Using Untargeted LC-MS Metabolomics to Identify the Association of Biomarkers in Cattle Feces with Marbling Standard Longissimus Lumborum
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Sample Collection
2.2. Fecal Sample Pretreatment
2.3. LC-MS Analysis
2.4. Data Analysis
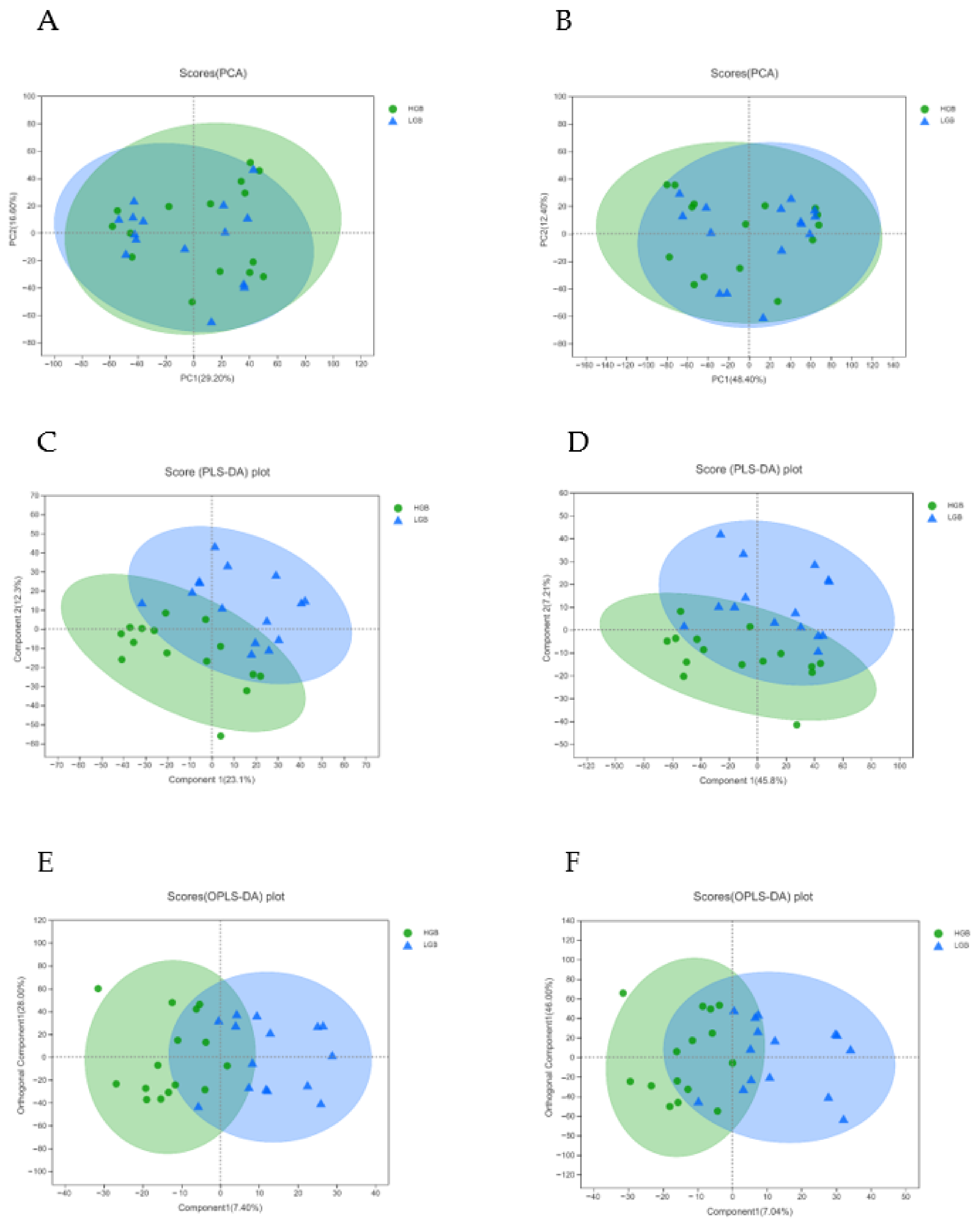
2.5. PCA, PLS-DA, and OPLS-DA Analysis
3. Results
3.1. Fecal Metabolomics Profiling
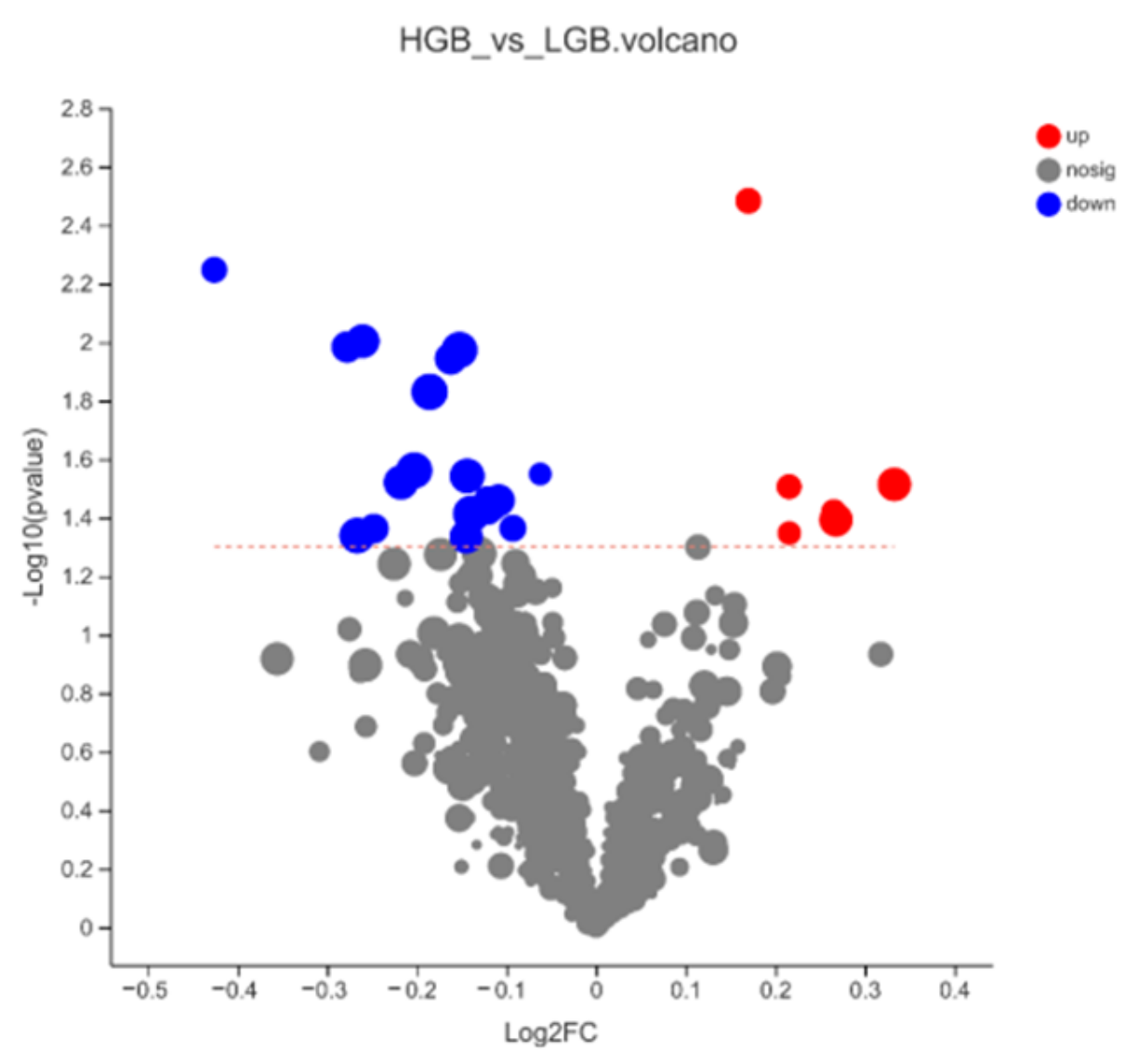
3.2. Differential Metabolite from OPLS-DA Analysis
3.3. Differential Metabolite from WGCNA Analysis
3.4. ROC Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nishimura, T.; Hattori, A.; Takahashi, K. Structural changes in intramuscular connective tissue during the fattening of Japanese black cattle: Effect of marbling on beef tenderization. J. Anim. Sci. 1999, 77, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, T.L.; Cundiff, L.V.; Koch, R.M. Effect of marbling degree on beef palatability in Bos taurus and Bos indicus cattle. J. Anim. Sci. 1994, 72, 3145–3151. [Google Scholar] [CrossRef] [PubMed]
- Joo, S.T.; Hwang, Y.H.; Frank, D. Characteristics of Hanwoo cattle and health implications of consuming highly marbled Hanwoo beef. Meat Sci. 2017, 132, 45–51. [Google Scholar] [CrossRef]
- Lee, S.H.; Gondro, C.; van der Werf, J.; Kim, N.K.; Lim, D.J.; Park, E.W.; Oh, S.J.; Gibson, J.P.; Thompson, J.M. Use of a bovine genome array to identify new biological pathways for beef marbling in Hanwoo (Korean Cattle). BMC Genom. 2010, 11, 623. [Google Scholar] [CrossRef] [PubMed]
- Onogi, A.; Ogino, A.; Komatsu, T.; Shoji, N.; Simizu, K.; Kurogi, K.; Yasumori, T.; Togashi, K.; Iwata, H. Genomic prediction in Japanese Black cattle: Application of a single-step approach to beef cattle. J. Anim. Sci. 2014, 92, 1931–1938. [Google Scholar] [CrossRef] [PubMed]
- Roudbari, Z.; Coort, S.L.; Kutmon, M.; Eijssen, L.; Melius, J.; Sadkowski, T.; Evelo, C.T. Identification of biological pathways contributing to marbling in skeletal muscle to improve beef cattle breeding. Front. Genet. 2020, 10, 1370. [Google Scholar] [CrossRef] [PubMed]
- Sadkowski, T.; Ciecierska, A.; Majewska, A.; Oprządek, J.; Dasiewicz, K.; Ollik, M.; Wicik, Z.; Motyl, T. Transcriptional background of beef marbling—Novel genes implicated in intramuscular fat deposition. Meat Sci. 2014, 97, 32–41. [Google Scholar] [CrossRef]
- Zhang, J.S.; Xu, H.Y.; Fang, J.C.; Yin, B.Z.; Wang, B.B.; Pang, Z.; Xia, G.J. Integrated microRNA–mRNA analysis reveals the roles of microRNAs in the muscle fat metabolism of Yanbian cattle. Anim. Genet. 2021, 52, 598–607. [Google Scholar] [CrossRef]
