Simple Summary
Synchronizing the energy and nitrogen supply in the rumen was reported to improve feed efficiency; however, synchronization indicators were rarely reported. The present study was conducted using a batch culture technique to evaluate the optimal rumen-degradable starch (RDS) and rumen-degradable protein (RDP) ratio (SPR) for synchronizing the energy and nitrogen supply for dairy cows. The in vitro disappearance of dry matter and neutral and acid detergent fiber, gas production and microbial crude protein synthesis quadratically increased with increasing the SPR and peaked at 2.3. Although an in vivo study could be further needed, our results provided an optimal SPR range for dairy cows to synchronize the energy and nitrogen supply.
Abstract
The objective of this study was to investigate the effects of dietary rumen-degradable starch (RDS, g/kg of DM) to rumen-degradable protein (RDP, g/kg of DM) ratios (SPR) on in vitro rumen fermentation characteristics and microbial protein synthesis (MCPS). Treatments were eight diets with SPR of 1.9, 2.0, 2.1, 2.2, 2.3, 2.4, 2.5 and 2.6 and were formulated to be isoenergetic, isonitrogenous, and isostarch. Substrates were anaerobically incubated in sealed culture vials (100 mL) for 6, 24 or 48 h. Three incubation runs were conducted within two consecutive weeks. With the increase of the dietary SPR, the gas production (GP), in vitro dry matter disappearance (IVDMD) and concentration of MCPS and total volatile fatty acids (TVFA) linearly increased after 6 h of incubation (p ≤ 0.01), whereas they quadratically increased and peaked at the SPR of 2.3 after 24 and 48 h of incubation (p < 0.05). In response to dietary SPR increasing, the in vitro neutral detergent fiber disappearance (IVNDFD) quadratically increased (p < 0.01), and the ammonia nitrogen (NH3-N) concentration linearly decreased (p < 0.01) after 6, 24 and 48 h of incubation. Based on the presented results, an SPR of 2.3 is recommended for formulating a diet due to its greatest IVDMD, IVNDFD, GP, TVFA and MCPS. However, as the results obtained are strictly dependent on the in vitro conditions, further in vivo studies are needed to verify our findings.
1. Introduction
The rumen is regarded as a natural anaerobic fermenter inhabited by many microorganisms. There is a strong link between rumen fermentation efficiency and microbial viability [1]. Synchronizing energy and nitrogen (N) supply in the rumen could maximize the growth and activity of rumen microorganisms, thereby potentiating the efficiency of rumen fermentation and microbial protein synthesis (MCPS) [2]. In addition, as microbial protein is the primary source of essential amino acids for dairy cows [3], the increase in MCPS is not only beneficial for optimizing milk protein but also for improving N utilization in dairy cows [4].
Previous experiments have been conducted to explore the effects of a synchronous diet using different indicators, including non-fibrous carbohydrates to degradable intake protein, non-structural carbohydrates to rumen-degradable protein (RDP) and rumen-degradable nitrogen to fermentable organic matter [5,6,7]. However, the results were not consistent, which was likely because the rate of energy or protein availability is often confounded with the total amount of energy or protein availability [8]. Therefore, more attention should be paid to the characteristics of energy and N sources used in diet [9]. Recent studies have demonstrated that rumen-degradable starch (RDS) and RDP could affect rumen fermentation, microbial community compositions, and MCPS [10,11,12,13]. Since MCPS was recognized as the important criterion for determining the degree of dietary synchronization [14,15,16], it is reasonable to infer that the RDS and RDP may be more effective indicators for evaluating the synchrony of energy and N supply. However, the optimal RDS to RDP ratio (SPR) in the diet is unclear.
As little research accurately reported the RDS and RDP contents in the diets of lactating cows, we previously analyzed the chemical compositions of diets for high-production dairy cows from 15 commercial dairy farms and found the value of SPR ranged from 1.9 to 2.6 (unpublished). Therefore, we hypothesized that the SPR could influence microbial activity, thus affecting rumen fermentation and MCPS efficiency. The objective of this study was to evaluate the response of different SPRs to rumen fermentation characteristics and microbial protein synthesis to screen out the optimal SPR.
2. Materials and Methods
All animal usage and experimental procedures (JGL 202102) were approved by the Animal Care Committee of Hebei Agriculture University (Baoding, China).
2.1. Treatments
Eight experimental diets were formulated according to different SPRs, which were 1.9, 2.0, 2.1, 2.2, 2.3, 2.4, 2.5 and 2.6. The diets contained 31.48% whole-plant corn silage, 12.42% alfalfa hay, 4.94% oat hay and 51.16% concentrate on a dry matter (DM) basis (Table 1). The chemical compositions and effective rumen degradability of the feed ingredients are shown in Table 2. The content of starch, crude protein (CP) and energy were similar among the diets.

Table 1.
Ingredients and chemical compositions of diets with different rumen-degradable starch to rumen-degradable protein ratio (SPR).

Table 2.
Effective ruminal degradability and content for starch and crude protein (CP) of principal dietary ingredients.
2.2. Animals and Diet
Three Holstein steers (850 ± 45 kg of BW) equipped with a permanent rumen cannula were used for in situ incubation and served as donor animals for the in vitro experiment. The steers were fed ad libitum a total mixed ration twice daily at 07:00 and 19:00 and were provided free access to water. The diet of the donors contained 40.00% whole-plant corn silage, 20.00% Chinese wild rye, 20.50% ground corn, 4.36% soybean meal, 9.36% cottonseed meal, 3.69% DDGS and 2.09% vitamin and mineral supplement (DM basis), to provide 13.93% of CP, 36.80% of neutral detergent fiber (aNDF) and 28.80% of starch.
