites each year poses an escalating risk of tick-borne diseases. Dermacentor silvarum, Ixodes persulcatus, and Haemaphysalis concinna (Acari: Ixodidae) are abundant and epidemiologically important tick species in China. All three tick species have a wide range of hosts and different host preferences at different life stages, and all have been reported to bite humans and animals [8]. Thus, the structure of their microbiota should be studied carefully. In addition to being the principal vector for the Lyme disease pathogen, Borreliella burgdorferi, these three species carry numerous other medically important pathogens: Anaplasma spp., Babesia spp., Ehrlichia spp., Rickettsia spp., and tick-borne encephalitis virus [9]. Moreover, alongside the tick-borne pathogens, ticks also harbour non-pathogenic microorganisms such as endosymbiotic, commensal, mutualistic, and parasitic bacteria [10]. There is evidence that tick microbial communities may affect their vectorial capacities.
Some representative bacterial genera may behave as subtle symbionts engaged in intricate interactions with ticks. In certain ticks, a large number of vertically transmitted endosymbionts are found, which may play a trophic role by providing essential nutrients to the host [11,12,13]. These nonpathogenic bacteria may interact with a variety of tick-borne pathogens, including Anaplasma marginale, B. burgdorferi, and other Rickettsia species [14,15,16].
Due to the recent resurgence of tick-borne infectious diseases worldwide, research on tick microbial composition is crucial. To understand the relationship between microbes and hosts, it is important to unravel the mystery of the composition of microbial communities. However, to date, there is limited information on the microbial interactions of these three species of ticks. In this study, we investigated the structure and composition of I. persulcatus, H. concinna, and D. silvarum collected from the field as major tick vector communities to better understand the forces shaping tick microbiota. In order to gain a better understanding of tick microbial interactions, we analysed microbial interaction networks. This study examined the composition of tick microbial communities and investigated the symbiotic and antagonistic relationships between microorganisms, which are conducive to providing basic data for the prevention of ticks and tick-borne diseases.
2. Materials and Methods
2.1. Tick Collection and DNA Extraction
Three tick species were collected in the forest of Harbin, Jiamusi, and Great Khingan in Heilongjiang Province of China from March to June 2021, according to their active areas and months. Unfed female and male ticks were collected from the wild forest using dragging, and engorged ticks were collected from cattle in the same locations. A total of 2464 ticks were collected, including 2375 unfed and 89 engorged ticks. Among them, 85 (22 engorged) ticks were used in the study, including 27 (9 engorged) I. persulcatus, 36 (9 engorged) D. silvarum, and 22 (4 engorged) H. concinna. All ticks were adults, and the detailed sample information is shown in Table 1. The tick species were identified by their morphological characteristics and molecular data based on mitochondrial 16S rDNA sequences [10,17]. In order to clean the microorganisms from the surface of ticks to reduce environmental contamination, a method involving bleach was used [18]. Briefly, a 1% commercial bleach solution was used on the first batch of ticks to process them for 30 s, followed by three rinses with DNA-free water, each lasting 1 min. The cleaned ticks were preserved intact at −20 °C for DNA extraction.

Table 1.
The information of the ticks collected in this study.
The ticks were analysed individually. Before DNA extraction, the ticks were dried on sterile filter paper and, under liquid nitrogen, ground with steel balls for five minutes. A DNA Mini kit (QiAGEN, Shanghai, China) was used to isolate DNA. Nucleic acid was stored at −80 °C until use.
2.2. High-Throughput Sequencing and Bioinformatics Analysis
As for the tick microbiota, the ribosome 16S V3–V4 region is commonly used for accurate taxonomic differentiation [16,19]. Therefore, according to previous reports, the V3–V4 region of the bacterial 16S rRNA gene was amplified. We sequenced the DNA using an Illumina Hiseq 2500 platform following a standard protocol. The NCBI accession number for the raw sequencing data reported here is PRJNA849745.
