The Sense of Number in Fish, with Particular Reference to Its Neurobiological Bases
Abstract
:Simple Summary
Abstract
1. Introduction
2. Numerical Abilities in Fish
3. Spontaneous Choice Tests
4. Operant Training Procedures
5. From Behavior to Neural Circuits
6. Neural Correlates of a Sense of Continuous Magnitude in Zebrafish
7. Neural Correlates of a Sense of Discrete Magnitude (Number) in Zebrafish
8. Implications of Neurobiological Research of Number Cognition in Zebrafish
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Key Words | Web of Science |
|---|---|
| Fish Numerosity | 44 |
| Fish Numerical Discrimination | 107 |
| Fish Size Discrimination | 585 |
| Fish Numerosity & Fish Numerical Discrimination | 32 |
| Fish Numerosity & Fish Size Discrimination | 20 |
| Fish Numerical Discrimination & Fish Size Discrimination | 53 |
| Fish Numerosity & Fish Numerical Discrimination & Fish Size Discrimination | 19 |
| Articles derived from double and triple matches | 124 |
| Relevant Articles | 58 |
References
- Piazza, M.; Izard, V. How Humans Count: Numerosity and the Parietal Cortex. Neuroscientist 2009, 15, 261–273. [Google Scholar] [CrossRef]
- Dehaene, S. Varieties of numerical abilities. Cognition 1992, 44, 1–42. [Google Scholar] [CrossRef]
- Piazza, M.; Pica, P.; Izard, V.; Spelke, E.S.; Dehaene, S. Education Enhances the Acuity of the Nonverbal Approximate Number System. Psychol. Sci. 2013, 24, 1037–1043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antell, S.E.; Keating, D.P. Perception of Numerical Invariance in Neonates. Child Dev. 1983, 54, 695–701. [Google Scholar] [CrossRef]
- Izard, V.; Sann, C.; Spelke, E.S.; Streri, A. Newborn infants perceive abstract numbers. Proc. Natl. Acad. Sci. USA 2009, 106, 10382–10385. [Google Scholar] [CrossRef] [Green Version]
- Agrillo, C.; Piffer, L.; Bisazza, A.; Butterworth, B. Evidence for Two Numerical Systems That Are Similar in Humans and Guppies. PLoS ONE 2012, 7, e31923. [Google Scholar] [CrossRef] [Green Version]
- Carey, S.; Xu, F. Infants’ knowledge of objects: Beyond object files and object tracking. Cognition 2001, 80, 179–213. [Google Scholar] [CrossRef]
- Vallortigara, G. Born Knowing; MIT Press: Cambridge, MA, USA, 2021. [Google Scholar]
- Xu, F.; Carey, S. Infants’ Metaphysics: The Case of Numerical Identity. Cogn. Psychol. 1996, 30, 111–153. [Google Scholar] [CrossRef] [PubMed]
- Cantlon, J.F.; Brannon, E.M. Basic Math in Monkeys and College Students. PLoS Biol. 2007, 5, e328. [Google Scholar] [CrossRef] [PubMed]
- Stancher, G.; Rugani, R.; Regolin, L.; Vallortigara, G. Numerical discrimination by frogs (Bombina orientalis). Anim. Cogn. 2014, 18, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Gazzola, A.; Vallortigara, G.; Pellitteri-Rosa, D. Continuous and discrete quantity discrimination in tortoises. Biol. Lett. 2018, 14, 20180649. [Google Scholar] [CrossRef] [Green Version]
- Rugani, R.; Vallortigara, G.; Regolin, L. Numerical Abstraction in Young Domestic Chicks (Gallus gallus). PLoS ONE 2013, 8, e65262. [Google Scholar] [CrossRef]
- Rugani, R.; Vallortigara, G.; Regolin, L. From small to large: Numerical discrimination by young domestic chicks (Gallus gallus). J. Comp. Psychol. 2014, 128, 163–171. [Google Scholar] [CrossRef] [Green Version]
