Effect of Dietary Rumen-Degradable Starch to Rumen-Degradable Protein Ratio on In Vitro Rumen Fermentation Characteristics and Microbial Protein Synthesis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Treatments
2.2. Animals and Diet
2.3. In Situ Ruminal Degradation
2.4. Batch Culture Procedure
2.5. Gas Production and Sampling
2.6. Chemical Analysis
2.7. Statistical Analysis
3. Results
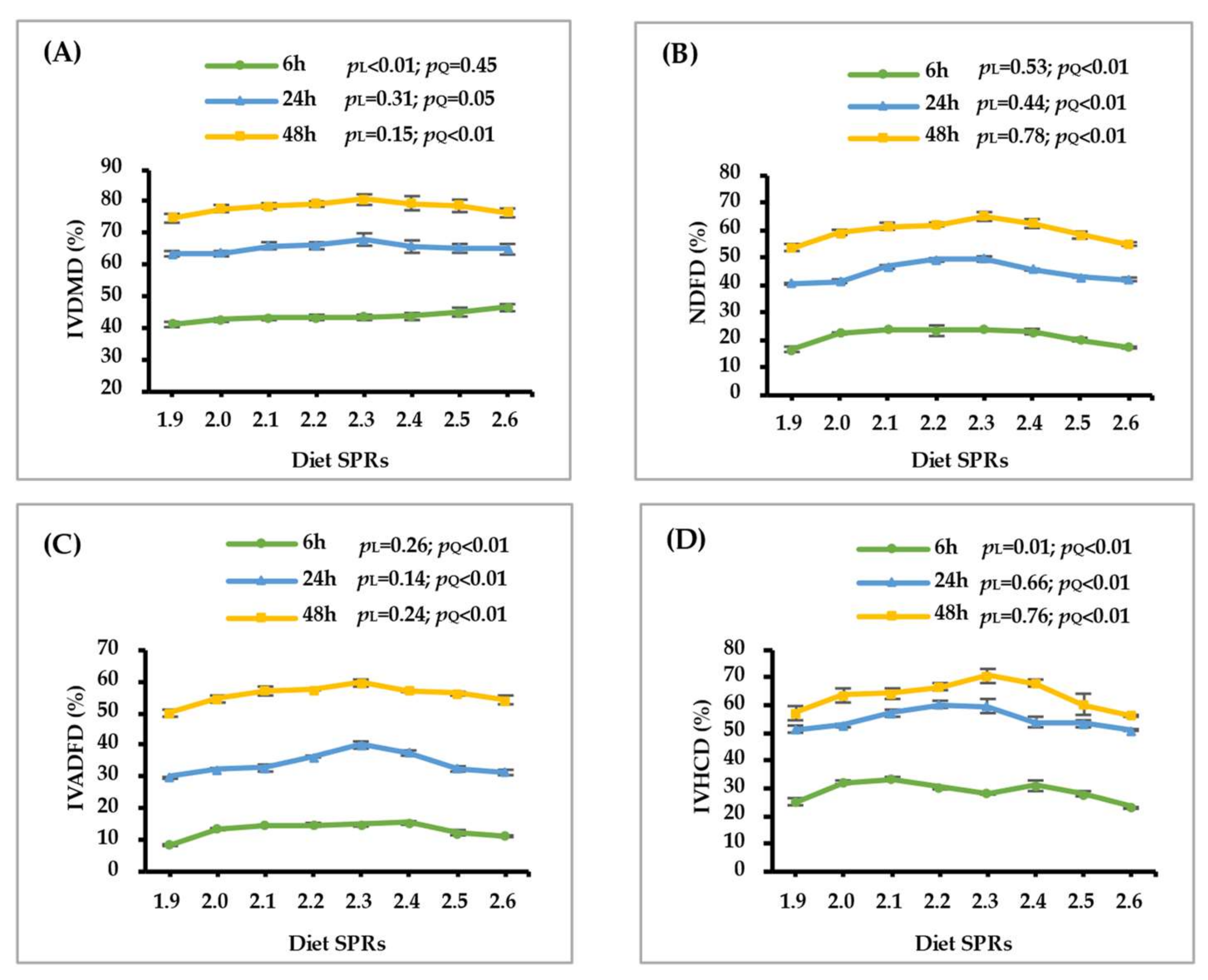
3.1. In Vitro Nutrients Disappearance and Gas Production
3.2. Rumen Ammonia Nitrogen Concentration and Microbial Protein Synthesis
3.3. Rumen Fermentation Characteristics
4. Discussion
4.1. In Vitro Nutrients Disappearance and Gas Production
4.2. Rumen Ammonia Nitrogen Concentration and Microbial Protein Synthesis
4.3. Rumen Fermentation Characteristics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matthews, C.; Crispie, F.; Lewis, E.; Reid, M.; O’Toole, P.W.; Cotter, P.D. The rumen microbiome: A crucial consideration when optimising milk and meat production and nitrogen utilisation efficiency. Gut Microbes 2019, 10, 115–132. [Google Scholar] [CrossRef] [PubMed]
- Belanche, A.; Doreau, M.; Edwards, J.E.; Moorby, J.M.; Pinloche, E.; Newbold, C.J. Shifts in the rumen microbiota due to the type of carbohydrate and level of protein ingested by dairy cattle are associated with changes in rumen fermentation. J. Nutr. 2012, 142, 1684–1692. [Google Scholar] [CrossRef] [PubMed]
- Schwab, C.G.; Satter, L.D.; Clay, A.B. Response of Lactating Dairy Cows to Abomasal Infusion of Amino Acids. J. Dairy Sci. 1976, 59, 1254–1270. [Google Scholar] [CrossRef]
- Schwab, C.G.; Broderick, G.A. A 100-Year Review: Protein and amino acid nutrition in dairy cows. J. Dairy Sci. 2017, 100, 10094–10112. [Google Scholar] [CrossRef]
- Mansfield, H.R.; Endres, M.I.; Stern, M.D. Influence of non-fibrous carbohydrate and degradable intake protein on fermentation by ruminal microorganisms in continuous culture. J. Anim. Sci. 1994, 72, 2464–2474. [Google Scholar] [CrossRef] [PubMed]
- Mabjeesh, S.J.; Arieli, A.; Bruckental, I.; Zamwell, S.; Tagari, H. Effect of Ruminal Degradability of Crude Protein and Nonstructural Carbohydrates on the Efficiency of Bacterial Crude Protein Synthesis and Amino Acid Flow to the Abomasum of Dairy Cows. J. Dairy Sci. 1997, 80, 2939–2949. [Google Scholar] [CrossRef]
- Sinclair, L.A.; Garnsworth, P.C.; Newbold, J.R.; Buttery, P.J. Effect of synchronizing the rate of dietary energy and nitrogen release on rumen fermentation and microbial protein synthesis in sheep. J. Agr. Sci. 1993, 120, 251–263. [Google Scholar] [CrossRef]
- Bach, A.; Calsamiglia, S.; Stern, M.D. Nitrogen Metabolism in the Rumen. J. Dairy Sci. 2005, 88, E9–E21. [Google Scholar] [CrossRef]
- Cabrita, A.R.J.; Dewhurst, R.J.; Abreu, J.M.F.; Fonseca, A.J.M. Evaluation of the effects of synchronising the availability of N and energy on rumen function and production responses of dairy cows—A review. Anim. Res. 2006, 55, 1–24. [Google Scholar] [CrossRef]