At 97% sequence identity, all effective tags clustered into operational taxonomic units (OTUs) [20]. We used the Mothur method and the SSUrRNA [21] database of SILVA [22] to conduct species annotation analysis (setting a threshold of 0.8–1), acquire information on taxonomy, and, at each classification level (kingdom, phylum, class, order, family, genus, and species), identify the community composition of each sample. MUSCLE 3.8.31 [23] software was used for multiple sequence alignment, and all OTUs were obtained on behalf of the sequence of the system. The statistical analysis involved in this work was implemented using the R 4.1.3 (http://www.r-project.org, accessed on 10 May 2022) platform. The Shannon and Chao1 indexes of samples were calculated using the ‘diversity’ and ‘estimateR’ functions in the vegan (v.2.6-2) package [24] (https://cran.r-project.org/package=vegan, accessed on 8 June 2022), respectively. The Bray–Curtis distance between samples was calculated using the ‘vegdist’ function in the vegan package [25]. The ‘cmdscale’ function was used to realize principal coordinate analysis (PCoA), and the results of PCoA were visualized by the scatterplot 3D package [26] (https://CRAN.R-project.org/package=scatterplot3d, accessed on 11 June 2022). The ‘adonis’ function in the vegan package was used for the permutational multivariate analysis of variance (PERMANOVA) analysis. The Linear discriminant analysis effect size (LEfSe) was used to find the genera of bacteria that differed between groups and was performed on the Galaxy online platform (https://huttenhower.sph.harvard.edu/galaxy, accessed on 21 June 2022). Comparisons of the differences in microbial diversity and classification level between the groups were obtained by Wilcoxon rank-sum test.
2.3. Detection of Complex Interaction Patterns
Microbial interaction analysis was conducted independently on each group of ticks. The Spearman correlation coefficient was used to evaluate the correlation between bacterial families based on the relative abundance spectrum [27]. Only strong inter-correlations (p > 0.01) were retained in order to clearly show associations between families. The visualization of correlations was achieved using the R package “ggplot2” and Gephi software (https://gephi.org/, accessed on 24 June 2022) [28,29].
3. Results
3.1. Microbiota Diversity
A total of 85 (22 engorged) adult ticks from three species were sequenced for the V3-V4 region of the 16S rRNA gene. After quality control, 8,869,556 clean reads were obtained. A total of 1810 OTUs were obtained through splicing and 97% identity threshold clustering (Table S1). Based on our rarefaction curve analysis, we were able to detect all OTUs in tick samples using our sequencing depth.
The α-diversity of the microbiota in the samples was demonstrated in two ways: using the Shannon index and Chao1 index. The diversity of the microbial communities varied significantly in H. concinna compared with D. silvarum and I. persulcatus in terms of the OTU level (Figure 1A). Female ticks from either the engorged or unfed samples had significantly higher diversity than male ticks within D. silvarum (Figure 1B). No difference was observed between groups in H. concinna, whereas I. persulcatus females had significantly lower diversity than males (Figure 1B). There were no significant differences in terms of male tick richness in any of the species (Figure 1B). Something more interesting was found when exploring the Chao1 index of the samples. Among the three tick species, H. concinna had the lowest Chao1 index, which differed from the trend reflected in the Shannon index (Figure 1C). This indicates that low-abundance bacterial OTUs are rare in H. concinna, and the number of OTUs was lower in H. concinna than in the other two tick species. In addition, we compared the samples of different sexes and feed stages in the same tick species (Figure 1D) and found that when the ticks were engorged, the Chao1 index significantly decreased.
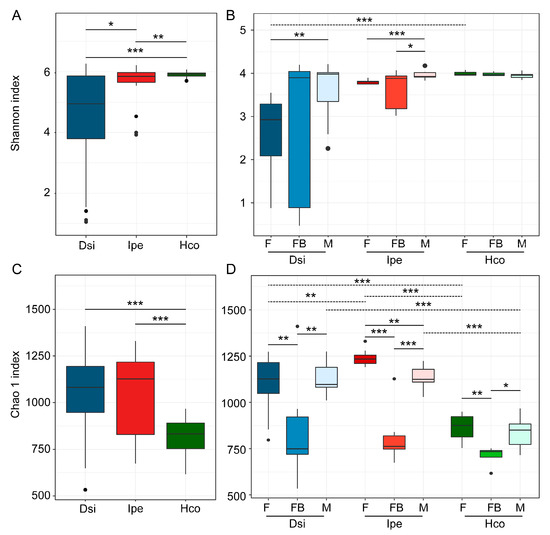
Figure 1.
Microbial diversity and richness in samples. (A,B) Shannon diversity indices at the OTU level for all tick species (A) or for each group within a species (B). (C,D) Chao1 indices at the OTU level for all tick species (C) or for each group within a species (D). The Wilcoxon rank test was used to determine significance (* p < 0.05, ** p < 0.01, *** p < 0.001) within species (full line) or groups (dotted line). Notes: F, female; FB, full of blood (engorged); M, male; Dsi, Dermacentor silvarum; Ipe, Ixodes spersulcatus; Hco, Haemaphysalis concinna.
3.2. Factors That Influence Microbiota Community Structure
To determine how sexes and feed stages influence the microbial composition, we compared the β-diversity of the microbiota of male and female (unfed or engorged) individuals within each tick species. All H. concinna samples showed a discrete distribution. Regarding I. spersulcatu, the microbial composition of engorged female samples was significantly different. The male and unfed female samples were clustered in different locations, and there was no significant difference within the group (Figure 2A). The β-diversity of males was more divergent in all three tick species but was not significantly different from that of engorged females; unfed females showed significant differences (Figure 2B).