- Kim, M.; Park, T.; Jeong, J.Y.; Baek, Y.; Lee, H.-J. Association between rumen microbiota and marbling score in Korean native beef cattle. Animals 2020, 10, 712. [Google Scholar] [CrossRef]
- Sato, Y.; Takebe, H.; Tominaga, K.; Oishi, K.; Kumagai, H.; Yoshida, T.; Hirooka, H. Taxonomic and functional characterization of the rumen microbiome of Japanese Black cattle revealed by 16S rRNA gene amplicon and metagenome shotgun sequencing. FEMS Microbiol. Ecol. 2021, 97, fiab152. [Google Scholar] [CrossRef]
- De Vadder, F.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Bäckhed, F.; Mithieux, G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 2014, 156, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ye, Y.; Quan, J.; Ding, R.; Wang, X.; Zhuang, Z.; Zhou, S.; Geng, Q.; Xu, C.; Hong, L.; et al. Using nontargeted LC-MS metabolomics to identify the Association of Biomarkers in pig feces with feed efficiency. Porc. Health Manag. 2021, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Shin, Y.; Kim, T.H.; Kim, D.-H.; Lee, A. Plasma metabolites as possible biomarkers for diagnosis of breast cancer. PLoS ONE 2019, 14, e0225129. [Google Scholar] [CrossRef]
- Zhgun, E.S.; Ilyina, E.N. Fecal metabolites as non-invasive biomarkers of gut diseases. Acta Nat. (Англoязычная Версия) 2020, 12, 2–45. [Google Scholar]
- Jansson, J.; Willing, B.; Lucio, M.; Fekete, A.; Dicksved, J.; Halfvarson, J.; Tysk, C.; Schmitt-Kopplin, P. Metabolomics reveals metabolic biomarkers of Crohn’s disease. PLoS ONE 2009, 4, e6386. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Ma, C.; Liu, C.; Wang, Z.; Yang, J.; Liu, X.; Shen, Z.; Wu, R. NMR-based fecal metabolomics fingerprinting as predictors of earlier diagnosis in patients with colorectal cancer. Oncotarget 2016, 7, 29454. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Luo, Y.; Su, R.; Yao, D.; Hou, Y.; Liu, C.; Du, R.; Jin, Y. Impact of feeding regimens on the composition of gut microbiota and metabolite profiles of plasma and feces from Mongolian sheep. J. Microbiol. 2020, 58, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhong, G.; Su, H.; ur Rahman, M.A.; Chen, K.; Tang, J.; Li, F. Physiological Variation in Ruminal Microbiota under Altered Energy Levels in Starter Ration of Suckling Angus Calves. Pak. Vet. J. 2021, 41, 409–413. [Google Scholar]
- Qiu, Q.; Qiu, X.; Gao, C.; Muhammad, A.U.R.; Cao, B.; Su, H. High-density diet improves growth performance and beef yield but affects negatively on serum metabolism and visceral morphology of Holstein steers. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1197–1208. [Google Scholar] [CrossRef]