2.3. In Situ Ruminal Degradation
The effective rumen degradability of the starch (ERDST, Table 2) and protein (ERDCP) of the feed ingredients used in this study were determined using the in situ nylon bags technique [17]. Briefly, the feed ingredients were milled to pass through a 2-mm screen (stand model 4 Wily Mill, Arthur H. Thomas, Philadelphia, PA, USA). The nylon bags (10 cm × 20 cm with a 50 μm pore size) were pre-weighed and filled with either 7.0 g of concentrate or 4.0 g of forage (DM basis). Prior to incubation, the bags were soaked for 10 min in warm tap water (39 °C). Four replicates per time point were incubated in each steer. Bags with concentrate were taken out after 2, 4, 8, 16, 24 and 48 h of incubation, and bags with forage were taken out after 4, 8, 16, 24, 48 and 72 h of incubation. After each incubation time point, bags were removed from the rumen and immediately dispersed in ice water to stop further degradation and rinsed with cold tap water to remove rumen digestive residues. The rinsed nylon bags were dried in a forced-air oven at 55 °C for 48 h and weighed. Residues were stored at 4 °C for chemical analysis. The degradation parameters and effective ruminal degradability (ERD) were calculated according to Ørskov and McDonald [18]:
where Yt = disappearance proportion at time t; a = rapidly degradable fraction; b = slowly degradable fraction; k = constant rate of degradation of fraction b; t = time of incubation (h); kp = passage rate, the rumen outflow rate was set at 0.06/h according to Offner et al. [19].
Dietary RDS or RDP was calculated using the following equation [20]:
where Pi represents the proportion of dietary starch or protein of feed i in the diet, ERDi represents the effective starch or protein rumen degradability of feed i, and n is the number of ingredients containing starch or protein in the diet.
2.4. Batch Culture Procedure
Prior to in vitro incubation, 500 ± 0.5 mg (DM basis) of the substrate was weighed into an acetone-washed and pre-weighed Ankom filter bag (F57; Ankom Technology, Macedon, NY, USA), then bags were heat sealed and placed into 100 mL culture vials. Blank with a pre-treated filter bag was incubated without any substrate to correct the gas production (GP). Fresh rumen fluid was collected from the donor steers before morning feeding, squeezed through four-layer cheesecloth, transferred in a pre-warmed bottle at 39 °C after pH measurement, and immediately transferred to the laboratory, then kept at 39 °C water bath and bubbled with CO2. The time from rumen-fluid collection to inoculation was no more than 30 min. Fresh buffer was prepared according to Menke et al. [21], kept at 39 °C in a water bath, and bubbled with CO2. A total of 45 mL buffer and 15 mL of rumen fluid were added to each vial and bubbled with CO2 for 15 s to maintain anaerobic conditions. Then, the vials were immediately sealed with butyl rubber stoppers and aluminum crimp seals. Thereafter, all the vials were incubated at 39 °C and oscillated at 125 rpm in a gas bath shaker (GCTS-2018, Jingda, Inc., Jintan, China). The pH of the buffer and rumen fluid mixture was averaged at 7.0 ± 0.21. Both blanks and treatments had three replicates in each sampling time point (6, 24, and 48 h). Three batch culture runs were performed within two weeks.
2.5. Gas Production and Sampling
Headspace gas pressure was recorded at 3, 6, 9, 12, 24, 36 and 48 h using the pressure meter as described by Theodorou et al. [22]. The gas was then released by leaving the needle in the vial after pressure measurement. The cumulative GP was calculated according to Mauricio et al. [23]:
where GPt is the GP volume at time ‘t’ (h), Pt is the gas pressure measured at the time ‘t’ (h). Gas production kinetics was calculated according to Ørskov and McDonald [18]:
where ‘y’ is the cumulative volume of gas produced after ‘t’ hours, ‘GV’ is the asymptotic gas volume, ‘C’ is the rate constant of GP and ‘lag’ is the time (h) between inoculation and commencement of GP. The parameters ‘GV’ and ‘C’ were used to calculate the absolute initial GP during the first hour (Abs):
GPt (mL) = 0.18 + 3.697Pt + 0.0824Pt2
y = GV × (1 − e− C × [t − lag])
Abs (mL/g DM) = GV × (1 − e− C)
At the end of each incubation time (6, 24 and 48 h), the vials were put on ice to stop fermentation, and the pH of the fermentation liquid was immediately determined. Approximately 20 mL fermentation liquid of each bottle was sampled, mixed with 1 mL 0.25 (wt/vol) HPO3, and kept at −20 °C for volatile fatty acids (VFAs) analysis. Approximately 5 mL fermentation liquid was sampled for MCPS determination, and another 5 mL liquid sample was mixed with 1 mL 0.01 (vol/vol) H2SO4 solution for ammoniacal nitrogen (NH3-N) determination. The Ankom filter bags were washed with cold water and dried at 55 °C for 48 h to determine the undigested substrate CP concentration, the in vitro dry matter disappearance (IVDMD), neutral detergent fiber disappearance (IVNDFD), acid detergent fiber disappearance (IVADFD), and hemicellulose disappearance (IVHCD).