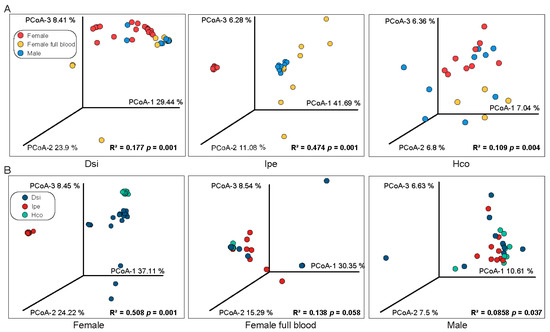
Figure 2.
PCA analyses for detecting similarities between different samples. Principal coordinate analysis (OTU level) using Bray–Curtis dissimilarity, comparing identified OTUs within a group (A) or species (B). Notes: The R2 and p values generated by the PERMANOVA analysis are shown in the lower right of the plot. Dsi, Dermacentor silvarum; Ipe, Ixodes spersulcatus; Hco, Haemaphysalis concinna.
3.3. Common and Differentially Abundant Bacteria between and within Tick Species
The data were examined to determine if tick species share or possess unique bacterial genera. The bacteria were largely common in all species. No matter which group, I. spersulcatus had more unique bacterial genera, but among all of the engorged female samples, D. silvarum had the most specific bacterial genera. Similarly, we analysed the genera of bacteria that were unique or shared among females, males, and engorged females. Unfed females had the most unique bacterial genera in samples of D. silvarum and I. spersulcatus, whereas engorged females had the most unique bacterial genera in samples of H. concinna (Figure 3A).
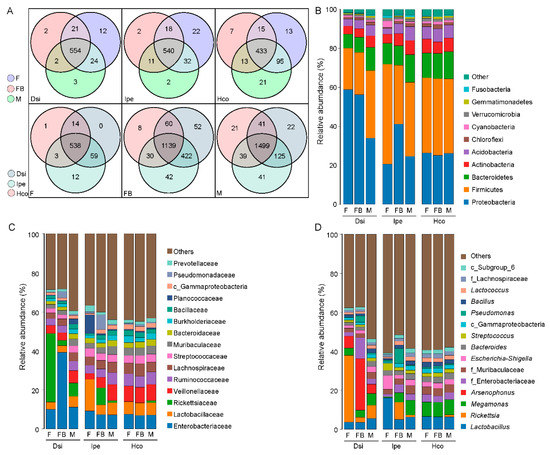
Figure 3.
Common and differentially abundant bacteria (different level) within ticks. Venn diagram showing number of common bacterial genera between tick groups and species (A). Histogram of species distribution at different taxonomic levels (B–D). Notes: F, female; FB, full of blood (engorged); M, male; Dsi, Dermacentor silvarum; Ipe, Ixodes spersulcatus; Hco, Haemaphysalis concinna.
All of the OTUs were assigned to 26 phyla, 67 classes, 159 orders, 279 families, and 627 genera, the detailed information of which is presented in Table S1. At the phylum level, Proteobacteria and Firmicutes were the dominant phyla in all ticks. Additionally, Bacteroidetes, Actinobacteria, and Acidobacteria were the main symbiotic bacteria in all ticks (Figure 3B). In D. silvarum, the relative abundance of Proteobacteria in all samples was higher than that in Firmicutes, and the relative abundance of Proteobacteria in the female samples was higher than that in the male samples. The relative abundance of Proteobacteria showed a decreasing trend after the ticks were filled with blood. In contrast, the relative abundance of Proteobacteria decreased after I. persulcatus ticks were filled with blood. The bacteria of different phyla in H. concinna showed a relatively stable state, consistent with the α-diversity results (Figure 3B).
The histogram of sample distribution at the family level showed that bacteria in all samples were mainly distributed in Lactobacillaceae, Veillonellaceae, Streptococcaceae, Ruminococcaceae, Bacteroidaceae, Muribaculaceae, and Rickettsiaceae. Enterobacteriaceae was the most common family. The relative abundance of Enterobacteriaceae increased, and that of Rickettsiae decreased after D. silvarum was engorged. Different from D. silvarum, the relative abundance of Rickettsiae increased significantly after I. persulcatus was engorged. The relative abundance of Lachnospiraceae increased after H. concinna was engorged (Figure 3C).
