Counting on Numbers—Numerical Abilities in Grey Bamboo Sharks and Ocellate River Stingrays
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
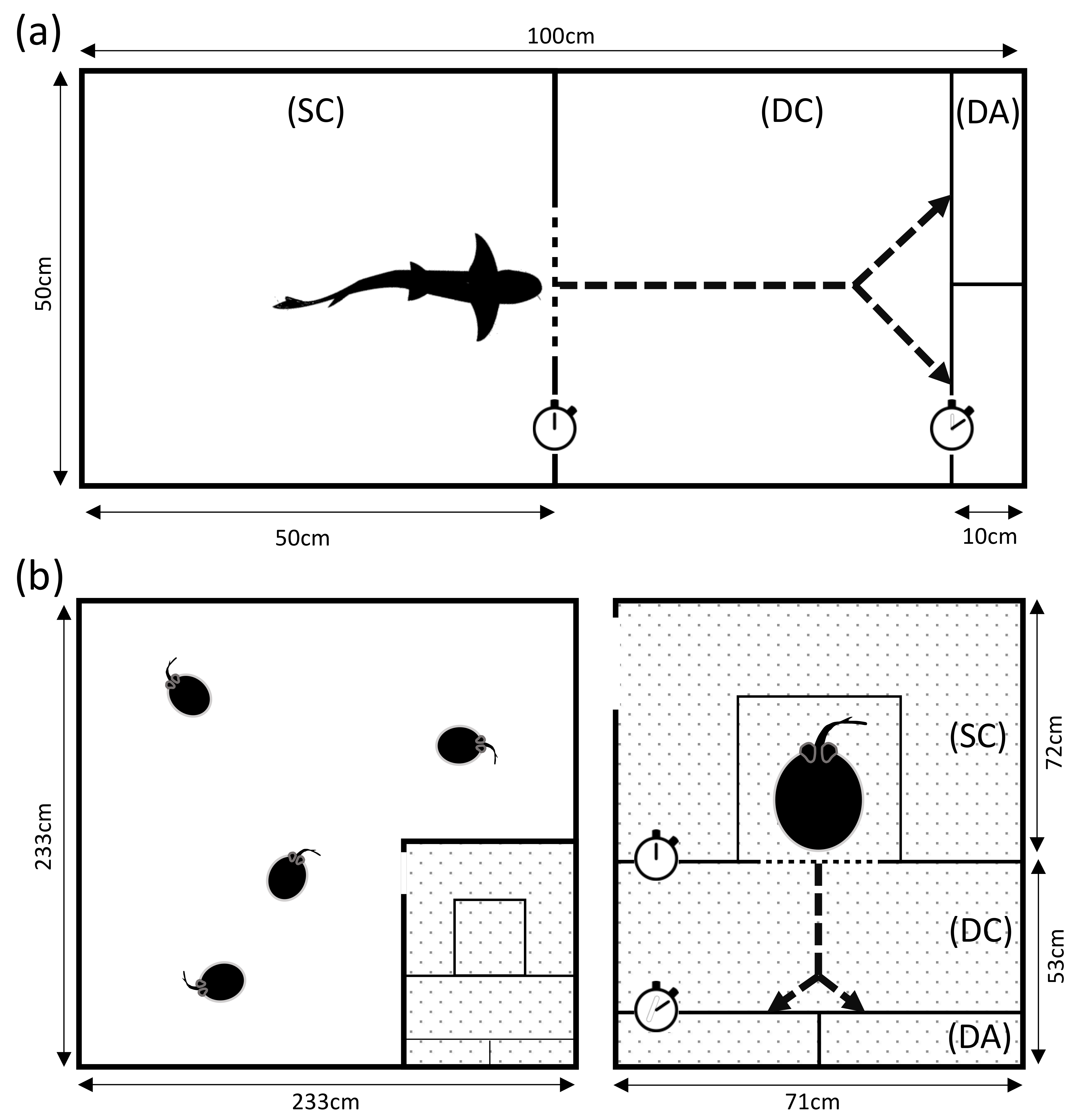
2.1. Housing and Experimental Facilities
2.2. Experimental Procedure
2.3. Experimental Animals
2.3.1. Grey Bamboo Shark (Chiloscyllium griseum)
2.3.2. Ocellate River Stingray (Potamotrygon Motoro)
2.4. Experiments
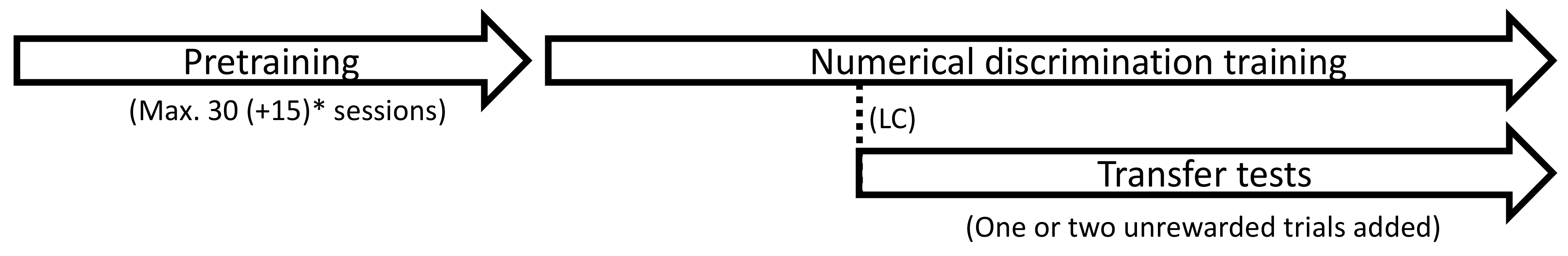
2.4.1. Pretraining
2.4.2. Numerical Discrimination Training
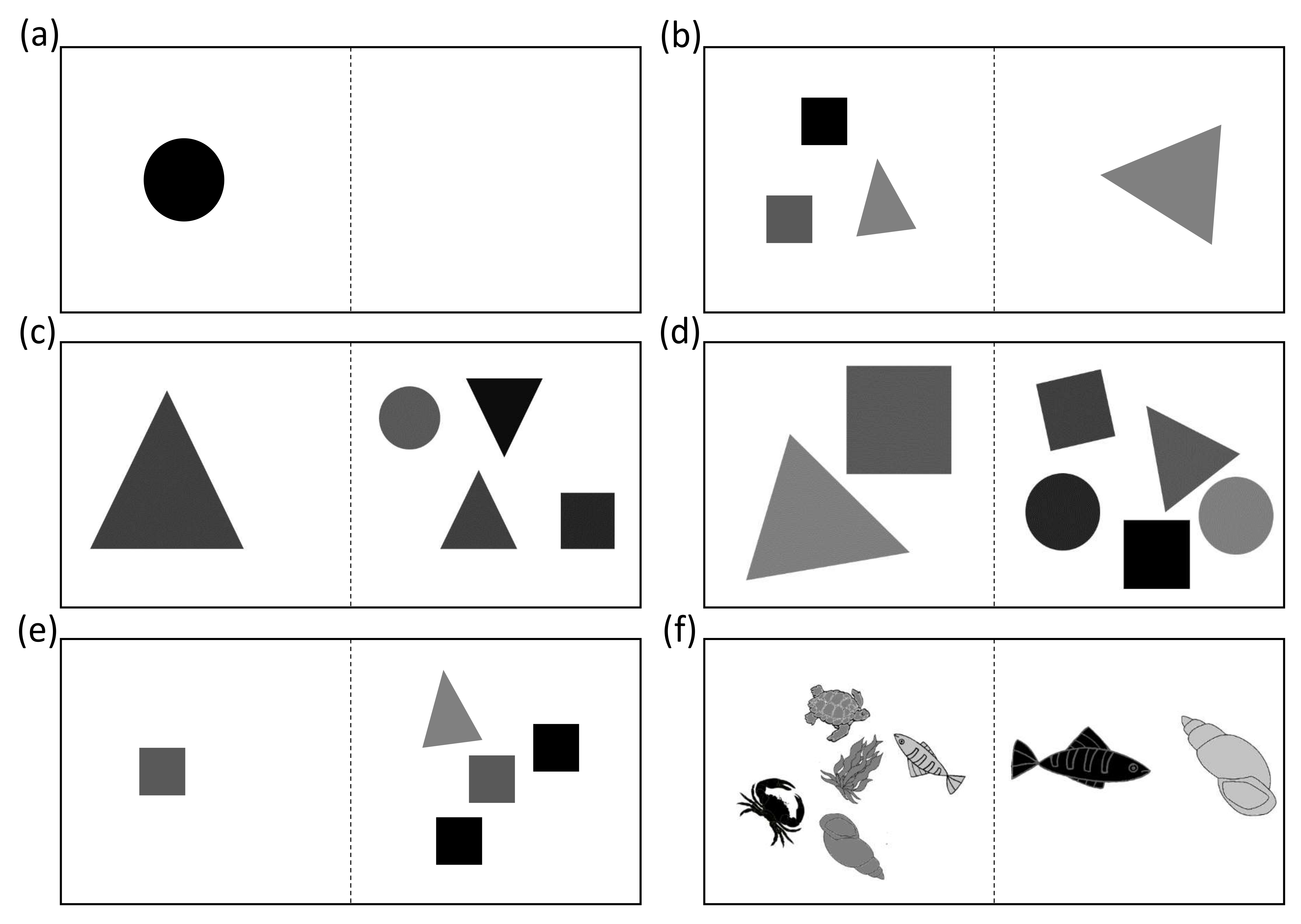
2.4.3. Transfer Tests
3. Results
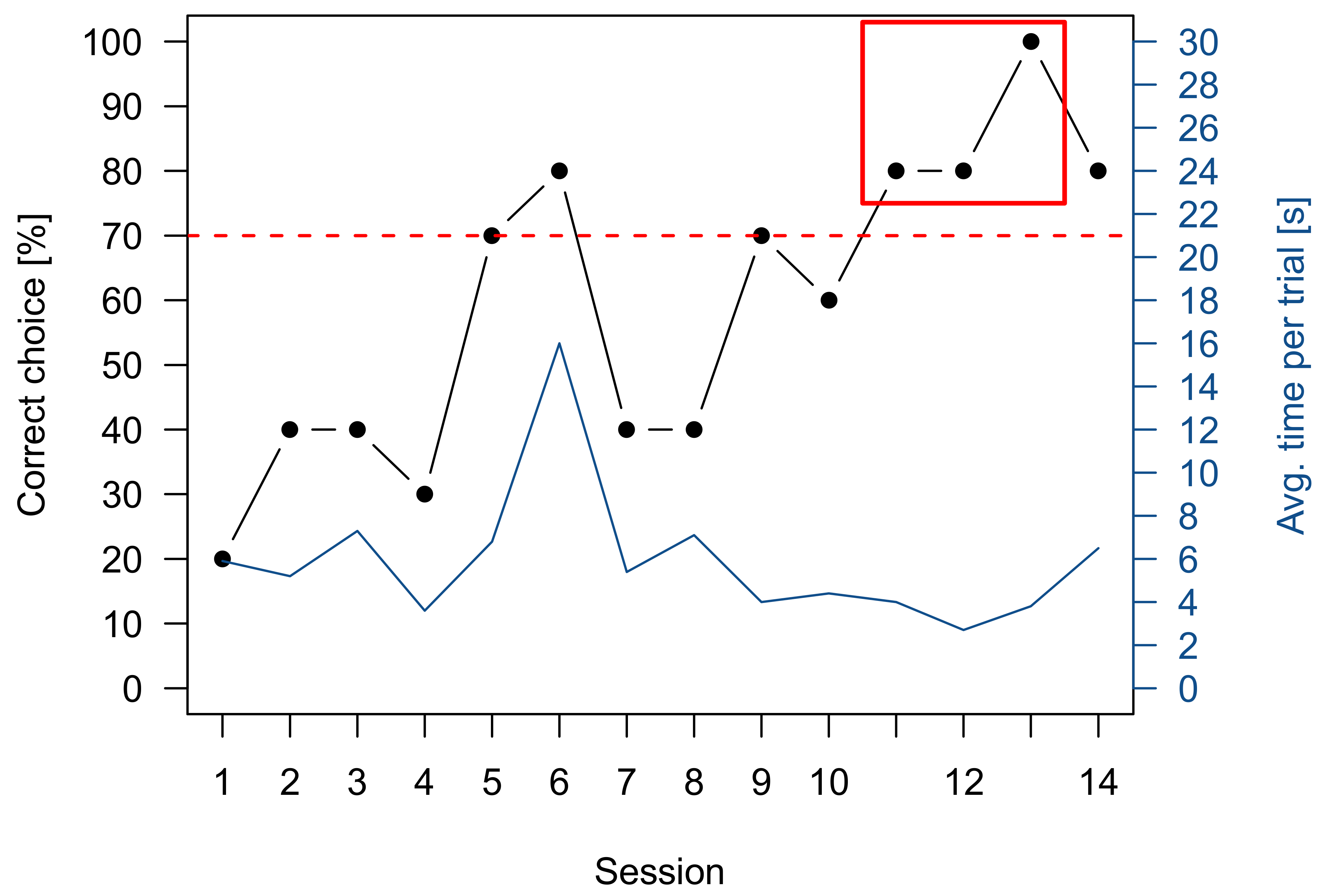
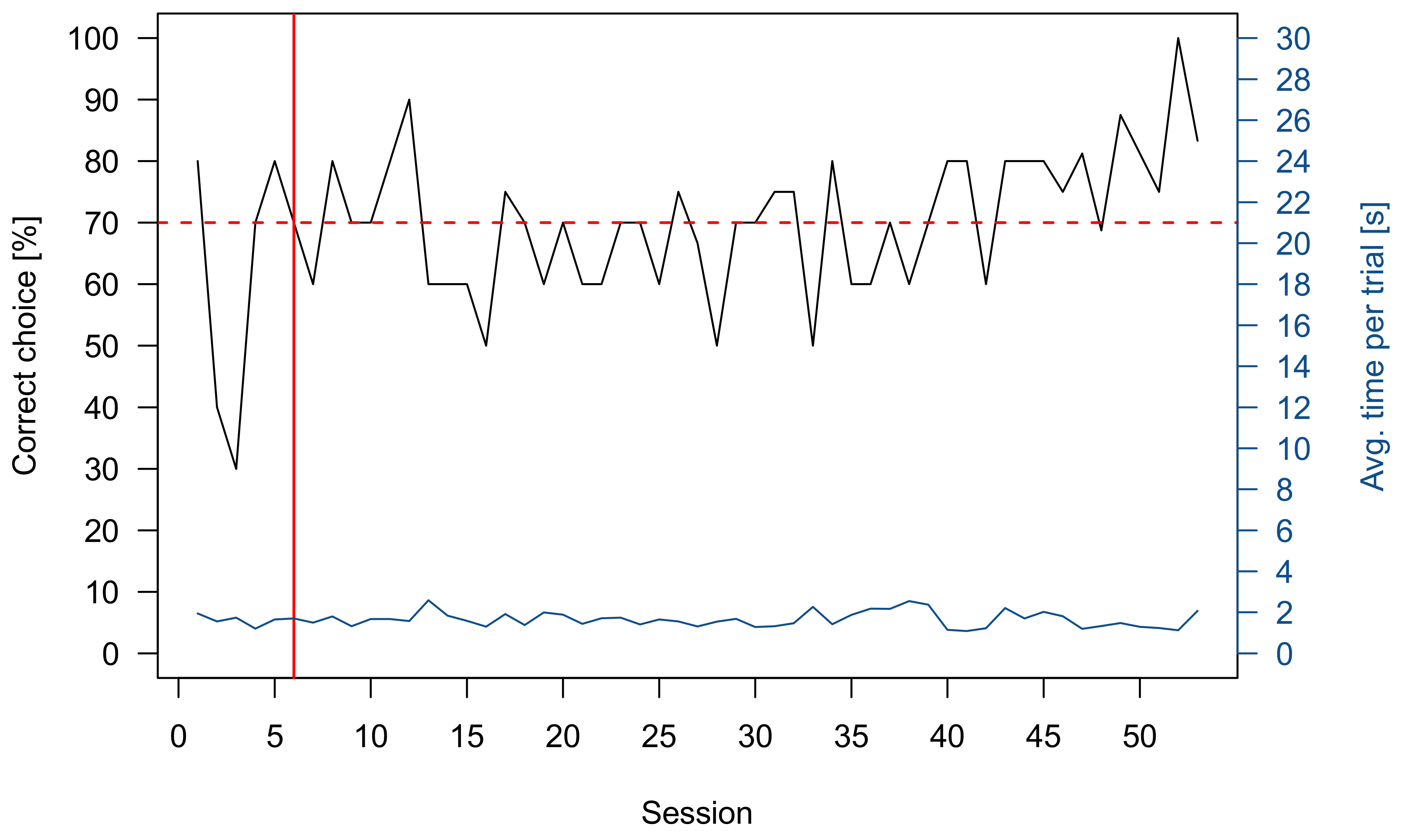
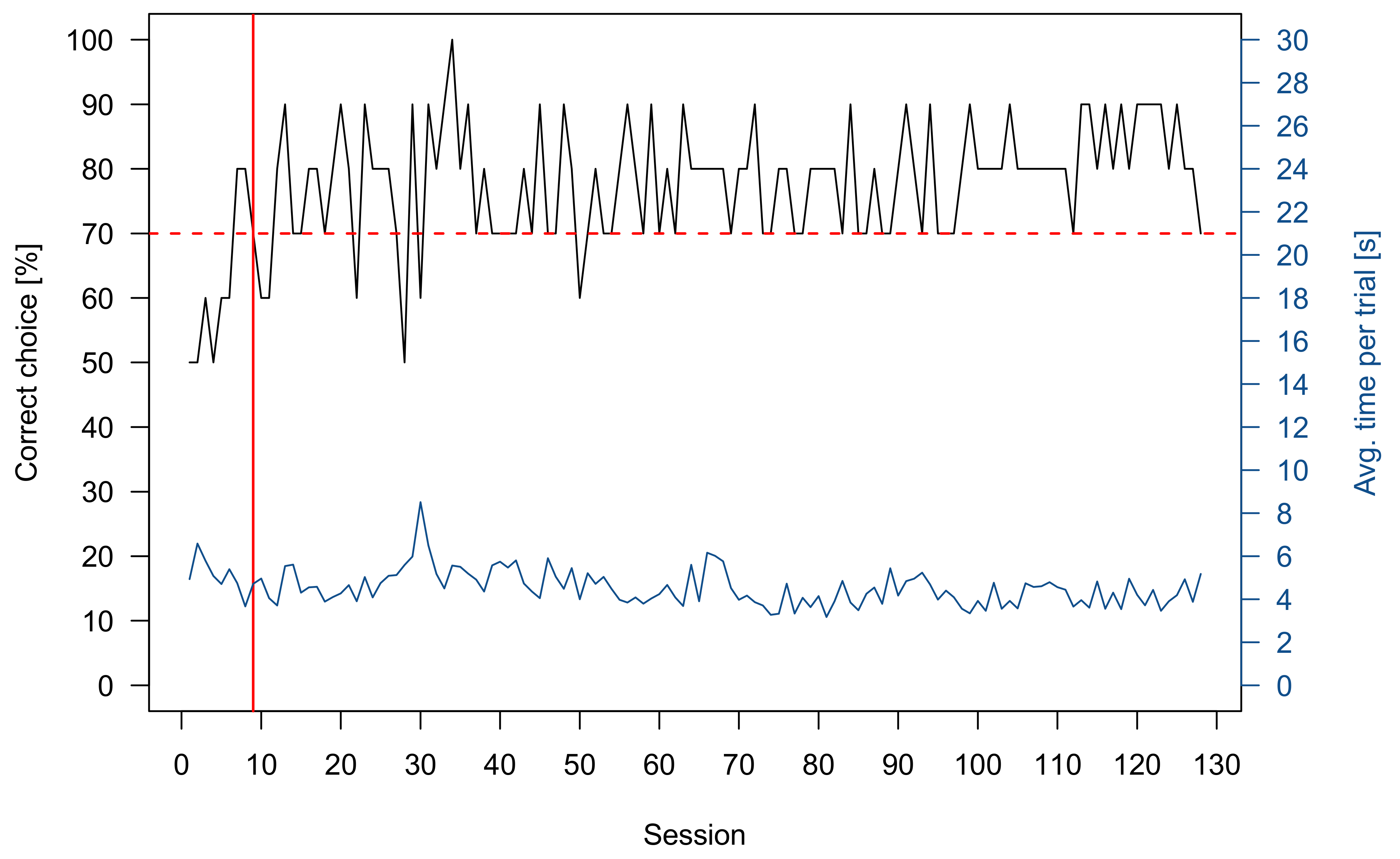
3.1. Pretraining
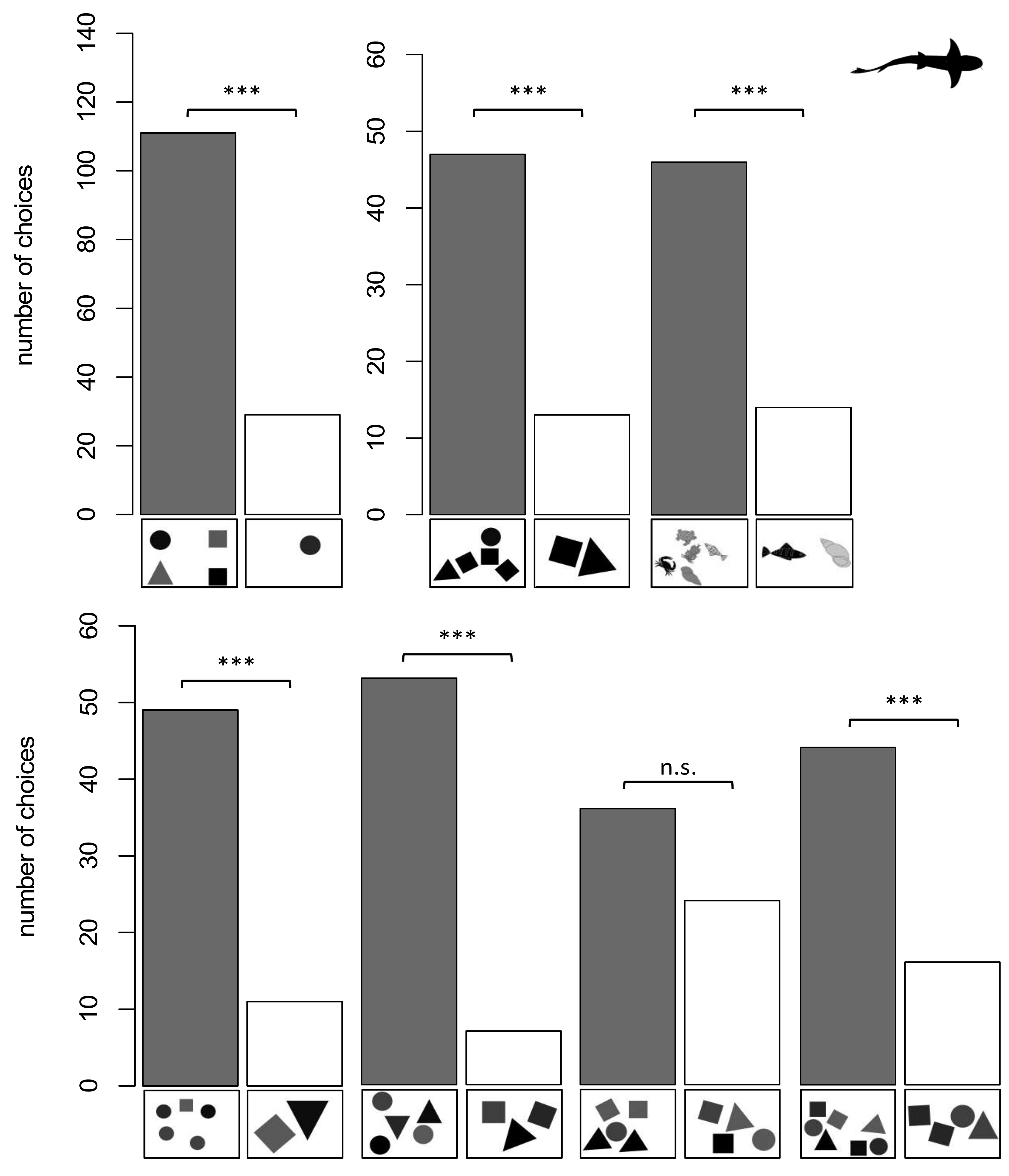
3.2. Sharks
3.2.1. Group 1
3.2.2. Group 2
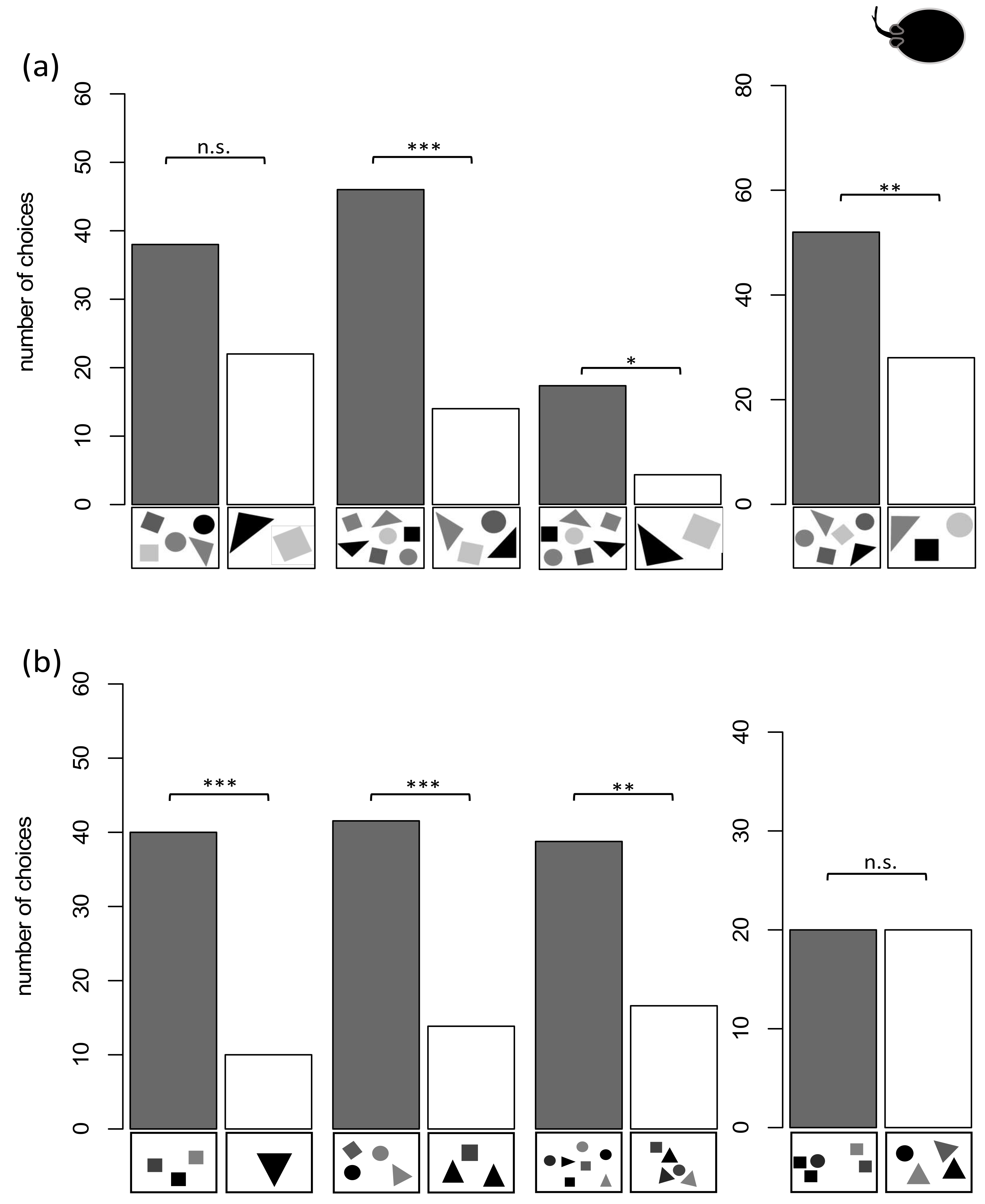
3.3. Rays
3.3.1. Group 3
3.3.2. Group 4
4. Discussion
4.1. Grey Bamboo Shark (Chiloscyllium griseum)
4.2. Ocellate River Stingray (Potamotrygon motoro)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Hauser, M.D.; Carey, S.; Hauser, L.B. Spontaneous Number Representation in Semi-Free-Ranging Rhesus Monkeys. Proc. R. Soc. London. Ser. B Biol. Sci. 2000, 267, 829–833. [Google Scholar] [CrossRef]
- Howard, S.R.; Avarguès-Weber, A.; Garcia, J.E.; Greentree, A.D.; Dyer, A.G. Numerical Cognition in Honeybees Enables Addition and Subtraction. Sci. Adv. 2019, 5, eaav0961. [Google Scholar] [CrossRef]
- Bogale, B.A.; Kamata, N.; Mioko, K.; Sugita, S. Quantity Discrimination in Jungle Crows, Corvus Macrorhynchos. Anim. Behav. 2011, 82, 635–641. [Google Scholar] [CrossRef]
- Agrillo, C.; Dadda, M.; Serena, G.; Bisazza, A. Do Fish Count? Spontaneous Discrimination of Quantity in Female Mosquitofish. Anim. Cogn. 2008, 11, 495–503. [Google Scholar] [CrossRef]
- Uller, C.; Jaeger, R.; Guidry, G.; Martin, C. Salamanders (Plethodon cinereus) Go for More: Rudiments of Number in an Amphibian. Anim. Cogn. 2003, 6, 105–112. [Google Scholar] [CrossRef]
- Gazzola, A.; Vallortigara, G.; Pellitteri-Rosa, D. Continuous and Discrete Quantity Discrimination in Tortoises. Biol. Lett. 2018, 14, 20180649. [Google Scholar] [CrossRef]