- Chen, D.; Yan, J.; Shen, W.; Song, Y.; Lan, X.; Yi, K.; Muhammad, A.U.R. Effect of inclusion of HMBi in the ration of goats on feed intake, nutrient digestibility, rumen bacteria community and blood serum parameters. J. Anim. Physiol. Anim. Nutr. 2020, 104, 987–997. [Google Scholar] [CrossRef]
- Backhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiotaas an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Xiong, X.; Su, Y.; Huang, L.; Chen, C. 16S rRNA gene-based association study identified microbial taxa associated with pork intramuscular fat content in feces and cecum lumen. BMC Microbiol. 2017, 17, 162. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Ahn, J.; Sampson, J.N.; Shi, J.; Yu, G.; Xiong, X.; Hayes, R.B.; Goedert, J.J. Fecal Microbiota, Fecal Metabolome, and Colorectal Cancer Interrelations. PLoS ONE 2016, 11, e0152126. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, T.; Nishimura, T.; Kuchida, K.; Mannen, H. The Japanese Wagyu beef industry: Current situation and future prospects—a review. Asian-Australas. J. Anim. Sci. 2018, 31, 933. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, T.; Albrecht, E.; Teuscher, F.; Kawabata, K.; Sakashita, K.; Iwamoto, H.; Wegner, J. Differences in muscle and fat accretion in Japanese Black and European cattle. Meat Sci. 2009, 82, 300–308. [Google Scholar] [CrossRef]
- Ge, F.; Li, H.P.; Li, J.Y.; Gao, H.J.; Sun, B.Z.; Gao, H.; Wang, X.J.; Cai, W.T.; Liu, X.; Gao, X.; et al. Analysis of growth and slaughter performances and meat quality of Luxi cattle. Shandong Agric. Sci. 2022, 54, 112–120. [Google Scholar]
- Shi, M.Y.; Ren, M.Y.; Fan, T.T.; Gao, X. Comparative analysis on production performance of different hybrid combinations of Luxi Cattle. China Anim. Husb. Vet. Med. 2019, 46, 3674–3679. [Google Scholar]
- Nguyen, D.V.; Nguyen, O.C.; Malau-Aduli, A.E.O. Main regulatory factors of marbling level in beef cattle. Vet. Anim. Sci. 2021, 14, 100219. [Google Scholar] [CrossRef]
- Su, M.; Chen, D.; Zhou, J.; Shen, Q. Effects of Different Dietary Carbohydrate Sources on the Meat Quality and Flavor Substances of Xiangxi Yellow Cattle. Animals 2022, 12, 1136. [Google Scholar] [CrossRef]
- Chen, D.; Wang, X.; Guo, Q.; Deng, H.; Luo, J.; Yi, K.; Sun, A.; Chen, K.; Shen, Q. Muscle Fatty Acids, Meat Flavor Compounds and Sensory Characteristics of Xiangxi Yellow Cattle in Comparison to Aberdeen Angus. Animals 2022, 12, 1161. [Google Scholar] [CrossRef]