2.6. Chemical Analysis
The DM (method 930.15), ether extract (method 991.36), CP (method 968.06), starch (method 996.11) and ash (method 942.05) were determined following the methods of AOAC (2005) [24]. The aNDF, analyzed using a heat-stable amylase, and ADF were expressed as the inclusion of residual ash according to the method described by Van Soest et al. [25]. The IVDMD, IVNDFD, IVADFD and IVHCD were calculated as the weight difference of DM, aNDF, ADF and HC in the substrate before and after incubation. The concentration of NH3-N and VFAs were analyzed as described by Broderick and Kang [26] and Erwin et al. [27], respectively. The MCPS was determined as described by Makkar et al. [28].
2.7. Statistical Analysis
All data were analyzed using the PROC MIXED model procedure of SAS (SAS Inst. Inc., Cary, NC, USA) with a model including SPR as a fixed effect and the incubation run as a random effect. Significant differences between the treatment means were identified using the least significant means. Polynomial contrasts were used to determine linear (L) and quadratic (Q) responses to the SPR. Significant effects were declared at p ≤ 0.05.
3. Results
3.1. In Vitro Nutrients Disappearance and Gas Production
As shown in Figure 1 and Table 3, with the increase of dietary SPR, the IVDMD and GP linearly increased after 6 h (p ≤ 0.01) of incubation and quadratically increased after 24 h (p ≤ 0.05) and 48 h (p < 0.01) of incubation. Increasing dietary SPR caused a quadratic increase in the IVNDFD, IVADFD and IVHCD after 6, 24 and 48 h of incubation (p < 0.01). Additionally, the GV, C, Lag time and Abs quadratically increased with the dietary SPR increasing (p ≤ 0.01).
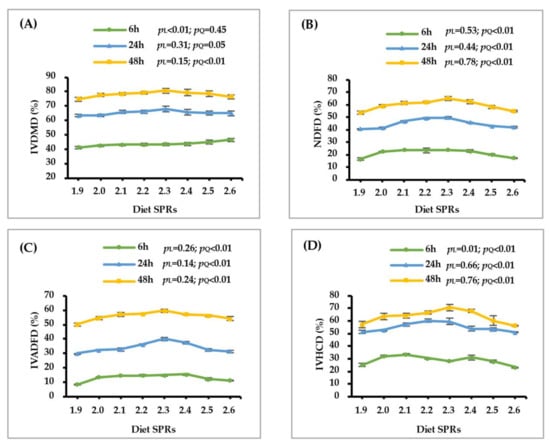
Figure 1.
Effect of dietary rumen-degradable starch to rumen-degradable protein ratio (SPR) on in vitro dry matte disappearance (IVDMD, (A), in vitro neutral detergent fiber disappearance (IVNDFD, (B)), in vitro acid detergent fiber disappearance (IVADFD, (C), and in vitro hemicellulose disappearance (IVHCD, (D)) after 6, 24, and 48 h of incubation. Error bars indicate measure of variation within the dietary SPRs. L is linear, and Q is quadratic effects for diet SPR.

Table 3.
Effects of dietary rumen-degradable starch to rumen-degradable protein ratio (SPR) on in vitro gas production parameters.
3.2. Rumen Ammonia Nitrogen Concentration and Microbial Protein Synthesis
As shown in Table 4, a linear reduction in NH3-N concentration was detected after 6, 24 and 48 h of incubation (p < 0.01) with the increase of dietary SPR, whereas a linear increase was observed in undegraded CP content after 6 and 48 h of incubation (p < 0.01). When expressed as mg N/mmol of VFA, the MCPS concentration was not affected by treatments, except for a quadratic increase after 24 h of incubation (p = 0.02). However, when expressed as mg N/g DM of the incubated substrate, the MCPS linearly increased with the increasing dietary SPR after 6 h of incubation (p < 0.01) and quadratically after 24 and 48 h of incubation (p < 0.01).

Table 4.
Effects of dietary rumen-degradable starch to rumen-degradable protein ratio (SPR) on the concentration of ammonia nitrogen (NH3-N), microbial protein synthesis (MCPS), and undegraded crude protein (CP) at different in vitro incubation times.
3.3. Rumen Fermentation Characteristics
As shown in Table 5, although the rumen pH was not affected by the dietary SPR, the Total VFA (TVFA) concentration showed a linear increase after 6 h of incubation (p < 0.01) and a quadratic increase after 24 h (p = 0.02) and 48 h (p < 0.01) of incubation with the increasing dietary SPR. Furthermore, after 24 h of incubation, the molar proportion of butyrate and acetate to propionate ratio (A:P) quadratically increased (p ≤ 0.02), and the molar proportion of valerate and branched-chain VFA (BCVFA, including isobutyrate and isovalerate) linearly increased (p < 0.01), whereas, the molar proportion of propionate quadratically decreased with the increasing dietary SPR (p < 0.01). In addition, after 48 h of incubation, increasing dietary SPR linearly increased the A:P ratio and the molar proportions of acetate and isovalerate (p ≤ 0.02), and quadratically increased the molar proportion of butyrate (p = 0.03), while it linearly decreased the molar proportion of propionate (p < 0.01).

Table 5.
Effects of dietary rumen-degradable starch to rumen-degradable protein ratio (SPR) on in vitro rumen fermentation parameters after 6, 24, and 48 h of incubation.