According to the genus-level analysis of sequencing data, the microbiota of the three species were dominated by Lactobacillus, Rickettsia, Megamonas, and Escherichia-Shigella. Arsenophonus was a highly abundant genus in D. silvarum. The relative abundance of Rickettsia was higher in unfed D. silvarum. The relative abundances of Arsenophonus and Pseudomonas increased after the ticks were engorged. The relative abundance of Lactobacillus was higher in unfed I. persulcatus. The relative abundance of Rickettsia and Pseudomonas increased, whereas the relative abundance of Lactobacillus decreased when the ticks were engorged. No significant changes were observed in the sex or feed stage of H. concinna (Figure 3D).
To detect the microbial signatures in each factor, LEfSe was conducted. When H. concinna was compared with the other two species, the microbiota showed the greatest differences, in agreement with the results of our β-diversity analysis (Figures S1–S3). Different bacterial genera were discovered between species regardless of feed stage or sex. It seems that feed stage and sex are not very influential on these specific bacterial genera. For example, Megamonas and Serratia were more abundant in H. concinna than in I. persulcatus and D. silvarum, regardless of sex (Figures S1–S3). Based on differences in abundance within the same species, we found infection gradients between sexes and feed stages. For example, Coxiella and Arsenophonus were found in higher abundance in female D. silvarum than in male ticks and engorged ticks (Figure S4). We were able to determine the bacterial abundance of specific microbes across species by comparing differential abundances within a group. For example, in I. persulcatus and D. silvarum, the abundance of Serratia and Macromonas was higher in unfed females than in males and engorged females (Figures S4–S6).
3.4. Microbial Interaction within Ticks
In order to identify potential patterns of microbial interaction, histograms and networks of microbial interactions were created based on data from 16S rRNA sequencing. The Computational model used presence/absence and relative abundance data to identify pairwise relationships between bacteria families. In general, microbial interactions with D. silvarum had more connections than with I. persulcatus and H. concinna (Figure 4, Figure S7). All networks showed antagonism and synergy interaction patterns for all tick species except for the male D. silvarum. The number of positive interactions was higher than that of negative interactions (Figure S7). We were able to identify taxa within these interactions that appeared to be important to the overall structure of the microbiota. Interestingly, we found that bacteria with high abundance were not important families in network interactions. Only three of the five most abundant families showed interactions. Rickettsiaceae and Lactobacillaceae did not show interactions in all samples. Positive interactions of Bacteroidaceae and F_Solibacteraceae Subgroup 3 with other bacterial families were detected in all groups (Figure 4).
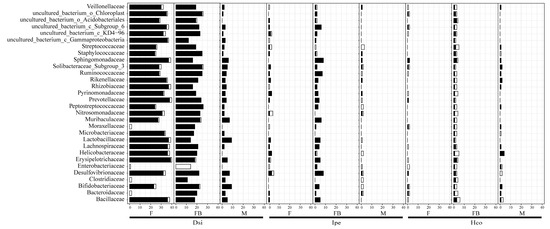
Figure 4.
Microbial interactions within ticks. The top 30 bacterial families are included in the chart. White represents co-exclusion/negative correlation, black represents co-occurrence/positive correlation interactions. Notes: F, female; FB, full of blood (engorged); M, male; Dsi, Dermacentor silvarum; Ipe, Ixodes spersulcatus; Hco, Haemaphysalis concinna.
4. Discussion
In order to gain a deeper understanding of the factors that influence the tick microbiota, we sequenced the microbiota of field-caught I. persulcatus, D. silvarum, and H. concinna adult ticks, which are common species in China. Evidently, the factors that make up the microbiota are complex. The Shannon index showed significant differences between the three species of ticks in terms of their microbiota diversity. The total bacterial diversity was similar between different sexes and feed stages in H. concinna, but it was different in I. persulcatus and D. silvarum. The tick species was also found to affect bacterial diversity, as in other studies [30,31]. The Chao1 index significantly decreased when the ticks were engorged, which suggests that some low-abundance bacteria were killed or expelled after a tick was fed. Some bacteria that initially inhabit the blood of animals or humans enter ticks through blood-feeding. Ticks have complex life cycles, and the off-host period is long; therefore, the microbiota inhabiting ticks could be shaped by the environment and, through interactions with blood meals, have an influence on the tick microbiota. Some studies of tick microbes have reported similar results [32,33,34]. When possible, blood feeding activates the immune system of the tick or insect, inhibited by the immune deficiency (IMD) pathway, which results in antimicrobial peptides (AMP) being expressed and bacteria surviving. The microorganisms that can adapt to this changed microenvironment have a greatly increased abundance in the gut bacteria. The diversity of microorganisms was significantly reduced due to some species being eliminated or greatly reduced [16]. However, the groupings of individuals from H. concinna showed less variation when comparing sexes and feed stages. At the same time, these results indicate that the bacterial diversity of male individuals was higher than that of female individuals in D. silvarum and I. persulcatus. There is no doubt that tick sex influences the diversity of microbial communities. We found that male ticks have a more diverse bacterial microbiota than female ticks in D. silvarum and I. persulcatus, which was consistent with other studies. For example, male Dermacentor reticulatus and Dermacentor marginatus have relatively richer and more diverse microbiotas than female ticks [35]. Additionally, similar results were found in Haemaphysalis longicornis [35,36], Rhipicephalus (Boophilus) microplus [37], and Rhipicephalus turanicus [38]. However, there was no significant difference in H. concinna in our study. The reason for this finding needs to be determined by further analysis.