- Agrillo, C.; Petrazzini, M.E.M.; Tagliapietra, C.; Bisazza, A. Inter-Specific Differences in Numerical Abilities Among Teleost Fish. Front. Psychol. 2012, 3, 483. [Google Scholar] [CrossRef] [Green Version]
- Stancher, G.; Sovrano, V.A.; Potrich, D.; Vallortigara, G. Discrimination of small quantities by fish (redtail splitfin, Xenotoca eiseni). Anim. Cogn. 2013, 16, 307–312. [Google Scholar] [CrossRef]
- Potrich, D.; Sovrano, V.A.; Stancher, G.; Vallortigara, G. Quantity discrimination by zebrafish (Danio rerio). J. Comp. Psychol. 2015, 129, 388–393. [Google Scholar] [CrossRef] [Green Version]
- Hersh, R.; Dehaene, S. The Number Sense: How the Mind Creates Mathematics. Am. Math. Mon. 1998, 105, 975. [Google Scholar] [CrossRef]
- Feigenson, L.; Dehaene, S.; Spelke, E. Core systems of number. Trends Cogn. Sci. 2004, 8, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Gallistel, C.; Gelman, R. Non-verbal numerical cognition: From reals to integers. Trends Cogn. Sci. 2000, 4, 59–65. [Google Scholar] [CrossRef]
- Nieder, A. A Brain for Numbers: The Biology of the Number Instinct; MIT Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Moyer, R.S.; Landauer, T.K. Time required for Judgements of Numerical Inequality. Nature 1967, 215, 1519–1520. [Google Scholar] [CrossRef] [PubMed]
- Restle, F. Moon Illusion Explained on the Basis of Relative Size. Science 1970, 167, 1092–1096. [Google Scholar] [CrossRef] [PubMed]
- Longo, M.R.; Lourenco, S.F. Spatial attention and the mental number line: Evidence for characteristic biases and compression. Neuropsychologia 2007, 45, 1400–1407. [Google Scholar] [CrossRef]
- Starkey, P.; Cooper, R.G. Perception of Numbers by Human Infants. Science 1980, 210, 1033–1035. [Google Scholar] [CrossRef]
- Starkey, P.; Spelke, E.S.; Gelman, R. Numerical abstraction by human infants. Cognition 1990, 36, 97–127. [Google Scholar] [CrossRef]
- Rugani, R.; Regolin, L.; Vallortigara, G. Discrimination of small numerosities in young chicks. J. Exp. Psychol. Anim. Behav. Process. 2008, 34, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Rugani, R.; Regolin, L.; Vallortigara, G. Summation of Large Numerousness by Newborn Chicks. Front. Psychol. 2011, 2, 179. [Google Scholar] [CrossRef] [Green Version]
- Rugani, R.; Cavazzana, A.; Vallortigara, G.; Regolin, L. One, two, three, four, or is there something more? Numerical discrimination in day-old domestic chicks. Anim. Cogn. 2013, 16, 557–564. [Google Scholar] [CrossRef]
- Piffer, L.; Petrazzini, M.E.M.; Agrillo, C. Large Number Discrimination in Newborn Fish. PLoS ONE 2013, 8, e62466. [Google Scholar] [CrossRef] [Green Version]
- Gatto, E.; Lucon-Xiccato, T.; Savaşçı, B.B.; Dadda, M.; Bisazza, A. Experimental setting affects the performance of guppies in a numerical discrimination task. Anim. Cogn. 2016, 20, 187–198. [Google Scholar] [CrossRef]
- Sheardown, E.; Torres-Perez, J.V.; Anagianni, S.; Fraser, S.E.; Vallortigara, G.; Butterworth, B.; Miletto-Petrazzini, M.E.; Brennan, C.H. Characterising Ontogeny of Numerosity Discrimination in Zebrafish Reveals Use of Multiple, Numerical and Non- Numerical Mechanisms. bioRxiv 2021. [Google Scholar] [CrossRef]
- Stephens, D.W.; Krebs, J.R. Foraging Theory; Princeton University Press: Princeton, NJ, USA, 1986. [Google Scholar]