- Howard, S.R.; Schramme, J.; Garcia, J.E.; Ng, L.; Avarguès-Weber, A.; Greentree, A.D.; Dyer, A.G. Spontaneous Quantity Discrimination of Artificial Flowers by Foraging Honeybees. J. Exp. Biol. 2020, 223. [Google Scholar] [CrossRef]
- Panteleeva, S.; Reznikova, Z.; Vygonyailova, O. Quantity Judgments in the Context of Risk/Reward Decision Making in Striped Field Mice: First “Count,” Then Hunt. Front. Psychol. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Agrillo, C.; Dadda, M.; Serena, G. Choice of Female Groups by Male Mosquitofish (Gambusia holbrooki). Ethology 2008, 114, 479–488. [Google Scholar] [CrossRef]
- Carazo, P.; Font, E.; Forteza-Behrendt, E.; Desfilis, E. Quantity Discrimination in Tenebrio Molitor: Evidence of Numerosity Discrimination in an Invertebrate? Anim. Cogn. 2009, 12, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Hager, M.C.; Helfman, G.S. Safety in Numbers: Shoal Size Choice by Minnows under Predatory Threat. Behav. Ecol. Sociobiol. 1991, 29, 271–276. [Google Scholar] [CrossRef]
- Mehlis, M.; Thünken, T.; Bakker, T.C.M.; Frommen, J.G. Quantification Acuity in Spontaneous Shoaling Decisions of Three-Spined Sticklebacks. Anim. Cogn. 2015, 18, 1125–1131. [Google Scholar] [CrossRef]
- Agrillo, C.; Miletto Petrazzini, M.E.; Bisazza, A. At the Root of Math: Numerical Abilities in Fish. In Mathematical Cognition and Learning; Geary, D.C., Berch, D.B., Koepke, K.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 1, pp. 3–33. [Google Scholar]
- Cantlon, J.F.; Brannon, E.M. Shared System for Ordering Small and Large Numbers in Monkeys and Humans. Psychol. Sci. 2006, 17, 401–406. [Google Scholar] [CrossRef]
- Feigenson, L.; Dehaene, S.; Spelke, E. Core Systems of Number. Trends Cogn. Sci. 2004, 8, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Piazza, M. Neurocognitive Start-Up Tools for Symbolic Number Representations Reprinted from Trends in Cognitive Sciences, Vol 14, Manuela Piazza, Neurocognitive Start-up Tools for Symbolic Number Representations, pp. 542–551, 2010, with Permission from Elsevier. In Space, Time and Number in the Brain; Dehaene, S., Brannon, E.M., Eds.; Academic Press: San Diego, CA, USA, 2011; pp. 267–285. [Google Scholar]
- Xu, F. Numerosity Discrimination in Infants: Evidence for Two Systems of Representations. Cognition 2003, 89, B15–B25. [Google Scholar] [CrossRef]