4. Discussion
4.1. In Vitro Nutrients Disappearance and Gas Production
The IVDMD after 6 h of incubation has generally been considered as the loss of the rapid degradation fraction of the substrate [29]. Since starch could be the dominant rapid degradation fraction in the present study [30], the IVDMD after 6 h of incubation could be mainly affected by the RDS content in the diet. Wheat has a higher RDS content than corn due to its lower crystallinity of starch granule and looser starch–protein linkage in the endosperm [31]. In this study, the higher SPR diet had a higher proportion of wheat; thus, the IVDMD after 6 h of incubation increased with the increasing dietary SPR. Whereas, after 24 h and 48 h of incubation, the IVDMD was more related to rumen microbial activity. Under adequate N source supply conditions, evaluating the supply of carbohydrates could increase the availability of ATP and carbon skeleton, thus improving microbial growth efficiency and increasing the digestibility of nutrients [32]. The results of this study corroborate the above findings; the IVDMD elevated as increasing dietary SPR from 1.9 to 2.3. When dietary SPR was greater than 2.3, the higher proportion of rumen-protected soybean in the diet induced a lower dietary RDP content. The deficiency in nitrogen supply could limit microbial growth [12], consequently leading to decreased IVDMD.
As we know, a diet of high fermentable carbohydrates can depress fiber degradation by decreasing the ruminal pH [33]. However, in the current study, there was no change in pH value among diets with different SPR. As previously reported by Lechartier and Peyraud [34], the high content of highly degradable starch in the diet could impair fibrolytic activity independently of its effect on ruminal pH. The evaluated dietary RDS content may increase the competition between amylolytic bacteria and cellulolytic bacteria in the rumen. Ren et al. [10] also reported that the relative abundance of amylolytic bacteria was increased, whereas the cellulolytic bacteria were decreased in the heifers fed a high RDS diet compared with a low RDS diet. Moreover, cellulolytic bacteria are also sensitive to a shortage of N [2]. When dietary SPR was at a high level, the higher RDS content and lower RDP may depress the growth of cellulolytic bacteria, consequently leading to lower fiber degradation. Therefore, the quadratic response of the increasing dietary SPR to the fiber degradation in the present study could be acceptable.
Gas is always released along with the microbial degradation of the substrate, and in a batch culture system, the GP was reported to be strongly related to IVDMD [35]. Thus, the variations in GP and GP kinetics in this study were mainly caused by the change in IVDMD. With the increase of the dietary SPR, the GP linearly increased after 6 h of incubation, which indicated the initial rate of digestion was enhanced, whereas the GP rate and GP after 24 h and 48 h of incubation quadratically increased, which reflected the change in the extent of diet digestion on the whole incubation [29,36].
4.2. Rumen Ammonia Nitrogen Concentration and Microbial Protein Synthesis
In ruminants, rumen concentration of NH3-N could be an important indicator to reflect rumen fermentation. The rumen NH3-N is mainly produced by CP degradation of the substrate and eliminated by outflowing of rumen content, absorption through the rumen wall and the use of microbial. As the main nitrogen source for rumen microbes [37], NH3-N concentration was commonly recognized as an indicator reflecting the synthesis ability of microbial protein in vivo, and the lower NH3-N concentration usually indicates that more NH3-N is used for MCPS [38]. However, in the present study, there was no rumen content outflow or rumen wall absorption in the batch culture system; thus, the rumen NH3-N concentration could only be affected by feed degradation and microbial use [39]. In the current study, since the linearly decreased NH3-N concentration was in consistent with the linearly increased undegradable CP, not the quadratically increased MCPS, the changes in NH3-N concentration among treatments could mainly be affected by different RDP content in different diets.
In addition, the NH3-N concentration of diets in the present study were all more than 5 mg/dL. That seems to suggest that each diet could provide sufficient N for MCPS [40]. However, Schwab et al. [41] suggested that the optimum concentration of ruminal NH3-N seemed to be diet-dependent and influenced by carbohydrate fermentability, and a greater ruminal NH3-N concentration may be required if more rapidly fermentable carbohydrates are supplied. Odle and Schaefer [42] reported that greater ruminal NH3-N concentrations were required for ground barley (8.9 mM) than for ground maize (4.3 mM) to maximize in situ digestion rate. In the present study, due to the increased RDS content with increasing dietary SPR, the demand for NH3-N and the ability to capture N by rumen microbes may increase. The high SPR diet may need more rumen-degradable N and greater minimum NH3-N concentration thresholds. Therefore, although the NH3-N concentration is greater than 5 mg/dL, it does not mean that the requirement of N for maximizing MCPS can be satisfied.
Microbial protein is dairy cows’ primary source of essential amino acids [3]. Synchronizing energy and nitrogen supply could evaluate MCPS efficiency via potentiate enzyme activities of ammonia assimilation and increase the abundance of the rumen microbes of nitrogen metabolism [43]. MCPS was recognized as the important criterion for determining if a treatment is more synchronous or not [14,15,16]. In the current study, the MCPS (mg N/g DM) increased with increasing dietary SPR after 6 h incubation. That result suggested that, in the initial stage of fermentation, energy supplementation can enhance the degrees of synchroneity of diet. However, after 24 h incubation, the MCPS (mg N/g DM) and MCPS (mg N/mmol TVFA) decreased when dietary SPR was greater than 2.3. That might be caused by the asynchronous supply of energy and nitrogen. As the dietary SPR exceeded 2.3, the degraded rate of proteins might be slower than the degraded rate of carbohydrates; thus, the insufficient supply of N made the production of ATP unavailable for MCPS [9].