- Park, S.J.; Beak, S.-H.; Jung, D.J.S.; Kim, S.Y.; Jeong, I.H.; Piao, M.Y.; Kang, H.J.; Fassah, D.M.; Na, S.W.; Yoo, S.P.; et al. Genetic, management, and nutritional factors affecting intramuscular fat deposition in beef cattle—A review. Asian-Australas. J. Anim. Sci. 2018, 31, 1043. [Google Scholar] [CrossRef] [PubMed]
- Monelli, E.; Villacampa, P.; Zabala-Letona, A.; Martinez-Romero, A.; Llena, J.; Beiroa, D.; Gouveia, L.; Chivite, I.; Zagmutt, S.; Gama-Perez, P.; et al. Angiocrine polyamine production regulates adiposity. Nat. Metab. 2022, 4, 327–343. [Google Scholar] [CrossRef] [PubMed]
- Sadasivan, S.K.; Vasamsetti, B.; Singh, J.; Marikunte, V.V.; Oommen, A.M.; Jagannath, M.; Rao, R.P. Exogenous administration of spermine improves glucose utilization and decreases bodyweight in mice. Eur. J. Pharmacol. 2014, 729, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Zhang, L.; Cao, Y.; Gao, W.; Zhao, C.; Fang, Y.; Zahedi, K.; Soleimani, M.; Lu, X.; Fang, Z.; et al. Spermidine/spermine N1-acetyltransferase-mediated polyamine catabolism regulates beige adipocyte biogenesis. Metabolism 2018, 85, 298–304. [Google Scholar] [CrossRef]
- Vuohelainen, S.; Pirinen, E.; Cerrada-Gimenez, M.; Keinänen, T.A.; Uimari, A.; Pietilä, M.; Khomutov, A.R.; Jänne, J.; Alhonen, L. Spermidine is indispensable in differentiation of 3T3-L1 fibroblasts to adipocytes. J. Cell. Mol. Med. 2010, 14, 1683–1692. [Google Scholar] [CrossRef]
- Amatruda, J.M.; Lockwood, D.H. Insulin-like effects of polyamines spermine binding to fat cells and fat cell membranes. Biochim. Biophys. Acta (BBA)—Gen. Subj. 1974, 372, 266–273. [Google Scholar] [CrossRef]
- Ishii, I.; Ikeguchi, Y.; Mano, H.; Wada, M.; Pegg, A.E.; Shirahata, A. Polyamine metabolism is involved in adipogenesis of 3T3-L1 cells. Amino Acids 2012, 42, 619–626. [Google Scholar] [CrossRef]
- Wang, D.; Yin, J.; Zhou, Z.; Tao, Y.; Jia, Y.; Jie, H.; Zhao, J.; Li, R.; Li, Y.; Guo, C.; et al. Oral Spermidine Targets Brown Fat and Skeletal Muscle to Mitigate Diet-Induced Obesity and Metabolic Disorders. Mol. Nutr. Food Res. 2021, 65, 2100315. [Google Scholar] [CrossRef]
- Pegg, A.E. Spermidine/spermine-N 1-acetyltransferase: A key metabolic regulator. Am. J. Physiol.—Endocrinol. Metab. 2008, 294, E995–E1010. [Google Scholar] [CrossRef]
- Gao, M.; Zhao, W.; Li, C.; Xie, X.; Li, M.; Bi, Y.; Fang, F.; Du, Y.; Liu, X. Spermidine ameliorates non-alcoholic fatty liver disease through regulating lipid metabolism via AMPK. Biochem. Biophys. Res. Commun. 2018, 505, 93–98. [Google Scholar] [CrossRef]