- Ren, H.; Su, X.; Bai, H.; Yang, Y.; Wang, H.; Dan, Z.; Lu, J.; Wu, S.; Cai, C.; Cao, Y.; et al. Specific enrichment of microbes and increased ruminal propionate production: The potential mechanism underlying the high energy efficiency of Holstein heifers fed steam-flaked corn. AMB Express 2019, 9, 209. [Google Scholar] [CrossRef]
- Martins, C.; Fonseca, D.C.M.; Alves, B.G.; Arcari, M.A.; Ferreira, G.C.; Welter, K.C.; Oliveira, C.A.F.; Renno, F.P.; Santos, M.V. Effect of dietary crude protein degradability and corn processing on lactation performance and milk protein composition and stability. J. Dairy Sci. 2019, 102, 4165–4178. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.B. Dietary starch source and protein degradability in diets containing sucrose: Effects on ruminal measures and proposed mechanism for degradable protein effects. J. Dairy Sci. 2013, 96, 7093–7109. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.L.; McKinnon, J.J.; Mutsvangwa, T. Effects of dietary ruminally degradable starch and ruminally degradable protein levels on urea recycling, microbial protein production, nitrogen balance, and duodenal nutrient flow in beef heifers fed low crude protein diets. Can. J. Anim. Sci. 2013, 93, 123–136. [Google Scholar] [CrossRef]
- Nocek, J.E.; Russell, J.B. Protein and Energy as an Integrated System. Relationship of Ruminal Protein and Carbohydrate Availability to Microbial Synthesis and Milk Production. J. Dairy Sci. 1988, 71, 2070–2107. [Google Scholar] [CrossRef]
- Hall, M.B.; Huntington, G.B. Nutrient synchrony: Sound in theory, elusive in practice. J. Anim. Sci. 2008, 86, E287–E292. [Google Scholar] [CrossRef]
- Chanjula, P.; Wanapat, M.; Wachirapakorn, C.; Rowlinson, P. Effect of Synchronizing Starch Sources and Protein (NPN) in the Rumen on Feed Intake, Rumen Microbial Fermentation, Nutrient Utilization and Performance of Lactating Dairy Cows. Asian-Australas J. Anim. Sci. 2004, 17, 1400–1410. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Dairy Cattle; National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- Ørskov, E.R.; McDonald, I. The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. J. Agric. Sci. 1979, 92, 499–503. [Google Scholar] [CrossRef]
- Offner, A.; Bach, A.; Sauvant, D. Quantitative review of in situ starch degradation in the rumen. Anim. Feed Sci. Technol. 2003, 106, 81–93. [Google Scholar] [CrossRef]
- Zebeli, Q.; Dijkstra, J.; Tafaj, M.; Steingass, H.; Ametaj, B.N.; Drochner, W. Modeling the adequacy of dietary fiber in dairy cows based on the responses of ruminal pH and milk fat production to composition of the diet. J. Dairy Sci. 2008, 91, 2046–2066. [Google Scholar] [CrossRef]
- Menke, K.H.; Raab, L.; Salewski, A.; Steingass, H.; Fritz, D.; Schneider, W. The estimation of the digestibility and metabolizable energy content of ruminant feedingstuffs from the gas production when they are incubated with rumen liquor in vitro. J. Agric. Sci. 1979, 93, 217–222. [Google Scholar] [CrossRef]