Moreover, increasing the abundance of carbohydrate species contributes to elevating the diversity of rumen microorganisms, thus increasing the MPCS [2]. In the present study, the differences in the content of beet pellets among diets may be a factor causing the change in MCPS. However, as reported by Münnich et al. [44], no significant changes in most rumen microorganisms were observed when the dietary beet pellets content was increased from 8.00% to 16.00%. Voelker and Allen [45] reported no change in MCPS even with the application of beet pellets at 24% of the diet. In the present study, though the content of beet pellets increased with the increase of dietary SPR, the content of beet pellets ranged from 9.26 to 12.64%, which were all in the range of 8.00 to 16.00%. Thus, the difference in the content of beet pellets was not a major influencing factor for MCPS.
Furthermore, the undegraded CP increased with declining RDP proportion suggesting that lowering dietary RDP could conserve protein and make it more available for the small intestine [46]. Combined with the results of MCPS, the appropriate dietary SPR may increase the supply of metabolic proteins for the dairy cow, and the ratio of 2.3 may achieve the balance.
4.3. Rumen Fermentation Characteristics
Rumen pH is a key factor in the regulation of microbial activity. Due to higher acid production for high RDS content and lower bicarbonates production for low RDP content [33,34], a high SPR diet was expected to diminish ruminal buffering capacity and then reduce ruminal pH. However, for the batch culture technique used in the present study, the buffer should be sufficiently added at the beginning, and thus, the pH should be less sensitive to TVFA production [47]. Thus, the TVFA concentration significantly changed in the fermentation liquid, while no differences observed in pH should be acceptable, and similar results were also previously reported by Shen et al. [48].
The TVFA concentration followed a similar response with the IVDMD and MCPS. That may contribute to the variation of rumen microorganisms’ abundance, which led to the change in IVDMD, consequently influencing TVFA production. Regarding VFAs, after 24 h of incubation, the quadratic increase for the A:P ratio by increasing dietary SPR was caused by the change of proportion of propionate. And after 48 h of incubation, as dietary SPR increased, the increment in the proportion of acetate and reduction in the proportion of propionate resulted in the linear increase in the A:P ratio. A similar result was observed by Kand et al. [49], who also found that acetate proportion was higher and propionate proportion was lower in corn starch diets than in cellulose diets by comparing three carbohydrate sources (sucrose, corn starch and cellulose). One possible explanation was that, as incubation time increased, the rapidly degradable carbohydrates (i.e., RDS) decreased, whereas the slow-degradable carbohydrates increased in the substrate [50]. Moreover, as fibrous carbohydrates are a major precursor of butyrate [51], the quadratically increased molar proportion of butyrate with the increase of SPR after 24 and 48 h of incubation may be caused by the variation of fibers degradation in this study. Additionally, the increase in valerate proportions is more associated with the proportion of ground wheat in the diet. Wheat is rich in proline [52], which is a precursor for valerate; thus, a diet with more wheat could produce more valerate.
BCVFAs are derived from the deamination and decarboxylation of branched-chain AA, such as valine, leucine, and isoleucine [53]. A reduction in protein degradation in the rumen has been associated with a decrease in the concentration of BCVFA [54,55]. However, in the present study, the molar proportion of BCVFA increased with the dietary RDP content decreased. Calsamiglia et al. [56] found that the flow of the branched-chain AA from a continuous culture fermenter was higher with lignosulfonate-treated soybean meal than with untreated soybean meal. Kand et al. [54] suggested that, in response to a lack of N, the predation and autolysis of microbes might increase to support more amino acids for BCVFA production. In this experiment, at a high SPR diet, the increase in BCVFA from the breakdown of microbial protein was presumably greater than the decrease in BCVFA from the degradation of dietary protein, leading to an overall increment in BCFA proportions. In addition, the amount of rumen-protected soybean meal increased with the dietary SPR increased, which could also be an important consideration for the increase in BCVFA.
5. Conclusions
The changes in rumen fermentation characteristics and MCPS suggested that the SPR could affect rumen microbial activity. According to the greater IVDMD, IVNDFD, GP, TVFA and MCPS in the present study, an SPR of 2.3 could be an optimal ratio for dairy cows. However, further research on rumen microbial composition is expected to get a clearer explanation for those changes. As our results are restricted to in vitro conditions, in vivo studies are needed to investigate the practical benefits to animal performances.