- Feigenson, L.; Carey, S.; Hauser, M. The Representations Underlying Infants’ Choice of More: Object Files vs. Analog Magnitudes. Psychol. Sci. 2002, 13, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Pylyshyn, Z.W.; Storm, R.W. Tracking Multiple Independent Targets: Evidence for a Parallel Tracking Mechanism. Spat. Vis. 1988, 3, 179–197. [Google Scholar] [CrossRef] [PubMed]
- Trick, L.M.; Pylyshyn, Z.W. Why Are Small and Large Numbers Enumerated Differently? A Limited-Capacity Preattentive Stage in Vision. Psychol. Rev. 1994, 101, 80–102. [Google Scholar] [CrossRef]
- Jordan, K.E.; Brannon, E.M. Weber’s Law Influences Numerical Representations in Rhesus Macaques (Macaca Mulatta). Anim. Cogn. 2006, 9, 159–172. [Google Scholar] [CrossRef]
- Lipton, J.S.; Spelke, E.S. Origins of Number Sense: Large-Number Discrimination in Human Infants. Psychol. Sci. 2003, 14, 396–401. [Google Scholar] [CrossRef]
- Brannon, E.M.; Roitman, J.D. Nonverbal Representations of Time and Number in Animals and Human Infants. In Functional and Neural Mechanisms of Interval Timing; CRC Press/Routledge/Taylor & Francis Group: Boca Raton, FL, USA, 2003; pp. 143–182. [Google Scholar]
- Beran, M.J. Rhesus Monkeys (Macaca Mulatta) Enumerate Large and Small Sequentially Presented Sets of Items Using Analog Numerical Representations. J. Exp. Psychol. Anim. Behav. Process. 2007, 33, 42–54. [Google Scholar] [CrossRef]
- Barnard, A.M.; Hughes, K.D.; Gerhardt, R.R.; DiVincenti, L.J.; Bovee, J.M.; Cantlon, J.F. Inherently Analog Quantity Representations in Olive Baboons (Papio Anubis). Front. Psychol. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Bonanni, R.; Natoli, E.; Cafazzo, S.; Valsecchi, P. Free-Ranging Dogs Assess the Quantity of Opponents in Intergroup Conflicts. Anim. Cogn. 2011, 14, 103–115. [Google Scholar] [CrossRef]
- Rugani, R.; Cavazzana, A.; Vallortigara, G.; Regolin, L. One, Two, Three, Four, or Is There Something More? Numerical Discrimination in Day-Old Domestic Chicks. Anim. Cogn. 2013, 16, 557–564. [Google Scholar] [CrossRef]
- Agrillo, C.; Bisazza, A. Understanding the Origin of Number Sense: A Review of Fish Studies. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20160511. [Google Scholar] [CrossRef] [PubMed]