- Ward, C.; Smuts, B.B. Quantity-based judgments in the domestic dog (Canis lupus familiaris). Anim. Cogn. 2006, 10, 71–80. [Google Scholar] [CrossRef]
- Mc Comb, K.; Packer, C.; Pusey, A. Roaring and numerical assessment in contests between groups of female lions, Panthera leo. Anim. Behav. 1994, 47, 379–387. [Google Scholar] [CrossRef] [Green Version]
- Wilson, D.J.; Bromley, R.G. Functional and numerical responses of predators to cyclic lemming abundance: Effects on loss of goose nests. Can. J. Zool. 2001, 79, 525–532. [Google Scholar] [CrossRef]
- Hager, M.C.; Helfman, G.S. Safety in numbers: Shoal size choice by minnows under predatory threat. Behav. Ecol. Sociobiol. 1991, 29, 271–276. [Google Scholar] [CrossRef]
- Cresswell, W. Predation in bird populations. J. Ornithol. 2010, 152, 251–263. [Google Scholar] [CrossRef]
- Wedell, N.; Gage, M.J.; Parker, G.A. Sperm competition, male prudence and sperm-limited females. Trends Ecol. Evol. 2002, 17, 313–320. [Google Scholar] [CrossRef]
- Nieder, A. Neuroethology of number sense across the animal kingdom. J. Exp. Biol. 2021, 224. [Google Scholar] [CrossRef]
- Nieder, A. The Evolutionary History of Brains for Numbers. Trends Cogn. Sci. 2021, 25, 608–621. [Google Scholar] [CrossRef]
- Piazza, M.; Eger, E. Neural foundations and functional specificity of number representations. Neuropsychologia 2016, 83, 257–273. [Google Scholar] [CrossRef]
- Viswanathan, P.; Nieder, A. Neuronal correlates of a visual “sense of number” in primate parietal and prefrontal cortices. Proc. Natl. Acad. Sci. USA 2013, 110, 11187–11192. [Google Scholar] [CrossRef] [Green Version]
- Nieder, A. The neuronal code for number. Nat. Rev. Neurosci. 2016, 17, 366–382. [Google Scholar] [CrossRef]
- Viswanathan, P.; Nieder, A. Spatial Neuronal Integration Supports a Global Representation of Visual Numerosity in Primate Association Cortices. J. Cogn. Neurosci. 2020, 32, 1184–1197. [Google Scholar] [CrossRef] [PubMed]
- Nieder, A.; Freedman, D.J.; Miller, E.K. Representation of the Quantity of Visual Items in the Primate Prefrontal Cortex. Science 2002, 297, 1708–1711. [Google Scholar] [CrossRef] [PubMed]
- Nieder, A.; Merten, K. A Labeled-Line Code for Small and Large Numerosities in the Monkey Prefrontal Cortex. J. Neurosci. 2007, 27, 5986–5993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ditz, H.M.; Nieder, A. Neurons selective to the number of visual items in the corvid songbird endbrain. Proc. Natl. Acad. Sci. USA 2015, 112, 7827–7832. [Google Scholar] [CrossRef] [Green Version]
- Ditz, H.M.; Nieder, A. Numerosity representations in crows obey the Weber–Fechner law. Proc. R. Soc. B Boil. Sci. 2016, 283, 20160083. [Google Scholar] [CrossRef] [PubMed]
- Vallortigara, G. Foundations of Number and Space Representations in Non-Human Species. In Evolutionary Origins and Early Development of Number Processing; Geary, D.C., Bearch, D.B., Mann, K., Eds.; Elsevier: New York, NY, USA, 2014; pp. 35–66. [Google Scholar]
- Vallortigara, G. An animal’s sense of number. In The Nature and Development of Mathematics. Cross Disciplinary Perspective on Cognition, Learning and Culture; Adams, J.W., Barmby, P., Mesoudi, A., Eds.; Routledge: New York, NY, USA, 2017; pp. 43–65. [Google Scholar]
- Svensson, P.A.; Barber, I.; Forsgren, E. Shoaling behaviour of the two-spotted goby. J. Fish Biol. 2000, 56, 1477–1487. [Google Scholar] [CrossRef]