Our findings reveal that the phylum Proteobacteria is the most dominant tick microbiota, followed by Firmicutes, Bacteroidetes, and Acinetobacter, which is consistent with previous research in ticks [31,38,39]. Several bacterial families were the same in different tick species, such as Enterobacteriaceae, Lactobacillaceae, Ruminococcaceae, etc., which was similar to findings in other tick species [39,40]. New microbial control strategies for ticks could use these common taxa as candidate bacteria, especially those causing infection at high abundances. Additionally, these core bacterial florae play indispensable roles in the growth and development of arthropods [41]. In comparison with D. silvarum and I. persulcatus, H. concinna had more differentially abundant genera, which explains the greater divergence of its microbiota. We observed a large number of different bacterial genera in ticks, but the genera that have been reported to have important effects on tick growth and development were not found in all tick samples; the reason may be that other symbiotic bacteria perform the same function in different tick species. For example, the Coxiella-LE genome was shown to encode the major pathways responsible for the synthesis of B vitamins, such as biotin (B7 vitamin), folic acid (B9), riboflavin (B2), and their cofactors, which are not usually obtainable in sufficient quantities from a uniquely blood-based diet [42]. However, Coxiella-LE was not detected in all the samples in this study. Other bacteria that code for the same gene also constitute an area for future research.
It is unknown how ticks interact with microorganisms. The majority of evidence for microbial interactions within blood-sucking arthropods comes from Wolbachia and its colonies [43,44,45,46] or transmitted symbionts in other arthropods [14,47,48]. Therefore, we are relatively familiar with inherited symbionts, whereas we are less familiar with the magnitude of interactions between the microbes found in arthropod guts. Our analysis identified synergistic and antagonistic interactions, which substantially increased the number of bacterial interactions observed in ticks. The α-diversity of the microbiota may explain the differences observed in the network structure. More complex networks generally had more OTUs. Network analysis using the entire dataset revealed that most of the detected interactions among members of the D. silvarum microbiota were positive, whereas those in other tick species were different. Taxa with positive associations are often interpreted as bacteria that perform similar or complementary functions [49,50]. Negative associations may also reflect interactions such as competition and niche partitioning. Accordingly, the majority of correlations being positive indicates that D. silvarum microbial communities perform similar or complementary functions and favour mutualism; this is not the case with the other two species.
Many factors may influence the microbiota network structure. In particular, the microbial networks could differ in tissues such as the salivary glands, germline, and gut. For example, microbial network analysis from the human microbiota project revealed that microbial networks were different depending on the site of the body [51,52]. Within the same individual, the microbiota of the reproductive organs was more diverse than that of the gut and salivary glands. Salivary glands, mostly in Anophelines, showed higher diversity indices when compared with the guts, similar to that reported in Anophelines Culicifacies [53]. Here, the whole tick was used for sampling, whereas bacteria can reside in different organs of a tick. Although there is no direct interaction, it is possible for co-occurrence at the tick level to result in indirect interactions, such as the host immune system. In the future, the analysis of distinct tissues may uncover further information.
5. Conclusions
The bacterial richness and microbiota structures of D. silvarum, H. concinna, and I. persulcatus were significantly different, and the bacterial richness of all ticks decreased significantly after they became engorged. There were synergistic or antagonistic relationships among co-occurring bacteria of different tick species, which are conducive to providing basic data for the prevention of ticks and tick-borne diseases.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani12233237/s1, Figure S1: LEfSe conducted based on bacterial community in unfed female ticks; Figure S2: LEfSe conducted based on bacterial community in engorged female ticks; Figure S3: LEfSe conducted based on bacterial community in male ticks; Figure S4: LEfSe conducted based on bacterial community in D. silvarum; Figure S5: LEfSe conducted based on bacterial community in I. spersulcatus; Figure S6: LEfSe conducted based on bacterial community in H. concinna; Figure S7: Microbial interaction networks within ticks; Table S1: Complete OTU table with read counts from each individual ticks.