- Lucon-Xiccato, T.; Dadda, M.; Gatto, E.; Bisazza, A. Development and Testing of a Rapid Method for Measuring Shoal Size Discrimination. Anim. Cogn. 2017, 20, 149–157. [Google Scholar] [CrossRef]
- Agrillo, C.; Piffer, L.; Bisazza, A. Large Number Discrimination by Mosquitofish. PLoS ONE 2010, 5, e15232. [Google Scholar] [CrossRef]
- Agrillo, C.; Miletto Petrazzini, M.E.; Bisazza, A. Numerical Acuity of Fish is Improved in the Presence of Moving Targets, but Only in the Subitizing Range. Anim. Cogn. 2014, 17, 307–316. [Google Scholar] [CrossRef]
- Beran, M.J.; Parrish, A.E. Going for More: Discrete and Continuous Quantity Judgments by Nonhuman Animals. In Continuous Issues in Numerical Cognition; Henik, A., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 175–192. [Google Scholar]
- Agrillo, C.; Piffer, L.; Bisazza, A. Number vs. Continuous Quantity in Numerosity Judgments by Fish. Cognition 2011, 119, 281–287. [Google Scholar] [CrossRef]
- Agrillo, C.; Bisazza, A. Spontaneous versus Trained Numerical Abilities. A Comparison between the Two Main Tools to Study Numerical Competence in Non-Human Animals. J. Neurosci. Methods 2014, 234, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Agrillo, C.; Dadda, M.; Serena, G.; Bisazza, A. Use of Number by Fish. PLoS ONE 2009, 4, e4786. [Google Scholar] [CrossRef]
- Bisazza, A.; Piffer, L.; Serena, G.; Agrillo, C. Ontogeny of Numerical Abilities in Fish. PLoS ONE 2010, 5, e15516. [Google Scholar] [CrossRef] [PubMed]
- Miletto Petrazzini, M.E.; Agrillo, C.; Izard, V.; Bisazza, A. Relative vs. Absolute Numerical Representation in Fish: Can Guppies Represent “Fourness”? Anim. Cogn. 2015, 18, 1007–1017. [Google Scholar] [CrossRef]
- Miletto Petrazzini, M.E.; Agrillo, C.; Izard, V.; Bisazza, A. Do Humans (Homo Sapiens) and Fish (Pterophyllum Scalare) Make Similar Numerosity Judgments? J. Comp. Psychol. 2016, 130, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Agrillo, C.; Miletto Petrazzini, M.E.; Tagliapietra, C.; Bisazza, A. Inter-Specific Differences in Numerical Abilities Among Teleost Fish. Front. Psychol. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- DeLong, C.M.; Barbato, S.; O’Leary, T.; Wilcox, K.T. Small and Large Number Discrimination in Goldfish (Carassius Auratus) with Extensive Training. Behav. Process. 2017, 141, 172–183. [Google Scholar] [CrossRef]