- Brown, C.; Laland, K.; Krause, J. Fish Cognition and Behavior; Wiley Online Library: New York, NY, USA, 2006. [Google Scholar] [CrossRef]
- Botham, M.S.; Kerfoot, C.J.; Louca, V.; Krause, J. Predator choice in the field; grouping guppies, Poecilia reticulata, receive more attacks. Behav. Ecol. Sociobiol. 2005, 59, 181–184. [Google Scholar] [CrossRef]
- Lindström, K.; Ranta, E. Social preferences by male guppies, Poecilia reticulata, based on shoal size and sex. Anim. Behav. 1993, 46, 1029–1031. [Google Scholar] [CrossRef] [Green Version]
- Smith, C.C.; Sargent, R.C. Female fitness declines with increasing female density but not male harassment in the western mosquitofish, Gambusia affinis. Anim. Behav. 2006, 71, 401–407. [Google Scholar] [CrossRef]
- Forsatkar, M.N.; Nematollahi, M.A.; Bisazza, A. Quantity discrimination in parental fish: Female convict cichlid discriminate fry shoals of different sizes. Anim. Cogn. 2016, 19, 959–964. [Google Scholar] [CrossRef]
- Agrillo, C.; Dadda, M.; Serena, G.; Bisazza, A. Do fish count? Spontaneous discrimination of quantity in female mosquitofish. Anim. Cogn. 2008, 11, 495–503. [Google Scholar] [CrossRef]
- Agrillo, C.; Dadda, M.; Serena, G.; Bisazza, A. Use of Number by Fish. PLoS ONE 2009, 4, e4786. [Google Scholar] [CrossRef]
- Gómez-Laplaza, L.M.; Gerlai, R. Quantification abilities in angelfish (Pterophyllum scalare): The influence of continuous variables. Anim. Cogn. 2012, 16, 373–383. [Google Scholar] [CrossRef]
- Jung, R.E.; Richard, J.H. The Parieto-Frontal Integration Theory (P-FIT) of intelligence: Converging neuroimaging evi-dence. Behav. Brain Sci. 2007, 30, 135–187. [Google Scholar] [CrossRef] [PubMed]
- Beran, M.J.; Smith, J.D.; Redford, J.S.; Washburn, D.A. Rhesus macaques (macaca mulatta) monitor uncertainty during numerosity judgments. J. Exp. Psychol. Anim. Behav. Process. 2006, 32, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Jordan, K.E.; MacLean, E.; Brannon, E.M. Monkeys match and tally quantities across senses. Cognition 2008, 108, 617–625. [Google Scholar] [CrossRef] [Green Version]
- Beran, M.J.; Parrish, A.E. Visual nesting of stimuli affects rhesus monkeys’ (Macaca mulatta) quantity judgments in a bisection task. Atten. Percept. Psychophys. 2013, 75, 1243–1251. [Google Scholar] [CrossRef] [Green Version]
- Al Aïn, S.; Giret, N.; Grand, M.; Kreutzer, M.; Bovet, D. The discrimination of discrete and continuous amounts in African grey parrots (Psittacus erithacus). Anim. Cogn. 2008, 12, 145–154. [Google Scholar] [CrossRef]
- Rugani, R.; Fontanari, L.; Simoni, E.; Regolin, L.; Vallortigara, G. Arithmetic in newborn chicks. Proc. R. Soc. B Boil. Sci. 2009, 276, 2451–2460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rugani, R.; Regolin, L.; Vallortigara, G. Imprinted numbers: Newborn chicks’ sensitivity to number vs. continuous extent of objects they have been reared with. Dev. Sci. 2010, 13, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Bertamini, M.; Guest, M.; Vallortigara, G.; Rugani, R.; Regolin, L. The effect of clustering on perceived quantity in humans (Homo sapiens) and in chicks (Gallus gallus). J. Comp. Psychol. 2018, 132, 280–293. [Google Scholar] [CrossRef]
- Hamilton, W. Geometry for the selfish herd. J. Theor. Biol. 1971, 31, 295–311. [Google Scholar] [CrossRef]