- Theodorou, M.K.; Williams, B.A.; Dhanoa, M.S.; McAllan, A.B.; France, J. A simple gas production method using a pressure transducer to determine the fermentation kinetics of ruminant feeds. Anim. Feed Sci. Technol. 1994, 48, 185–197. [Google Scholar] [CrossRef]
- Mauricio, R.M.; Mould, F.L.; Dhanoa, M.S.; Owen, E.; Channa, K.S.; Theodorou, M.K. A semi-automated in vitro gas production technique for ruminant feedstuff evaluation. Anim. Feed Sci. Technol. 1999, 79, 321–330. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 17th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Broderick, G.A.; Kang, J.H. Automated Simultaneous Determination of Ammonia and Total Amino Acids in Ruminal Fluid and In Vitro Media. J. Dairy Sci. 1980, 63, 64–75. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Sharma, O.P.; Dawra, R.K.; Negi, S.S. Simple Determination of Microbial Protein in Rumen Liquor. J. Dairy Sci. 1982, 65, 2170–2173. [Google Scholar] [CrossRef]
- Erwin, E.S.; Marco, G.J.; Emery, E.M. Volatile Fatty Acid Analyses of Blood and Rumen Fluid by Gas Chromatography. J. Dairy Sci. 1961, 44, 1768–1771. [Google Scholar] [CrossRef]
- Saleem, A.M.; Nyachiro, J.; Gomaa, W.M.S.; Yang, W.; Oatway, L.; McAllister, T.A. Effects of barley type and processing method on rumen fermentation, dry matter disappearance and fermentation characteristics in batch cultures. Anim. Feed Sci. Technol. 2020, 269, 114625. [Google Scholar] [CrossRef]
- Gallo, A.; Giuberti, G.; Masoero, F. Gas production and starch degradability of corn and barley meals differing in mean particle size. J. Dairy Sci. 2016, 99, 4347–4359. [Google Scholar] [CrossRef]
- Xu, N.N.; Wang, D.M.; Wang, B.; Wang, J.K.; Liu, J.X. Different endosperm structures in wheat and corn affected in vitro rumen fermentation and nitrogen utilization of rice straw-based diet. Animal 2019, 13, 1607–1613. [Google Scholar] [CrossRef]
- Jacobs, J.L. Challenges in ration formulation in pasture-based milk production systems. Anim. Prod. Sci. 2014, 54, 1130–1140. [Google Scholar] [CrossRef]
- Leddin, C.M.; Stockdale, C.R.; Hill, J.; Heard, J.W.; Doyle, P.T. Increasing amounts of crushed wheat fed with pasture hay reduced dietary fiber digestibility in lactating dairy cows. J. Dairy Sci. 2009, 92, 2747–2757. [Google Scholar] [CrossRef] [PubMed]
- Lechartier, C.; Peyraud, J.L. The effects of starch and rapidly degradable dry matter from concentrate on ruminal digestion in dairy cows fed corn silage-based diets with fixed forage proportion. J. Dairy Sci. 2011, 94, 2440–2454. [Google Scholar] [CrossRef]
- Tan, H.Y.; Sieo, C.C.; Abdullah, N.; Liang, J.B.; Huang, X.D.; Ho, Y.W. Effects of condensed tannins from Leucaena on methane production, rumen fermentation and populations of methanogens and protozoa in vitro. Anim. Feed Sci. Technol. 2011, 169, 185–193. [Google Scholar] [CrossRef]