- Bisazza, A.; Tagliapietra, C.; Bertolucci, C.; Foà, A.; Agrillo, C. Non-Visual Numerical Discrimination in a Blind Cavefish (Phreatichthys Andruzzii). J. Exp. Biol. 2014, 217, 1902–1909. [Google Scholar] [CrossRef]
- Guttridge, T.L.; Myrberg, A.A.; Porcher, I.F.; Sims, D.W.; Krause, J. The Role of Learning in Shark Behaviour. Fish Fish. 2009, 10, 450–469. [Google Scholar] [CrossRef]
- Guttridge, T.L.; Yopak, K.E.; Schluessel, V. Sharks-Elasmobranch Cognition. In Field and Laboratory Methods in Animal Cognition: A Comparative Guide; Cambridge University Press: Cambridge, UK, 2018; pp. 354–380. [Google Scholar]
- Schluessel, V.; Rick, I.P.; Seifert, F.D.; Baumann, C.; Lee Davies, W.I. Not Just Shades of Grey: Life Is Full of Colour for the Ocellate River Stingray (Potamotrygon Motoro). J. Exp. Biol. 2021, 224. [Google Scholar] [CrossRef]
- Daniel, M.M.M.; Alvermann, L.; Böök, I.; Schluessel, V. Visual Discrimination and Resolution in Freshwater Stingrays (Potamotrygon Motoro). J. Comp. Physiol. A 2021, 207, 43–58. [Google Scholar] [CrossRef]
- Daniel, M.M.M.; Schluessel, V. Serial Reversal Learning in Freshwater Stingrays (Potamotrygon motoro). Anim. Cogn. 2020, 23, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Schluessel, V.; Ober, C. How to Get out of a Maze? Stingrays (Potamotrygon motoro) Use Directional over Landmark Information When Provided with Both in a Spatial Task. Evol. Ecol. Res. 2018, 19, 591–617. [Google Scholar]
- Fuss, T.; John, L.; Schluessel, V. Same or Different? Abstract Relational Concept Use in Juvenile Bamboo Sharks and Malawi Cichlids. Curr. Zool. 2018. [Google Scholar] [CrossRef]
- Fuss, T.; Russnak, V.; Stehr, K.; Schluessel, V. World in Motion: Perception and Discrimination of Movement in Juvenile Grey Bamboo Sharks (Chiloscyllium griseum). AB&C 2017, 4, 223–241. [Google Scholar] [CrossRef][Green Version]