- Fernández, F.; Bárcena, C.; Martínez-García, G.G.; Tamargo-Gómez, I.; Suárez, M.F.; Pietrocola, F.; Castoldi, F.; Esteban, L.; Sierra-Filardi, E.; Boya, P.; et al. Autophagy couteracts weight gain, lipotoxicity and pancreatic β-cell death upon hypercaloric pro-diabetic regimens. Cell Death Dis. 2017, 8, e2970. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.A.; Mariño, G.; BenYounès, A.; Shen, S.; Harper, F.; Maiuri, M.C.; Kroemer, G. Neuroendocrine regulation of autophagy by leptin. Cell Cycle 2011, 10, 2917–2923. [Google Scholar] [CrossRef] [PubMed]
- Schipke, J.; Vital, M.; Schnapper-Isl, A.; Pieper, D.H.; Mühlfeld, C. Spermidine and voluntary activity exert differential effects on sucrose-compared with fat-induced systemic changes in male mice. J. Nutr. 2019, 149, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Wahl, S.; Yu, Z.; Kleber, M.; Singmann, P.; Holzapfel, C.; He, Y.; Mittelstrass, K.; Polonikov, A.; Prehn, C.; Römisch-Margl, W.; et al. Childhood obesity is associated with changes in the serum metabolite profile. Obes. Facts 2012, 5, 660–670. [Google Scholar] [CrossRef]
- Liu, L.; Lin, Y.; Lei, S.; Zhang, Y.; Zeng, H. Synergistic Effects of Lotus Seed Resistant Starch and Sodium Lactate on Hypolipidemic Function and Serum Nontargeted Metabolites in Hyperlipidemic Rats. J. Agric. Food Chem. 2021, 69, 14580–14592. [Google Scholar] [CrossRef]
- Noh, S.K.; Koo, S.I. Egg sphingomyelin lowers the lymphatic absorption of cholesterol and α-tocopherol in rats. J. Nutr. 2003, 133, 3571–3576. [Google Scholar] [CrossRef]
- Noh, S.K.; Koo, S.I. Milk sphingomyelin is more effective than egg sphingomyelin in inhibiting intestinal absorption of cholesterol and fat in rats. J. Nutr. 2004, 134, 2611–2616. [Google Scholar] [CrossRef]
- Norris, G.H.; Jiang, C.; Ryan, J.; Porter, C.M.; Blesso, C.N. Milk sphingomyelin improves lipid metabolism and alters gut microbiota in high fat diet-fed mice. J. Nutr. Biochem. 2016, 30, 93–101. [Google Scholar] [CrossRef]
- Fehr, A.R.; Singh, S.A.; Kerr, C.M.; Mukai, S.; Higashi, H.; Aikawa, M. The impact of PARPs and ADP-ribosylation on inflammation and host–pathogen interactions. Genes Dev. 2020, 34, 341–359. [Google Scholar] [CrossRef]
- Miotto, P.M.; LeBlanc, P.J.; Holloway, G.P. High-fat diet causes mitochondrial dysfunction as a result of impaired ADP sensitivity. Diabetes 2018, 67, 2199–2205. [Google Scholar] [CrossRef]
- Pethick, D.W.; McIntyre, L.; Tudor, G.; Rowe, J.B. The partitioning of fat in ruminants—Can nutrition be used as a tool to regulate marbling? Recent Adv. Anim. Nutr. Aust. 1997, 151–158. Available online: http://www.livestocklibrary.com.au/bitstream/handle/1234/19831/97_151.pdf?sequence=1 (accessed on 13 April 2022).