- Pang, D.G.; Yang, H.J.; Cao, B.B.; Wu, T.T.; Wang, J.Q. The beneficial effect of Enterococcus faecium on the in vitro ruminal fermentation rate and extent of three typical total mixed rations in northern China. Livest. Sci. 2014, 167, 154–160. [Google Scholar] [CrossRef]
- Russell, J.B.; O’Connor, J.D.; Fox, D.G.; Van Soest, P.J.; Sniffen, C.J. A net carbohydrate and protein system for evaluating cattle diets: I. Ruminal fermentation. J. Anim. Sci. 1992, 70, 3551–3561. [Google Scholar] [CrossRef]
- Piao, M.Y.; Kim, H.J.; Seo, J.K.; Park, T.S.; Yoon, J.S.; Kim, K.H.; Ha, J.K. Effects of synchronization of carbohydrate and protein supply in total mixed ration with korean rice wine residue on ruminal fermentation, nitrogen metabolism and microbial protein synthesis in holstein steers. Asian-Australas J. Anim. Sci. 2012, 25, 1568–1574. [Google Scholar] [CrossRef]
- Agle, M.; Hristov, A.N.; Zaman, S.; Schneider, C.; Ndegwa, P.; Vaddella, V.K. The effects of ruminally degraded protein on rumen fermentation and ammonia losses from manure in dairy cows. J. Dairy Sci. 2010, 93, 1625–1637. [Google Scholar] [CrossRef]
- Satter, L.D.; Slyter, L.L. Effect of ammonia concentration of rumen microbial protein production in vitro. Br. J. Nutr. 1974, 32, 199–208. [Google Scholar] [CrossRef]
- Schwab, C.G.; Huhtanen, P.; Hunt, C.; Hvelplund, T. Nitrogen Requirements of Cattle; Pfeffer, A.H.E., Ed.; CABI Publishing: Wallingford, UK, 2005. [Google Scholar]
- Odle, J.; Schaefer, D.M. Influence of rumen ammonia concentration on the rumen degradation rates of barley and maize. Br. J. Nutr. 1987, 57, 127–138. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, N.; Shen, W.; Zhao, S.; Wang, J. Synchrony Degree of Dietary Energy and Nitrogen Release Influences Microbial Community, Fermentation, and Protein Synthesis in a Rumen Simulation System. Microorganisms 2020, 8, 231. [Google Scholar] [CrossRef]
- Munnich, M.; Khol-Parisini, A.; Klevenhusen, F.; Metzler-Zebeli, B.U.; Zebeli, Q. Graded replacement of maize grain with molassed sugar beet pulp modulated ruminal microbial community and fermentation profile in vitro. J. Sci. Food Agric. 2018, 98, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Voelker, J.A.; Allen, M.S. Pelletsed Beet Pulp Substituted for High-Moisture Corn: 3. Effects on Ruminal Fermentation, pH, and Microbial Protein Efficiency in Lactating Dairy Cows. J. Dairy Sci. 2003, 86, 3562–3570. [Google Scholar] [CrossRef]
- Savari, M.; Khorvash, M.; Amanlou, H.; Ghorbani, G.R.; Ghasemi, E.; Mirzaei, M. Effects of rumen-degradable protein:rumen-undegradable protein ratio and corn processing on production performance, nitrogen efficiency, and feeding behavior of Holstein dairy cows. J. Dairy Sci. 2018, 101, 1111–1122. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, J.; Ellis, J.L.; Kebreab, E.; Strathe, A.B.; López, S.; France, J.; Bannink, A. Ruminal pH regulation and nutritional consequences of low pH. Anim. Feed Sci. Technol. 2012, 172, 22–33. [Google Scholar] [CrossRef]