- Fuss, T.; Schluessel, V. The Ebbinghaus Illusion in the Gray Bamboo Shark (Chiloscyllium griseum) in Comparison to the Teleost Damselfish (Chromis Chromis). Zoology 2017, 123, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Schluessel, V.; Herzog, H.; Scherpenstein, M. Seeing the Forest before the Trees-Spatial Orientation in Freshwater Stingrays (Potamotrygon motoro) in a Hole-Board Task. Behav. Process. 2015, 119, 105–115. [Google Scholar] [CrossRef]
- Schluessel, V.; Duengen, D. Irrespective of Size, Scales, Color or Body Shape, All Fish Are Just Fish: Object Categorization in the Gray Bamboo Shark Chiloscyllium Griseum. Anim. Cogn. 2015, 18, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Fuss, T.; Schluessel, V. Something Worth Remembering: Visual Discrimination in Sharks. Anim. Cogn. 2015, 18, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Kuba, M.J.; Byrne, R.A.; Burghardt, G.M. A New Method for Studying Problem Solving and Tool Use in Stingrays (Potamotrygon castexi). Anim. Cogn. 2010, 13, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Compagno, L.J.V. Alternative Life-History Styles of Cartilaginous Fishes in Time and Space. Environ. Biol. Fish. 1990, 28, 33–75. [Google Scholar] [CrossRef]
- Vila Pouca, C.; Gervais, C.; Reed, J.; Michard, J.; Brown, C. Quantity Discrimination in Port Jackson Sharks Incubated under Elevated Temperatures. Behav. Ecol. Sociobiol. 2019, 73, 93. [Google Scholar] [CrossRef]
- Schluessel, V.; Beil, O.; Weber, T.; Bleckmann, H. Symmetry Perception in Bamboo Sharks (Chiloscyllium griseum) and Malawi Cichlids (Pseudotropheus sp.). Anim. Cogn. 2014, 17, 1187–1205. [Google Scholar] [CrossRef]
- Fuss, T.; Bleckmann, H.; Schluessel, V. The Brain Creates Illusions Not Just for Us: Sharks (Chiloscyllium griseum) Can “See the Magic” as Well. Front. Neural Circuits 2014, 8. [Google Scholar] [CrossRef]
- Schluessel, V.; Bleckmann, H. Spatial Learning and Memory Retention in the Grey Bamboo Shark (Chiloscyllium griseum). Zoology 2012, 115, 346–353. [Google Scholar] [CrossRef]
- Agrillo, C.; Miletto Petrazzini, M.E.; Bisazza, A. Numerical Abilities in Fish: A Methodological Review. Behav. Process. 2017, 141, 161–171. [Google Scholar] [CrossRef]
- Compagno, L.J.V.; Nations, F. and A.O. of the U. Sharks of the World: An. Annotated and Illustrated Catalogue of Shark Species Known to Date; Food & Agriculture Org: Rome, Italy, 2001. [Google Scholar]