- Morel, F.; Lauquin, G.; Lunardi, J.; Duszynski, J.; Vignais, P.V. An appraisal of the functional significance of the inhibitory effect of long chain acyl-CoAs on mitochondrial transports. Febs Lett. 1974, 39, 133–138. [Google Scholar] [CrossRef]
- Frisardi, V.; Panza, F.; Seripa, D.; Farooqui, T.; Farooqui, A.A. Glycerophospholipids and glycerophospholipid-derived lipid mediators: A complex meshwork in Alzheimer’s disease pathology. Prog. Lipid Res. 2011, 50, 313–330. [Google Scholar] [CrossRef] [PubMed]
- Marcucci, H.; Paoletti, L.; Jackowski, S.; Banchio, C. Phosphatidylcholine biosynthesis during neuronal differentiation and its role in cell fate determination. J. Biol. Chem. 2010, 285, 25382–25393. [Google Scholar] [CrossRef]
- Schuler, M.-H.; Di Bartolomeo, F.; Böttinger, L.; Horvath, S.E.; Wenz, L.-S.; Daum, G.; Becker, T. Phosphatidylcholine affects the role of the sorting and assembly machinery in the biogenesis of mitochondrial β-barrel proteins. J. Biol. Chem. 2015, 290, 26523–26532. [Google Scholar] [CrossRef]
- Gao, X.; Du, L.; Randell, E.; Zhang, H.; Li, K.; Li, D. Effect of different phosphatidylcholines on high fat diet-induced insulin resistance in mice. Food Funct. 2021, 12, 1516–1528. [Google Scholar] [CrossRef]
- Inoue, M.; Senoo, N.; Sato, T.; Nishimura, Y.; Nakagawa, T.; Miyoshi, N.; Goda, T.; Morita, A.; Miura, S. Effects of the dietary carbohydrate–fat ratio on plasma phosphatidylcholine profiles in human and mouse. J. Nutr. Biochem. 2017, 50, 83–94. [Google Scholar] [CrossRef]
- Guedes Rittes, P. The use of phosphatidylcholine for correction of localized fat deposits. Aesthet. Plast. Surg. 2003, 27, 315–318. [Google Scholar] [CrossRef]
- Young, V.L. Lipostabil: The effect of phosphatidylcholine on subcutaneous fat. Aesthet. Surg. J. 2003, 23, 413–417. [Google Scholar] [CrossRef]
- Lee, H.S.; Nam, Y.; Chung, Y.H.; Kim, H.R.; Park, E.S.; Chung, S.J.; Kim, J.H.; Sohn, U.D.; Kim, H.-C.; Oh, K.W.; et al. Beneficial effects of phosphatidylcholine on high-fat diet-induced obesity, hyperlipidemia and fatty liver in mice. Life Sci. 2014, 118, 7–14. [Google Scholar] [CrossRef]