Author Contributions
Data curation, T.W., S.L. and J.G.; Formal analysis, Q.L., X.L. and Y.Y.; Methodology, H.J.; Writing—original draft, H.Q.; Writing—review and editing, Q.C. and C.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (32072885), State Key Research Development Program of China (2019YFC1200501), Science and Technology Plan Medical and Health Category Project of Shantou (Grant No. 220511176491121), and Heilongjiang Bayi Agricultural University Support Program for San Heng San Zong (TDJH202002).
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee of Heilongjiang Bayi Agricultural University (protocol code HBAU-2018002).
Data Availability Statement
The data presented in this study are openly available in NCBI at https://www.ncbi.nlm.nih.gov/bioproject/PRJNA849745, accessed on 8 June 2022, accession number PRJNA849745.
Acknowledgments
We would like to acknowledge the staff and workers in the filed who helped in the collection of ticks. We thank MDPI AUTHOR services (https://www.mdpi.com/authors/english, accessed on 8 June 2022) for editing this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, Y.; Gilbreath, T.M., 3rd; Kukutla, P.; Yan, G.; Xu, J. Dynamic gut microbiome across life history of the malaria mosquito Anopheles gambiae in Kenya. PLoS ONE 2011, 6, e24767. [Google Scholar] [CrossRef] [PubMed]
- Osei-Poku, J.; Mbogo, C.M.; Palmer, W.J.; Jiggins, F.M. Deep sequencing reveals extensive variation in the gut microbiota of wild mosquitoes from Kenya. Mol. Ecol. 2012, 21, 5138–5150. [Google Scholar] [CrossRef] [PubMed]
- Buck, M.; Nilsson, L.K.; Brunius, C.; Dabiré, R.K.; Hopkins, R.; Terenius, O. Bacterial associations reveal spatial population dynamics in Anopheles gambiae mosquitoes. Sci. Rep. 2016, 6, 22806. [Google Scholar] [CrossRef] [PubMed]
- Muturi, E.J.; Ramirez, J.L.; Rooney, A.P.; Kim, C.H. Comparative analysis of gut microbiota of mosquito communities in central Illinois. PLoS Negl. Trop. Dis. 2017, 11, e0005377. [Google Scholar] [CrossRef]
- Yin, C.; Sun, P.; Yu, X.; Wang, P.; Cheng, G. Roles of symbiotic microorganisms in arboviral infection of arthropod vectors. Trends Parasitol. 2020, 36, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Couper, L.I.; Kwan, J.Y.; Ma, J.; Swei, A. Drivers and patterns of microbial community assembly in a Lyme disease vector. Ecol. Evol. 2019, 9, 7768–7779. [Google Scholar] [CrossRef]
- Wu, C.A.; Hodžić, A.; Mateos, H.L.; Estrada, P.A.; Obregon, D.; Cabezas, C.A. Current debates and advances in tick microbiome research. CRPVBD 2021, 6, 100036. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, X.J. Systematic Taxonomy of Ticks, 2nd ed.; China Science Publishing and Media: Beijing, China, 2021; pp. 201–596. [Google Scholar]
- Adegoke, A.; Kumar, D.; Bobo, C.; Rashid, M.I.; Durrani, A.Z.; Sajid, M.S.; Karim, S. Tick-borne pathogens shape the native microbiome within tick vectors. Microorganisms 2020, 8, 1299. [Google Scholar] [CrossRef]
- Jia, N.; Wang, J.; Shi, W.; Du, L.; Sun, Y.; Zhan, W.; Jiang, J.F.; Wang, Q.; Zhang, B.; Ji, P.; et al. Large-scale comparative analyses of tick genomes elucidate their genetic diversity and vector capacities. Cell 2020, 182, 1328–1340. [Google Scholar] [CrossRef] [PubMed]
- Klyachko, O.; Stein, B.D.; Grindle, N.; Clay, K.; Fuqua, C. Localization and visualization of a coxiella-type symbiont within the lone star tick, Amblyomma americanum. Appl. Environ. Microbiol. 2007, 73, 6584–6594. [Google Scholar] [CrossRef] [PubMed]
- Machado-Ferreira, E.; Dietrich, G.; Hojgaard, A.; Levin, M.; Piesman, J.; Zeidner, N.S.; Soares, C.A. Coxiella symbionts in the cayenne tick Amblyomma cajennense. Microb. Ecol. 2011, 62, 134–142. [Google Scholar] [CrossRef]