- Garrone Neto, D.; Uieda, V.S. Activity and Habitat Use of Two Species of Stingrays (Myliobatiformes: Potamotrygonidae) in the Upper Paraná River Basin, Southeastern Brazil. Neotrop. Ichthyol. 2012, 10, 81–88. [Google Scholar] [CrossRef]
- Kilian, A.; Yaman, S.; von Fersen, L.; Güntürkün, O. A Bottlenose Dolphin Discriminates Visual Stimuli Differing in Numerosity. Anim. Learn. Behav. 2003, 31, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Stancher, G.; Rugani, R.; Regolin, L.; Vallortigara, G. Numerical Discrimination by Frogs (Bombina orientalis). Anim. Cogn. 2015, 18, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Laplaza, L.M.; Gerlai, R. Spontaneous Discrimination of Small Quantities: Shoaling Preferences in Angelfish (Pterophyllum scalare). Anim. Cogn. 2011, 14, 565–574. [Google Scholar] [CrossRef]
- Agrillo, C.; Dadda, M. Discrimination of the Larger Shoal in the Poeciliid Fish Girardinus falcatus. Ethol. Ecol. Evol. 2007, 19, 145–157. [Google Scholar] [CrossRef]
- Stancher, G.; Sovrano, V.A.; Potrich, D.; Vallortigara, G. Discrimination of Small Quantities by Fish (Redtail Splitfin, Xenotoca Eiseni). Anim. Cogn. 2013, 16, 307–312. [Google Scholar] [CrossRef]
- Piffer, L.; Agrillo, C.; Hyde, D.C. Small and Large Number Discrimination in Guppies. Anim. Cogn. 2012, 15, 215–221. [Google Scholar] [CrossRef]
- Bisazza, A.; Agrillo, C.; Lucon-Xiccato, T. Extensive Training Extends Numerical Abilities of Guppies. Anim. Cogn. 2014, 17, 1413–1419. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Spelke, E.S.; Goddard, S. Number Sense in Human Infants. Dev. Sci. 2005, 8, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Lucon-Xiccato, T.; Bisazza, A. Individual Differences in Cognition among Teleost Fishes. Behav. Process. 2017, 141, 184–195. [Google Scholar] [CrossRef]
- Lucon-Xiccato, T.; Dadda, M. Individual Guppies Differ in Quantity Discrimination Performance across Antipredator and Foraging Contexts. Behav. Ecol. Sociobiol. 2016, 71, 13. [Google Scholar] [CrossRef]
- Gatto, E.; Lucon-Xiccato, T.; Savaşçı, B.B.; Dadda, M.; Bisazza, A. Experimental Setting Affects the Performance of Guppies in a Numerical Discrimination Task. Anim. Cogn. 2017, 20, 187–198. [Google Scholar] [CrossRef]