Author Contributions
Conceptualization, P.C. and Y.L.; methodology, Y.S. and Y.C.; software, Y.S.; validation, P.C., S.R., Z.H. and Z.W.; formal analysis, P.C. and Y.S.; investigation, M.L. and M.W.; resources, Y.G. and J.L.; data curation, P.C.; writing—original draft preparation, P.C.; writing—review and editing, Y.S. and Y.L.; visualization, P.C. and Y.S.; supervision, Q.L. and Y.S.; project administration, Y.G. and J.L.; funding acquisition, Y.G. and J.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the earmarked fund for CARS (CARS-36, Beijing, China), the Top Talent Project of Hebei Province (6012018, Shijiazhuang, China), Hebei Dairy Cattle Innovation Team of Modern Agro-industry Technology Research System (HBCT2018120203, Shijiazhuang, China), the Key Research and Development Project of Hebei (20326606D, Shijiazhuang, China) and Precision Animal Husbandry Discipline Group Construction Project of Hebei Agricultural University (1090064, Baoding, China).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Animal Care Committee of Hebei Agricultural University (protocol code JGL 202102 and date of approval 5 February 2021).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We are thankful for the help of members of the Institute of Cattle Science of Hebei Agricultural University for their assistance in sample collections.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Matthews, C.; Crispie, F.; Lewis, E.; Reid, M.; O’Toole, P.W.; Cotter, P.D. The rumen microbiome: A crucial consideration when optimising milk and meat production and nitrogen utilisation efficiency. Gut Microbes 2019, 10, 115–132. [Google Scholar] [CrossRef] [PubMed]
- Belanche, A.; Doreau, M.; Edwards, J.E.; Moorby, J.M.; Pinloche, E.; Newbold, C.J. Shifts in the rumen microbiota due to the type of carbohydrate and level of protein ingested by dairy cattle are associated with changes in rumen fermentation. J. Nutr. 2012, 142, 1684–1692. [Google Scholar] [CrossRef] [PubMed]
- Schwab, C.G.; Satter, L.D.; Clay, A.B. Response of Lactating Dairy Cows to Abomasal Infusion of Amino Acids. J. Dairy Sci. 1976, 59, 1254–1270. [Google Scholar] [CrossRef]
- Schwab, C.G.; Broderick, G.A. A 100-Year Review: Protein and amino acid nutrition in dairy cows. J. Dairy Sci. 2017, 100, 10094–10112. [Google Scholar] [CrossRef]
- Mansfield, H.R.; Endres, M.I.; Stern, M.D. Influence of non-fibrous carbohydrate and degradable intake protein on fermentation by ruminal microorganisms in continuous culture. J. Anim. Sci. 1994, 72, 2464–2474. [Google Scholar] [CrossRef] [PubMed]
- Mabjeesh, S.J.; Arieli, A.; Bruckental, I.; Zamwell, S.; Tagari, H. Effect of Ruminal Degradability of Crude Protein and Nonstructural Carbohydrates on the Efficiency of Bacterial Crude Protein Synthesis and Amino Acid Flow to the Abomasum of Dairy Cows. J. Dairy Sci. 1997, 80, 2939–2949. [Google Scholar] [CrossRef]
- Sinclair, L.A.; Garnsworth, P.C.; Newbold, J.R.; Buttery, P.J. Effect of synchronizing the rate of dietary energy and nitrogen release on rumen fermentation and microbial protein synthesis in sheep. J. Agr. Sci. 1993, 120, 251–263. [Google Scholar] [CrossRef]
- Bach, A.; Calsamiglia, S.; Stern, M.D. Nitrogen Metabolism in the Rumen. J. Dairy Sci. 2005, 88, E9–E21. [Google Scholar] [CrossRef]
- Cabrita, A.R.J.; Dewhurst, R.J.; Abreu, J.M.F.; Fonseca, A.J.M. Evaluation of the effects of synchronising the availability of N and energy on rumen function and production responses of dairy cows—A review. Anim. Res. 2006, 55, 1–24. [Google Scholar] [CrossRef]
- Ren, H.; Su, X.; Bai, H.; Yang, Y.; Wang, H.; Dan, Z.; Lu, J.; Wu, S.; Cai, C.; Cao, Y.; et al. Specific enrichment of microbes and increased ruminal propionate production: The potential mechanism underlying the high energy efficiency of Holstein heifers fed steam-flaked corn. AMB Express 2019, 9, 209. [Google Scholar] [CrossRef]
- Martins, C.; Fonseca, D.C.M.; Alves, B.G.; Arcari, M.A.; Ferreira, G.C.; Welter, K.C.; Oliveira, C.A.F.; Renno, F.P.; Santos, M.V. Effect of dietary crude protein degradability and corn processing on lactation performance and milk protein composition and stability. J. Dairy Sci. 2019, 102, 4165–4178. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.B. Dietary starch source and protein degradability in diets containing sucrose: Effects on ruminal measures and proposed mechanism for degradable protein effects. J. Dairy Sci. 2013, 96, 7093–7109. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.L.; McKinnon, J.J.; Mutsvangwa, T. Effects of dietary ruminally degradable starch and ruminally degradable protein levels on urea recycling, microbial protein production, nitrogen balance, and duodenal nutrient flow in beef heifers fed low crude protein diets. Can. J. Anim. Sci. 2013, 93, 123–136. [Google Scholar] [CrossRef]