- Almeida, A.P.; Marcili, A.; Leite, R.C.; Nieri-Bastos, F.A.; Domingues, L.N.; Martins, J.R.; Labruna, M.B. Coxiella symbiont in the tick Ornithodoros rostratus (Acari: Argasidae). Ticks Tick Borne Dis. 2012, 3, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Macaluso, K.R.; Sonenshine, D.E.; Ceraul, S.M.; Azad, A.F. Rickettsial infection in Dermacentor variabilis (Acari: Ixodidae) inhibits transovarial transmission of a second Rickettsia. J. Med. Entomol. 2002, 39, 809–813. [Google Scholar] [CrossRef] [PubMed]
- Gall, C.A.; Reif, K.E.; Scoles, G.A.; Mason, K.L.; Mousel, M.; Noh, S.M.; Brayton, K.A. The bacterial microbiome of Dermacentor andersoni ticks influences pathogen susceptibility. ISME J. 2016, 10, 1846–1855. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, S.I.; Binetruy, F.; Hernández-Jarguín, A.M.; Duron, O. The tick microbiome: Why non-pathogenic microorganisms matter in tick biology and pathogen transmission. Front. Cell Infect. Microbiol. 2017, 7, 236. [Google Scholar] [CrossRef]
- Black, W.C.; Piesman, J. Phylogeny of hard- and soft-tick taxa (Acari: Ixodida) based on mitochondrial 16S rDNA sequences. Proc. Natl. Acad. Sci. USA 1994, 21, 10034–10038. [Google Scholar] [CrossRef] [PubMed]
- Binetruy, F.; Dupraz, M.; Buysse, M.; Duron, O. Surface sterilization methods impact measures of internal microbial diversity in ticks. Parasit Vectors 2019, 12, 268. [Google Scholar] [CrossRef] [PubMed]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Solymos, P.; Stevens, M.; Szoecs, E.; et al. Vegan: Community Ecology Package, R Package Version 2.6-4. 2022. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 8 June 2022).
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Ligges, U.; Mächler, M. Scatterplot3d—an R Package for Visualizing Multivariate Data. J. Stat. Softw. 2003, 8, 1–20. [Google Scholar] [CrossRef]
- Sedgwick, P. Spearman’s rank correlation coefficient. BMJ 2014, 349, g7327. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Bastian, M.; Heymann, S.; Jacomy, M. Gephi: An open source software for exploring and manipulating networks. Icwsm 2009, 8, 361–362. [Google Scholar] [CrossRef]
- Van Treuren, W.; Ponnusamy, L.; Brinkerhoff, R.J.; Gonzalez, A.; Parobek, C.M.; Juliano, J.J.; Andreadis, T.G.; Falco, R.C.; Ziegler, L.B.; Hathaway, N.; et al. Variation in the microbiota of Ixodes ticks with regard to geography, species, and sex. Appl. Environ. Microbiol. 2015, 81, 6200–6209. [Google Scholar] [CrossRef] [PubMed]
- Sperling, J.L.; Silva-Brandão, K.L.; Brandão, M.M.; Lloyd, V.K.; Dang, S.; Davis, C.S.; Sperling, F.A.H.; Magor, K.E. Comparison of bacterial 16S rRNA variable regions for microbiome surveys of ticks. Ticks Tick Borne Dis. 2017, 8, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Kwan, J.Y.; Griggs, R.; Chicana, B.; Miller, C.; Swei, A. Vertical VS. horizontal transmission of the microbiome in a key disease vector, Ixodes pacificus. Mol. Ecol. 2017, 23, 6578–6589. [Google Scholar] [CrossRef] [PubMed]
- Swei, A.; Kwan, J.Y. Tick microbiome and pathogen acquisition altered by host blood meal. ISME J. 2017, 11, 813–816. [Google Scholar] [CrossRef] [PubMed]
- Lalzar, I.; Harrus, S.; Mumcuoglu, K.Y.; Gottlieb, Y. Composition and seasonal variation of Rhipicephalus turanicus and Rhipicephalus sanguineus bacterial communities. Appl. Environ. Microbiol. 2012, 78, 4110–4116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.K.; Yu, Z.J.; Wang, D.; Bronislava, V.; Branislav, P.; Liu, J.Z. The bacterial microbiome of field-collected Dermacentor marginatus and Dermacentor reticulatus from Slovakia. Parasit. Vectors 2019, 12, 325. [Google Scholar] [CrossRef] [PubMed]