| C. griseum | P. motoro | |
|---|---|---|
| Groups tested within the Transfer tests | Group 1 | Groups 3 and 4 |
| Geometrical two-dimensional objects presented as stimuli | Circle, Square, Triangle | |
| Tasks within the Training procedures | 1 vs. 4 2 vs. 5 | 1 vs. 3 (Group 4) |
| 1 vs. 4 (Groups 3 and 4) | ||
| 2 vs. 5 (Groups 3 and 4) | ||
| 3 vs. 6 (Group 3) | ||
| 4 vs. 7 (Group 3) | ||
| 5 vs. 7 (Group 3) | ||
| 6 vs. 7 (Group 3) | ||
| Successfully discriminated quantities in Transfer tests with controlled continuous variables | 4 vs. 1 5 vs. 2 (Same stimuluscolor) 5 vs. 2 (Non-geometrical stimuli) 5 vs. 2 (Positive stimuli reduced in size) 5 vs. 3 7 vs. 4 | 3 vs. 1 |
| 3 vs. 2 | ||
| 4 vs. 3 | ||
| 5 vs. 2 (Group 4) * | ||
| 5 vs. 3 | ||
| 6 vs. 3 | ||
| 7 vs. 2 | ||
| 7 vs. 4 | ||
| 7 vs. 5 (Group 4) ** | ||
| 9 vs. 5 | ||
| 12 vs. 6 | ||
| 12 vs. 8 | ||
| 15 vs. 5 | ||
| Unable to discriminate quantities in Transfer tests with controlled continuous variables | 5 vs. 4 | 5 vs. 2 (Group 3) * |
| 5 vs. 4 | ||
| 7 vs. 5 (Group 3) ** | ||
| 7 vs. 6 | ||
| 12 vs. 9 | ||
| Use of “relative” or “absolute” approach to discriminate quantities | Not tested | “Relative strategy” |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kreuter, N.; Christofzik, N.; Niederbremer, C.; Bollé, J.; Schluessel, V. Counting on Numbers—Numerical Abilities in Grey Bamboo Sharks and Ocellate River Stingrays. Animals 2021, 11, 2634. https://doi.org/10.3390/ani11092634
Kreuter N, Christofzik N, Niederbremer C, Bollé J, Schluessel V. Counting on Numbers—Numerical Abilities in Grey Bamboo Sharks and Ocellate River Stingrays. Animals. 2021; 11(9):2634. https://doi.org/10.3390/ani11092634
Chicago/Turabian StyleKreuter, Nils, Nele Christofzik, Carolin Niederbremer, Janik Bollé, and Vera Schluessel. 2021. "Counting on Numbers—Numerical Abilities in Grey Bamboo Sharks and Ocellate River Stingrays" Animals 11, no. 9: 2634. https://doi.org/10.3390/ani11092634
APA StyleKreuter, N., Christofzik, N., Niederbremer, C., Bollé, J., & Schluessel, V. (2021). Counting on Numbers—Numerical Abilities in Grey Bamboo Sharks and Ocellate River Stingrays. Animals, 11(9), 2634. https://doi.org/10.3390/ani11092634