- Shen, Y.Z.; Ran, T.; Saleem, A.M.; Wang, H.R.; Yang, W.Z. Short communication: Ground corn steeped in citric acid modulates in vitro gas production kinetics, fermentation patterns and dry matter digestibility. Anim. Feed Sci. Technol. 2019, 247, 9–14. [Google Scholar] [CrossRef]
- Kand, D.; Bagus Raharjo, I.; Castro-Montoya, J.; Dickhoefer, U. The effects of rumen nitrogen balance on in vitro rumen fermentation and microbial protein synthesis vary with dietary carbohydrate and nitrogen sources. Anim. Feed Sci. Technol. 2018, 241, 184–197. [Google Scholar] [CrossRef]
- Castro, M.M.D.; Cardoso, M.A.; Detmann, E.; Fonseca, M.A.; Sampaio, C.B.; Marcondes, M.I. In vitro ruminal fermentation and enteric methane production of tropical forage added nitrogen or nitrogen plus starch. Anim. Feed Sci. Technol. 2021, 275, 114878. [Google Scholar] [CrossRef]
- Wang, W.; Wu, Q.; Li, W.; Wang, Y.; Zhang, F.; Lv, L.; Li, S.; Yang, H. High-Gossypol Whole Cottonseed Exhibited Mediocre Rumen Degradability and Less Microbial Fermentation Efficiency than Cottonseed Hull and Cottonseed Meal with an In Vitro Gas Production Technique. Fermentation 2022, 8, 103. [Google Scholar] [CrossRef]
- McDonald, P. Animal Nutrition, 7th ed.; Prentice Hall: Harlow, UK, 2011. [Google Scholar]
- Davila, A.M.; Blachier, F.; Gotteland, M.; Andriamihaja, M.; Benetti, P.H.; Sanz, Y.; Tome, D. Intestinal luminal nitrogen metabolism: Role of the gut microbiota and consequences for the host. Pharmacol. Res. 2013, 68, 95–107. [Google Scholar] [CrossRef]
- Hristov, A.N.; Etter, R.P.; Ropp, J.K.; Grandeen, K.L. Effect of dietary crude protein level and degradability on ruminal fermentation and nitrogen utilization in lactating dairy cows. J. Anim. Sci. 2004, 82, 3219–3229. [Google Scholar] [CrossRef]
- Vastolo, A.; Calabro, S.; Pacifico, S.; Koura, B.I.; Cutrignelli, M.I. Chemical and nutritional characteristics of Cannabis sativa L. co-products. J. Anim. Physiol. Anim. Nutr. 2021, 105 (Suppl. S1), 1–9. [Google Scholar] [CrossRef] [PubMed]
- Calsamiglia, S.; Stern, M.D.; Firkins, J.L. Effects of protein source on nitrogen metabolism in continuous culture and intestinal digestion in vitro. J. Anim. Sci. 1995, 73, 1819–1827. [Google Scholar] [CrossRef] [PubMed]
| Item 1 | Treatments (SPRs) | |||||||
|---|---|---|---|---|---|---|---|---|
| 1.9 | 2.0 | 2.1 | 2.2 | 2.3 | 2.4 | 2.5 | 2.6 | |
| Ingredients (% DM) | ||||||||
| Whole-plant corn silage | 31.48 | 31.48 | 31.48 | 31.48 | 31.48 | 31.48 | 31.48 | 31.48 |
| Alfalfa hay | 12.42 | 12.42 | 12.42 | 12.42 | 12.42 | 12.42 | 12.42 | 12.42 |
| Oat hay | 4.94 | 4.94 | 4.94 | 4.94 | 4.94 | 4.94 | 4.94 | 4.94 |
| Ground corn | 19.76 | 17.71 | 13.77 | 11.80 | 7.87 | 5.90 | 3.93 | 0.00 |