- Eichhorn, E.J.; Gheorghiade, M. Digoxin. Prog. Cardiovasc. Dis. 2002, 44, 251–266. [Google Scholar] [CrossRef] [PubMed]
- Gheorghiade, M.; Adams, K.F., Jr.; Colucci, W.S. Digoxin in the management of cardiovascular disorders. Circulation 2004, 109, 2959–2964. [Google Scholar] [CrossRef] [PubMed]
- Schneider, E.A.; Wightman, F. Metabolism of auxin in higher plants. Annu. Rev. Plant Physiol. 1974, 25, 487–513. [Google Scholar] [CrossRef]
- Jirásko, R.; Idkowiak, J.; Wolrab, D.; Kvasnička, A.; Friedecký, D.; Polański, K.; Študentová, H.; Študent, V.; Melichar, B.; Holčapek, M. Altered plasma, urine, and tissue profiles of sulfatides and sphingomyelins in patients with renal cell carcinoma. medRxiv 2022. [Google Scholar] [CrossRef]
- Buschard, K.; Diamant, M.; Bovin, L.F.; Månsson, J.-E.; Fredman, R.; Bendtzen, K. Sulphatide and its precursor galactosylceramide influence the production of cytokines in human mononuclear cells. Apmis 1996, 104, 938–944. [Google Scholar] [CrossRef]
- Kim, H.S.; Han, M.; Park, I.H.; Park, C.H.; Kwak, M.S.; Shin, J.-S. Sulfatide inhibits HMGB1 secretion by hindering Toll-like receptor 4 localization within lipid rafts. Front. Immunol. 2020, 11, 1305. [Google Scholar] [CrossRef]
- Buschard, K.; Høy, M.; Bokvist, K.; Olsen, H.L.; Madsbad, S.; Fredman, P.; Gromada, J. Sulfatide controls insulin secretion by modulation of ATP-sensitive K+-channel activity and Ca2+-dependent exocytosis in rat pancreatic β-cells. Diabetes 2002, 51, 2514–2521. [Google Scholar] [CrossRef]
- Bello, A.R.; Bucalo, L.; Estébanez, S.A.; Martínez, A.V.; Núñez, D.B.; Lozano, C.Y.; de José, A.P.; López-Gómez, J.M. Fat tissue and inflammation in patients undergoing peritoneal dialysis. Clin. Kidney J. 2016, 9, 374–380. [Google Scholar] [CrossRef]



 : N, C, H, and their metabolic pathways (C).
: N, C, H, and their metabolic pathways (C).
 : N, C, H, and their metabolic pathways (C).
: N, C, H, and their metabolic pathways (C).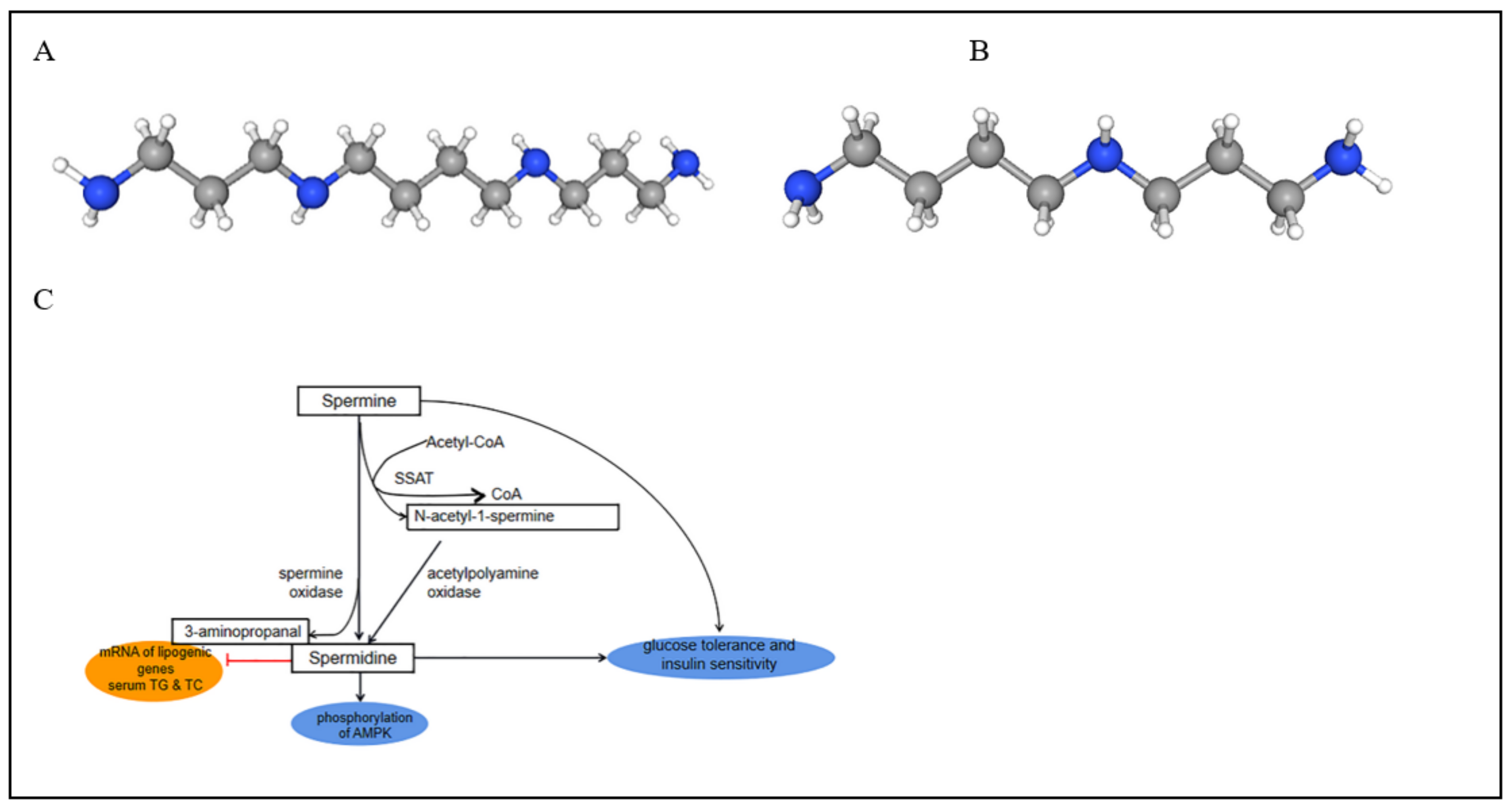