- Nocek, J.E.; Russell, J.B. Protein and Energy as an Integrated System. Relationship of Ruminal Protein and Carbohydrate Availability to Microbial Synthesis and Milk Production. J. Dairy Sci. 1988, 71, 2070–2107. [Google Scholar] [CrossRef]
- Hall, M.B.; Huntington, G.B. Nutrient synchrony: Sound in theory, elusive in practice. J. Anim. Sci. 2008, 86, E287–E292. [Google Scholar] [CrossRef]
- Chanjula, P.; Wanapat, M.; Wachirapakorn, C.; Rowlinson, P. Effect of Synchronizing Starch Sources and Protein (NPN) in the Rumen on Feed Intake, Rumen Microbial Fermentation, Nutrient Utilization and Performance of Lactating Dairy Cows. Asian-Australas J. Anim. Sci. 2004, 17, 1400–1410. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Dairy Cattle; National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- Ørskov, E.R.; McDonald, I. The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. J. Agric. Sci. 1979, 92, 499–503. [Google Scholar] [CrossRef]
- Offner, A.; Bach, A.; Sauvant, D. Quantitative review of in situ starch degradation in the rumen. Anim. Feed Sci. Technol. 2003, 106, 81–93. [Google Scholar] [CrossRef]
- Zebeli, Q.; Dijkstra, J.; Tafaj, M.; Steingass, H.; Ametaj, B.N.; Drochner, W. Modeling the adequacy of dietary fiber in dairy cows based on the responses of ruminal pH and milk fat production to composition of the diet. J. Dairy Sci. 2008, 91, 2046–2066. [Google Scholar] [CrossRef]
- Menke, K.H.; Raab, L.; Salewski, A.; Steingass, H.; Fritz, D.; Schneider, W. The estimation of the digestibility and metabolizable energy content of ruminant feedingstuffs from the gas production when they are incubated with rumen liquor in vitro. J. Agric. Sci. 1979, 93, 217–222. [Google Scholar] [CrossRef]
- Theodorou, M.K.; Williams, B.A.; Dhanoa, M.S.; McAllan, A.B.; France, J. A simple gas production method using a pressure transducer to determine the fermentation kinetics of ruminant feeds. Anim. Feed Sci. Technol. 1994, 48, 185–197. [Google Scholar] [CrossRef]
- Mauricio, R.M.; Mould, F.L.; Dhanoa, M.S.; Owen, E.; Channa, K.S.; Theodorou, M.K. A semi-automated in vitro gas production technique for ruminant feedstuff evaluation. Anim. Feed Sci. Technol. 1999, 79, 321–330. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 17th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Broderick, G.A.; Kang, J.H. Automated Simultaneous Determination of Ammonia and Total Amino Acids in Ruminal Fluid and In Vitro Media. J. Dairy Sci. 1980, 63, 64–75. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Sharma, O.P.; Dawra, R.K.; Negi, S.S. Simple Determination of Microbial Protein in Rumen Liquor. J. Dairy Sci. 1982, 65, 2170–2173. [Google Scholar] [CrossRef]
- Erwin, E.S.; Marco, G.J.; Emery, E.M. Volatile Fatty Acid Analyses of Blood and Rumen Fluid by Gas Chromatography. J. Dairy Sci. 1961, 44, 1768–1771. [Google Scholar] [CrossRef]
- Saleem, A.M.; Nyachiro, J.; Gomaa, W.M.S.; Yang, W.; Oatway, L.; McAllister, T.A. Effects of barley type and processing method on rumen fermentation, dry matter disappearance and fermentation characteristics in batch cultures. Anim. Feed Sci. Technol. 2020, 269, 114625. [Google Scholar] [CrossRef]
- Gallo, A.; Giuberti, G.; Masoero, F. Gas production and starch degradability of corn and barley meals differing in mean particle size. J. Dairy Sci. 2016, 99, 4347–4359. [Google Scholar] [CrossRef]
- Xu, N.N.; Wang, D.M.; Wang, B.; Wang, J.K.; Liu, J.X. Different endosperm structures in wheat and corn affected in vitro rumen fermentation and nitrogen utilization of rice straw-based diet. Animal 2019, 13, 1607–1613. [Google Scholar] [CrossRef]
- Jacobs, J.L. Challenges in ration formulation in pasture-based milk production systems. Anim. Prod. Sci. 2014, 54, 1130–1140. [Google Scholar] [CrossRef]
- Leddin, C.M.; Stockdale, C.R.; Hill, J.; Heard, J.W.; Doyle, P.T. Increasing amounts of crushed wheat fed with pasture hay reduced dietary fiber digestibility in lactating dairy cows. J. Dairy Sci. 2009, 92, 2747–2757. [Google Scholar] [CrossRef] [PubMed]
- Lechartier, C.; Peyraud, J.L. The effects of starch and rapidly degradable dry matter from concentrate on ruminal digestion in dairy cows fed corn silage-based diets with fixed forage proportion. J. Dairy Sci. 2011, 94, 2440–2454. [Google Scholar] [CrossRef]
- Tan, H.Y.; Sieo, C.C.; Abdullah, N.; Liang, J.B.; Huang, X.D.; Ho, Y.W. Effects of condensed tannins from Leucaena on methane production, rumen fermentation and populations of methanogens and protozoa in vitro. Anim. Feed Sci. Technol. 2011, 169, 185–193. [Google Scholar] [CrossRef]
- Pang, D.G.; Yang, H.J.; Cao, B.B.; Wu, T.T.; Wang, J.Q. The beneficial effect of Enterococcus faecium on the in vitro ruminal fermentation rate and extent of three typical total mixed rations in northern China. Livest. Sci. 2014, 167, 154–160. [Google Scholar] [CrossRef]
- Russell, J.B.; O’Connor, J.D.; Fox, D.G.; Van Soest, P.J.; Sniffen, C.J. A net carbohydrate and protein system for evaluating cattle diets: I. Ruminal fermentation. J. Anim. Sci. 1992, 70, 3551–3561. [Google Scholar] [CrossRef]