| Ground wheat | 0.00 | 2.07 | 6.00 | 7.97 | 11.90 | 13.87 | 15.83 | 19.77 |
| Soybean meal | 13.96 | 12.79 | 9.84 | 7.38 | 5.90 | 4.13 | 1.97 | 0.00 |
| Rumen-protected soybean meal | 0.00 | 0.98 | 2.95 | 4.92 | 5.71 | 6.89 | 8.85 | 10.33 |
| Wheat bran | 2.79 | 2.94 | 2.95 | 2.95 | 2.95 | 2.95 | 2.95 | 2.95 |
| Beet pellets | 9.26 | 9.28 | 10.26 | 10.75 | 11.44 | 12.03 | 12.16 | 12.64 |
| Fat powder | 2.36 | 2.36 | 2.36 | 2.36 | 2.36 | 2.36 | 2.44 | 2.44 |
| Premix 2 | 3.03 | 3.03 | 3.03 | 3.03 | 3.03 | 3.03 | 3.03 | 3.03 |
| Chemical compositions (% DM) | ||||||||
| CP | 15.75 | 15.83 | 15.71 | 15.66 | 15.65 | 15.56 | 15.61 | 15.68 |
| EE | 4.66 | 4.69 | 4.75 | 4.78 | 4.83 | 4.86 | 4.97 | 5.03 |
| Ash | 7.39 | 7.40 | 7.42 | 7.43 | 7.45 | 7.46 | 7.47 | 7.48 |
| Starch | 26.63 | 26.63 | 26.62 | 26.62 | 26.59 | 26.59 | 26.56 | 26.53 |
| aNDF | 32.95 | 33.18 | 33.84 | 34.25 | 34.73 | 35.11 | 35.38 | 35.84 |
| ADF | 17.18 | 17.21 | 17.41 | 17.52 | 17.67 | 17.79 | 17.82 | 17.94 |
| HC | 15.77 | 15.97 | 16.43 | 16.73 | 17.06 | 17.32 | 17.56 | 17.90 |
| RDS | 18.55 | 18.99 | 19.85 | 20.27 | 21.12 | 21.55 | 21.95 | 22.78 |
| RDP | 9.54 | 9.51 | 9.31 | 9.11 | 9.10 | 8.96 | 8.80 | 8.75 |
| SPR | 1.94 | 2.00 | 2.13 | 2.23 | 2.32 | 2.41 | 2.49 | 2.60 |
| NFC 3 | 39.25 | 38.90 | 38.27 | 37.88 | 37.33 | 37.01 | 36.58 | 35.97 |
| NEL (Mcal/kg of DM) | 1.70 | 1.70 | 1.69 | 1.69 | 1.68 | 1.67 | 1.67 | 1.67 |
| Item 1 | Whole-Plant Corn Silage | Oat Hay | Alfalfa Hay | Ground Wheat | Ground Corn | Soybean Meal | Rumen- Protected Soybean Meal | Wheat Bran | Beet Pellets |
|---|---|---|---|---|---|---|---|---|---|
| Chemical compositions (% DM) | |||||||||
| Starch | 34.05 | 6.67 | 3.76 | 63.90 | 65.62 | 3.50 | 3.52 | 22.60 | 9.62 |
| RDS | 27.80 | 4.32 | 2.26 | 60.19 | 39.55 | 2.28 | 1.65 | 14.85 | 8.14 |
| CP | 8.50 | 7.30 | 19.36 | 15.54 | 9.16 | 49.16 | 50.68 | 19.50 | 11.19 |
| RDP | 5.77 | 3.62 | 12.04 | 10.52 | 6.38 | 25.77 | 15.85 | 14.86 | 9.14 |
| ERD (%) | |||||||||
| ERDST | 81.65 | 64.81 | 60.00 | 94.19 | 60.27 | 65.12 | 46.86 | 65.70 | 84.57 |
| ERDCP | 67.92 | 49.64 | 62.17 | 67.06 | 58.08 | 54.42 | 31.27 | 76.18 | 81.72 |
| Items 1 | Treatments (SPRs) | SEM 2 | p-Value 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.9 | 2.0 | 2.1 | 2.2 | 2.3 | 2.4 | 2.5 | 2.6 | L | Q | ||
| GP, mL/g DM | |||||||||||
| 6 h | 18.4 | 20.7 | 21.0 | 21.7 | 22.6 | 23.9 | 24.1 | 24.2 | 0.78 | <0.01 | 0.10 |
| 24 h | 83.2 | 86.8 | 94.5 | 99.7 | 102.9 | 96.3 | 95.8 | 92.8 | 3.17 | 0.01 | <0.01 |
| 48 h | 117 | 122 | 130 | 136 | 139 | 137 | 133 | 129 | 3.6 | <0.01 | <0.01 |
| GP kinetics after 48 h of incubation | |||||||||||
| GV, mL/g DM | 114 | 119 | 126 | 134 | 135 | 136 | 130 | 126 | 3.4 | <0.01 | <0.01 |
| C,/h | 4.65 | 4.81 | 5.45 | 5.73 | 5.93 | 4.98 | 5.24 | 5.00 | 0.272 | 0.33 | <0.01 |
| Lag, h | 2.04 | 1.85 | 2.18 | 2.21 | 2.13 | 1.19 | 1.43 | 1.25 | 0.167 | <0.01 | 0.01 |