- Ruiling, Z.; Zhendong, H.; Guangfu, Y.; Zhong, Z. Characterization of the bacterial community in Haemaphysalis longicornis (Acari: Ixodidae) throughout developmental stages. Exp. Appl. Acarol. 2019, 77, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Andreotti, R.; Pérez de León, A.A.; Dowd, S.E.; Guerrero, F.D.; Bendele, K.G.; Scoles, G.A. Assessment of bacterial diversity in the cattle tick Rhipicephalus (Boophilus) microplus through tag-encoded pyrosequencing. BMC Microbiol. 2011, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Tekin, S.; Dowd, S.E.; Davinic, M.; Bursali, A.; Keskin, A. Pyrosequencing based assessment of bacterial diversity in Turkish Rhipicephalus annulatus and Dermacentor marginatus ticks (Acari: Ixodidae). Parasitol. Res. 2017, 116, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Brinkerhoff, R.J.; Clark, C.; Ocasio, K.; Gauthier, D.T.; Hynes, W.L. Factors affecting the microbiome of Ixodes scapularis and Amblyomma americanum. PLoS ONE 2020, 15, e0232398. [Google Scholar] [CrossRef] [PubMed]
- Sperling, J.L.H.; Fitzgerald, D.; Sperling, F.A.H.; Magor, K.E. Microbiome composition and Borrelia detection in Ixodes scapularis ticks at the northwestern edge of their range. Trop. Med. Infect. Dis. 2020, 5, 173. [Google Scholar] [CrossRef] [PubMed]
- Saldaña, M.A.; Hegde, S.; Hughes, G.L. Microbial control of arthropod-borne disease. Mem. Inst. Oswaldo. Cruz. 2017, 112, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.A.; Driscoll, T.; Gillespie, J.J.; Raghavan, R. A Coxiella-like endosymbiont is a potential vitamin source for the Lone Star tick. Genome. Biol. Evol. 2015, 7, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Zink, S.D.; Van Slyke, G.A.; Palumbo, M.J.; Kramer, L.D.; Ciota, A.T. Exposure to West Nile virus increases bacterial diversity and immune gene expression in Culex pipiens. Viruses 2015, 7, 5619–5631. [Google Scholar] [CrossRef]
- Audsley, M.D.; Seleznev, A.; Joubert, D.A.; Woolfit, M.; O’Neill, S.L.; McGraw, E.A. Wolbachia infection alters the relative abundance of resident bacteria in adult Aedes aegypti mosquitoes, but not larvae. Mol. Ecol. 2018, 27, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Hughes, G.L.; Dodson, B.L.; Johnson, R.M.; Murdock, C.C.; Tsujimoto, H.; Suzuki, Y.; Patt, A.A.; Cui, L.; Nossa, C.W.; Barry, R.M.; et al. Native microbiome impedes vertical transmission of Wolbachia in Anopheles mosquitoes. Proc. Natl. Acad Sci. USA 2014, 111, 12498–12503. [Google Scholar] [CrossRef] [PubMed]
- Rossi, P.; Ricci, I.; Cappelli, A.; Damiani, C.; Ulissi, U.; Mancini, M.V.; Valzano, M.; Capone, A.; Epis, S.; Crotti, E.; et al. Mutual exclusion of Asaia and Wolbachia in the reproductive organs of mosquito vectors. Parasit. Vectors 2015, 8, 278. [Google Scholar] [CrossRef] [PubMed]
- Kondo, N.; Shimada, M.; Fukatsu, T. Infection density of Wolbachia endosymbiont affected by co-infection and host genotype. Biol. Lett. 2005, 1, 488–491. [Google Scholar] [CrossRef] [PubMed]
- Rock, D.I.; Smith, A.H.; Joffe, J.; Albertus, A.; Wong, N.; O’Connor, M.; Oliver, K.M.; Russell, J.A. Context-dependent vertical transmission shapes strong endosymbiont community structure in the pea aphid, Acyrthosiphon pisum. Mol. Ecol. 2018, 27, 2039–2056. [Google Scholar] [CrossRef] [PubMed]
- Eiler, A.; Heinrich, F.; Bertilsson, S. Coherent dynamics and association networks among lake bacterioplankton taxa. ISME J. 2012, 6, 330–342. [Google Scholar] [CrossRef]
- Chow, C.E.; Kim, D.Y.; Sachdeva, R.; Caron, D.A.; Fuhrman, J.A. Top-down controls on bacterial community structure: Microbial network analysis of bacteria, T4-like viruses and protists. ISME J. 2014, 8, 816–829. [Google Scholar] [CrossRef] [PubMed]
- Faust, K.; Raes, J. Microbial interactions: From networks to models. Nat. Rev. Microbiol. 2012, 10, 538–550. [Google Scholar] [CrossRef]
- Faust, K.; Raes, J. CoNet app: Inference of biological association networks using cytoscape. F1000Research 2016, 5, 1519. [Google Scholar] [CrossRef]
- Sharma, P.; Sharma, S.; Maurya, R.K.; Das De, T.; Thomas, T.; Lata, S.; Singh, N.; Pandey, K.C.; Valecha, N.; Dixit, R. Salivary glands harbor more diverse microbial communities than gut in Anopheles culicifacies. Parasit. Vectors 2014, 7, 235. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).