- Piao, M.Y.; Kim, H.J.; Seo, J.K.; Park, T.S.; Yoon, J.S.; Kim, K.H.; Ha, J.K. Effects of synchronization of carbohydrate and protein supply in total mixed ration with korean rice wine residue on ruminal fermentation, nitrogen metabolism and microbial protein synthesis in holstein steers. Asian-Australas J. Anim. Sci. 2012, 25, 1568–1574. [Google Scholar] [CrossRef]
- Agle, M.; Hristov, A.N.; Zaman, S.; Schneider, C.; Ndegwa, P.; Vaddella, V.K. The effects of ruminally degraded protein on rumen fermentation and ammonia losses from manure in dairy cows. J. Dairy Sci. 2010, 93, 1625–1637. [Google Scholar] [CrossRef]
- Satter, L.D.; Slyter, L.L. Effect of ammonia concentration of rumen microbial protein production in vitro. Br. J. Nutr. 1974, 32, 199–208. [Google Scholar] [CrossRef]
- Schwab, C.G.; Huhtanen, P.; Hunt, C.; Hvelplund, T. Nitrogen Requirements of Cattle; Pfeffer, A.H.E., Ed.; CABI Publishing: Wallingford, UK, 2005. [Google Scholar]
- Odle, J.; Schaefer, D.M. Influence of rumen ammonia concentration on the rumen degradation rates of barley and maize. Br. J. Nutr. 1987, 57, 127–138. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, N.; Shen, W.; Zhao, S.; Wang, J. Synchrony Degree of Dietary Energy and Nitrogen Release Influences Microbial Community, Fermentation, and Protein Synthesis in a Rumen Simulation System. Microorganisms 2020, 8, 231. [Google Scholar] [CrossRef]
- Munnich, M.; Khol-Parisini, A.; Klevenhusen, F.; Metzler-Zebeli, B.U.; Zebeli, Q. Graded replacement of maize grain with molassed sugar beet pulp modulated ruminal microbial community and fermentation profile in vitro. J. Sci. Food Agric. 2018, 98, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Voelker, J.A.; Allen, M.S. Pelletsed Beet Pulp Substituted for High-Moisture Corn: 3. Effects on Ruminal Fermentation, pH, and Microbial Protein Efficiency in Lactating Dairy Cows. J. Dairy Sci. 2003, 86, 3562–3570. [Google Scholar] [CrossRef]
- Savari, M.; Khorvash, M.; Amanlou, H.; Ghorbani, G.R.; Ghasemi, E.; Mirzaei, M. Effects of rumen-degradable protein:rumen-undegradable protein ratio and corn processing on production performance, nitrogen efficiency, and feeding behavior of Holstein dairy cows. J. Dairy Sci. 2018, 101, 1111–1122. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, J.; Ellis, J.L.; Kebreab, E.; Strathe, A.B.; López, S.; France, J.; Bannink, A. Ruminal pH regulation and nutritional consequences of low pH. Anim. Feed Sci. Technol. 2012, 172, 22–33. [Google Scholar] [CrossRef]
- Shen, Y.Z.; Ran, T.; Saleem, A.M.; Wang, H.R.; Yang, W.Z. Short communication: Ground corn steeped in citric acid modulates in vitro gas production kinetics, fermentation patterns and dry matter digestibility. Anim. Feed Sci. Technol. 2019, 247, 9–14. [Google Scholar] [CrossRef]
- Kand, D.; Bagus Raharjo, I.; Castro-Montoya, J.; Dickhoefer, U. The effects of rumen nitrogen balance on in vitro rumen fermentation and microbial protein synthesis vary with dietary carbohydrate and nitrogen sources. Anim. Feed Sci. Technol. 2018, 241, 184–197. [Google Scholar] [CrossRef]
- Castro, M.M.D.; Cardoso, M.A.; Detmann, E.; Fonseca, M.A.; Sampaio, C.B.; Marcondes, M.I. In vitro ruminal fermentation and enteric methane production of tropical forage added nitrogen or nitrogen plus starch. Anim. Feed Sci. Technol. 2021, 275, 114878. [Google Scholar] [CrossRef]
- Wang, W.; Wu, Q.; Li, W.; Wang, Y.; Zhang, F.; Lv, L.; Li, S.; Yang, H. High-Gossypol Whole Cottonseed Exhibited Mediocre Rumen Degradability and Less Microbial Fermentation Efficiency than Cottonseed Hull and Cottonseed Meal with an In Vitro Gas Production Technique. Fermentation 2022, 8, 103. [Google Scholar] [CrossRef]
- McDonald, P. Animal Nutrition, 7th ed.; Prentice Hall: Harlow, UK, 2011. [Google Scholar]
- Davila, A.M.; Blachier, F.; Gotteland, M.; Andriamihaja, M.; Benetti, P.H.; Sanz, Y.; Tome, D. Intestinal luminal nitrogen metabolism: Role of the gut microbiota and consequences for the host. Pharmacol. Res. 2013, 68, 95–107. [Google Scholar] [CrossRef]
- Hristov, A.N.; Etter, R.P.; Ropp, J.K.; Grandeen, K.L. Effect of dietary crude protein level and degradability on ruminal fermentation and nitrogen utilization in lactating dairy cows. J. Anim. Sci. 2004, 82, 3219–3229. [Google Scholar] [CrossRef]
- Vastolo, A.; Calabro, S.; Pacifico, S.; Koura, B.I.; Cutrignelli, M.I. Chemical and nutritional characteristics of Cannabis sativa L. co-products. J. Anim. Physiol. Anim. Nutr. 2021, 105 (Suppl. S1), 1–9. [Google Scholar] [CrossRef] [PubMed]
- Calsamiglia, S.; Stern, M.D.; Firkins, J.L. Effects of protein source on nitrogen metabolism in continuous culture and intestinal digestion in vitro. J. Anim. Sci. 1995, 73, 1819–1827. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).