| Abs, mL/g DM | 5.22 | 5.59 | 6.66 | 7.36 | 7.79 | 6.59 | 6.63 | 6.15 | 0.421 | 0.04 | <0.01 |
| Items 1 | Treatments (SPRs) | SEM 2 | p-Value 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.9 | 2.0 | 2.1 | 2.2 | 2.3 | 2.4 | 2.5 | 2.6 | L | Q | ||
| NH3-N (mg/dL) | |||||||||||
| 6 h | 6.73 | 6.54 | 6.46 | 6.13 | 5.96 | 5.71 | 5.61 | 5.49 | 0.111 | <0.01 | 0.62 |
| 24 h | 7.58 | 7.31 | 7.03 | 6.66 | 6.39 | 6.32 | 6.28 | 6.06 | 0.151 | <0.01 | 0.07 |
| 48 h | 11.14 | 10.60 | 10.37 | 10.15 | 9.89 | 9.55 | 9.30 | 8.98 | 0.293 | <0.01 | 0.83 |
| MCPS (mg N/g DM of incubated substrate) | |||||||||||
| 6 h | 2.54 | 2.57 | 2.61 | 2.74 | 3.22 | 3.24 | 3.24 | 3.00 | 0.101 | <0.01 | 0.06 |
| 24 h | 6.90 | 7.19 | 7.62 | 7.81 | 8.07 | 7.32 | 7.23 | 6.89 | 0.187 | 0.85 | <0.01 |
| 48 h | 8.58 | 8.99 | 9.43 | 9.52 | 10.33 | 9.93 | 9.75 | 9.48 | 0.260 | 0.01 | <0.01 |
| MCPS (mg N/mmol of TVFA) | |||||||||||
| 6 h | 0.28 | 0.28 | 0.28 | 0.28 | 0.31 | 0.31 | 0.31 | 0.30 | 0.014 | 0.09 | 0.88 |
| 24 h | 0.51 | 0.52 | 0.52 | 0.53 | 0.54 | 0.50 | 0.49 | 0.48 | 0.016 | 0.02 | 0.02 |
| 48 h | 0.60 | 0.59 | 0.58 | 0.59 | 0.61 | 0.62 | 0.61 | 0.62 | 0.024 | 0.29 | 0.69 |
| Undegraded CP (g/100 g of CP) | |||||||||||
| 6 h | 58.9 | 59.5 | 60.0 | 59.6 | 61.0 | 63.6 | 63.5 | 62.6 | 0.78 | <0.01 | 0.99 |
| 24 h | 38.7 | 39.6 | 40.5 | 40.7 | 40.8 | 41.2 | 41.7 | 41.4 | 1.68 | 0.16 | 0.62 |
| 48 h | 11.2 | 14.7 | 15.2 | 14.5 | 14.7 | 17.6 | 18.7 | 18.6 | 1.87 | <0.01 | 0.90 |
| Items 1 | Treatments (SPRs) | SEM 2 | p-Value 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.9 | 2.0 | 2.1 | 2.2 | 2.3 | 2.4 | 2.5 | 2.6 | L | Q | ||
| pH | |||||||||||
| 6 h | 6.72 | 6.72 | 6.71 | 6.71 | 6.71 | 6.70 | 6.71 | 6.71 | 0.006 | 0.20 | 0.29 |
| 24 h | 6.61 | 6.60 | 6.58 | 6.58 | 6.60 | 6.59 | 6.60 | 6.58 | 0.007 | 0.14 | 0.30 |
| 48 h | 6.60 | 6.57 | 6.57 | 6.58 | 6.59 | 6.59 | 6.57 | 6.60 | 0.024 | 0.83 | 0.44 |
| TVFA, mmol/L | |||||||||||
| 6 h | 72.7 | 76.4 | 79.5 | 83.8 | 86.4 | 86.8 | 88.3 | 82.8 | 2.69 | <0.01 | 0.01 |
| 24 h | 113 | 115 | 122 | 123 | 126 | 123 | 122 | 121 | 3.4 | 0.03 | 0.02 |
| 48 h | 119 | 126 | 135 | 136 | 140 | 135 | 133 | 128 | 3.2 | 0.03 | <0.01 |
| Acetate, % | |||||||||||
| 6 h | 65.1 | 64.5 | 64.5 | 64.8 | 64.1 | 63.9 | 63.6 | 63.8 | 0.86 | 0.18 | 0.93 |
| 24 h | 62.4 | 62.4 | 62.6 | 62.2 | 61.7 | 62.0 | 62.2 | 61.6 | 0.70 | 0.29 | 0.94 |
| 48 h | 58.2 | 58.3 | 58.3 | 59.5 | 58.7 | 59.6 | 58.3 | 60.2 | 0.52 | 0.02 | 0.99 |
| Propionate, % | |||||||||||
| 6 h | 21.3 | 21.4 | 21.3 | 21.4 | 21.8 | 21.4 | 22.0 | 21.9 | 0.44 | 0.17 | 0.75 |
| 24 h | 22.3 | 22.1 | 21.2 | 20.9 | 20.7 | 21.2 | 21.4 | 21.6 | 0.28 | 0.04 | <0.01 |
| 48 h | 23.5 | 22.7 | 22.4 | 22.7 | 22.0 | 22.2 | 21.3 | 21.6 | 0.32 | <0.01 | 0.54 |
| Butyrate, % | |||||||||||
| 6 h | 9.7 | 10.3 | 10.3 | 10.0 | 10.2 | 10.8 | 10.7 | 10.4 | 0.45 | 0.12 | 0.51 |
| 24 h | 11.9 | 13.0 | 12.6 | 13.1 | 13.6 | 13.0 | 12.7 | 12.8 | 0.30 | 0.09 | <0.01 |