| Metabolite | Formula | VIP_Pred_OPLS-DA | FC(HGB/LGB) | p-Value | FDR | Mode | M/Z |
|---|---|---|---|---|---|---|---|
| ADP | C10H15N5O10P2 | 3.043 | 0.831 | 0.046 | 0.837 | neg | 426.021 |
| SM(d18:0/16:1(9Z)) | C39H79N2O6P | 3.136 | 0.869 | 0.027 | 0.896 | pos | 725.556 |
| PC(15:0/18:2(9Z,12Z)) | C41H78NO8P | 2.907 | 0.905 | 0.029 | 0.896 | pos | 766.536 |
| Digoxin | C41H64O14 | 2.731 | 0.835 | 0.010 | 0.896 | pos | 745.420 |
| Indoleacetaldehyde | C10H9NO | 1.749 | 0.938 | 0.043 | 0.856 | pos | 160.076 |
| Spermine | C10H26N4 | 2.121 | 0.842 | 0.043 | 0.896 | pos | 203.223 |
| Metabolite | M/Z | Mode | Metabolite and Trait Correlation | Formula |
|---|---|---|---|---|
| PC(16:0/16:0) | 756.551 | pos | −0.361 | C40H80NO8P |
| 3-O-Sulfogalactosylceramide (d18:1/18:0) | 846.522 | pos | −0.359 | C42H81NO11S |
| Spermine | 203.223 | pos | −0.239 | C10H26N4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, D.; Su, M.; Zhu, H.; Zhong, G.; Wang, X.; Ma, W.; Wanapat, M.; Tan, Z. Using Untargeted LC-MS Metabolomics to Identify the Association of Biomarkers in Cattle Feces with Marbling Standard Longissimus Lumborum. Animals 2022, 12, 2243. https://doi.org/10.3390/ani12172243
Chen D, Su M, Zhu H, Zhong G, Wang X, Ma W, Wanapat M, Tan Z. Using Untargeted LC-MS Metabolomics to Identify the Association of Biomarkers in Cattle Feces with Marbling Standard Longissimus Lumborum. Animals. 2022; 12(17):2243. https://doi.org/10.3390/ani12172243
Chicago/Turabian StyleChen, Dong, Minchao Su, He Zhu, Gang Zhong, Xiaoyan Wang, Weimin Ma, Metha Wanapat, and Zhiliang Tan. 2022. "Using Untargeted LC-MS Metabolomics to Identify the Association of Biomarkers in Cattle Feces with Marbling Standard Longissimus Lumborum" Animals 12, no. 17: 2243. https://doi.org/10.3390/ani12172243
APA StyleChen, D., Su, M., Zhu, H., Zhong, G., Wang, X., Ma, W., Wanapat, M., & Tan, Z. (2022). Using Untargeted LC-MS Metabolomics to Identify the Association of Biomarkers in Cattle Feces with Marbling Standard Longissimus Lumborum. Animals, 12(17), 2243. https://doi.org/10.3390/ani12172243