| 48 h | 12.9 | 13.2 | 13.7 | 12.7 | 13.5 | 12.4 | 14.3 | 12.6 | 0.13 | 0.97 | 0.03 |
| Valerate, % | |||||||||||
| 6 h | 1.75 | 1.75 | 1.73 | 1.69 | 1.72 | 1.71 | 1.66 | 1.74 | 0.063 | 0.50 | 0.60 |
| 24 h | 1.45 | 1.61 | 1.51 | 1.53 | 1.65 | 1.62 | 1.65 | 1.67 | 0.049 | <0.01 | 0.74 |
| 48 h | 2.34 | 2.27 | 2.10 | 2.06 | 2.20 | 2.42 | 2.37 | 2.43 | 0.089 | 0.07 | 0.01 |
| Isobutyrate, % | |||||||||||
| 6 h | 0.66 | 0.64 | 0.68 | 0.66 | 0.69 | 0.71 | 0.65 | 0.60 | 0.060 | 0.72 | 0.37 |
| 24 h | 0.61 | 0.61 | 0.62 | 0.70 | 0.73 | 0.79 | 0.62 | 0.83 | 0.035 | <0.01 | 1.00 |
| 48 h | 1.06 | 1.43 | 1.28 | 1.02 | 1.25 | 1.30 | 1.29 | 1.11 | 0.087 | 0.95 | 0.33 |
| Isovalerate, % | |||||||||||
| 6 h | 1.50 | 1.50 | 1.48 | 1.44 | 1.47 | 1.46 | 1.42 | 1.48 | 0.054 | 0.47 | 0.59 |
| 24 h | 1.36 | 1.63 | 1.39 | 1.48 | 1.58 | 1.42 | 1.42 | 1.53 | 0.034 | 0.46 | 0.34 |
| 48 h | 2.02 | 2.12 | 2.23 | 2.03 | 2.33 | 2.01 | 2.46 | 2.14 | 0.038 | <0.01 | 0.11 |
| BCVFA, % | |||||||||||
| 6 h | 2.16 | 2.14 | 2.16 | 2.10 | 2.16 | 2.17 | 2.07 | 2.09 | 0.087 | 0.49 | 0.78 |
| 24 h | 1.97 | 2.24 | 2.01 | 2.18 | 2.31 | 2.20 | 2.04 | 2.37 | 0.050 | <0.01 | 0.51 |
| 48 h | 3.08 | 3.55 | 3.51 | 3.05 | 3.58 | 3.31 | 3.75 | 3.25 | 0.121 | 0.20 | 0.23 |
| A:P | |||||||||||
| 6 h | 3.06 | 3.02 | 3.03 | 3.04 | 2.94 | 2.99 | 2.89 | 2.91 | 0.098 | 0.16 | 0.83 |
| 24 h | 2.81 | 2.83 | 2.95 | 2.97 | 2.97 | 2.93 | 2.91 | 2.85 | 0.062 | 0.45 | 0.02 |
| 48 h | 2.48 | 2.57 | 2.61 | 2.63 | 2.67 | 2.69 | 2.74 | 2.79 | 0.059 | <0.01 | 0.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.; Li, Y.; Shen, Y.; Cao, Y.; Li, Q.; Wang, M.; Liu, M.; Wang, Z.; Huo, Z.; Ren, S.; et al. Effect of Dietary Rumen-Degradable Starch to Rumen-Degradable Protein Ratio on In Vitro Rumen Fermentation Characteristics and Microbial Protein Synthesis. Animals 2022, 12, 2633. https://doi.org/10.3390/ani12192633
Chen P, Li Y, Shen Y, Cao Y, Li Q, Wang M, Liu M, Wang Z, Huo Z, Ren S, et al. Effect of Dietary Rumen-Degradable Starch to Rumen-Degradable Protein Ratio on In Vitro Rumen Fermentation Characteristics and Microbial Protein Synthesis. Animals. 2022; 12(19):2633. https://doi.org/10.3390/ani12192633
Chicago/Turabian StyleChen, Panliang, Yan Li, Yizhao Shen, Yufeng Cao, Qiufeng Li, Meimei Wang, Mingchao Liu, Zhiyuan Wang, Zihan Huo, Shuai Ren, and et al. 2022. "Effect of Dietary Rumen-Degradable Starch to Rumen-Degradable Protein Ratio on In Vitro Rumen Fermentation Characteristics and Microbial Protein Synthesis" Animals 12, no. 19: 2633. https://doi.org/10.3390/ani12192633
APA StyleChen, P., Li, Y., Shen, Y., Cao, Y., Li, Q., Wang, M., Liu, M., Wang, Z., Huo, Z., Ren, S., Gao, Y., & Li, J. (2022). Effect of Dietary Rumen-Degradable Starch to Rumen-Degradable Protein Ratio on In Vitro Rumen Fermentation Characteristics and Microbial Protein Synthesis. Animals, 12(19), 2633. https://doi.org/10.3390/ani12192633