- Wong, B.; Rosenthal, G.; Buckingham, J. Shoaling decisions in female swordtails: How do fish gauge group size? Behaviour 2007, 144, 1333–1346. [Google Scholar] [CrossRef] [Green Version]
- Mehlis, M.; Thünken, T.; Bakker, T.C.M.; Frommen, J.G. Quantification acuity in spontaneous shoaling decisions of three-spined sticklebacks. Anim. Cogn. 2015, 18, 1125–1131. [Google Scholar] [CrossRef]
- Agrillo, C.; Dadda, M.; Serena, G. Choice of Female Groups by Male Mosquitofish (Gambusia holbrooki). Ethology 2008, 114, 479–488. [Google Scholar] [CrossRef]
- Gómez-Laplaza, L.M.; Gerlai, R. Can angelfish (Pterophyllum scalare) count? Discrimination between different shoal sizes follows Weber’s law. Anim. Cogn. 2010, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, V.L.; Lawrence, J.; Butlin, R.K.; Krause, J. Shoal choice in zebrafish, Danio rerio: The influence of shoal size and activity. Anim. Behav. 2001, 62, 1085–1088. [Google Scholar] [CrossRef] [Green Version]
- Xiong, W.; Yi, L.-C.; Tang, Z.; Zhao, X.; Fu, S.-J. Quantity discrimination in fish species: Fish use non-numerical continuous quantity traits to select shoals. Anim. Cogn. 2018, 21, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Beran, M.J.; McIntyre, J.M.; Garland, A.; Evans, T.A. What counts for ‘counting’? Chimpanzees, Pan troglodytes, respond appropriately to relevant and irrelevant information in a quantity judgment task. Anim. Behav. 2013, 85, 987–993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dadda, M.; Piffer, L.; Agrillo, C.; Bisazza, A. Spontaneous number representation in mosquitofish. Cognition 2009, 112, 343–348. [Google Scholar] [CrossRef]
- Lucon-Xiccato, T.; Petrazzini, M.E.M.; Agrillo, C.; Bisazza, A. Guppies discriminate between two quantities of food items but prioritize item size over total amount. Anim. Behav. 2015, 107, 183–191. [Google Scholar] [CrossRef]
- Gómez-Laplaza, L.M.; Díaz-Sotelo, E.; Gerlai, R. Quantity discrimination in angelfish, Pterophyllum scalare: A novel approach with food as the discriminant. Anim. Behav. 2018, 142, 19–30. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Laplaza, L.M.; Romero, L.; Gerlai, R. The role of item size on choosing contrasted food quantities in angelfish (Pterophyllum scalare). Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Laplaza, L.M.; Gerlai, R. Food density and preferred quantity: Discrimination of small and large numbers in angelfish (Pterophyllum scalare). Anim. Cogn. 2020, 23, 509–522. [Google Scholar] [CrossRef]
- Gómez-Laplaza, L.M.; Gerlai, R. Food Quantity Discrimination in Angelfish (Pterophyllum scalare): The Role of Number, Density, Size and Area Occupied by the Food Items. Front. Behav. Neurosci. 2020, 14, 106. [Google Scholar] [CrossRef]
- Agrillo, C.; Piffer, L.; Bisazza, A. Large Number Discrimination by Mosquitofish. PLoS ONE 2010, 5, e15232. [Google Scholar] [CrossRef]
- Davis, H.; Pérusse, R. Numerical competence in animals: Definitional issues, current evidence, and a new research agenda. Behav. Brain Sci. 1988, 11, 561–579. [Google Scholar] [CrossRef]
- Agrillo, C.; Piffer, L.; Bisazza, A. Number versus continuous quantity in numerosity judgments by fish. Cognition 2011, 119, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Leibovich-Raveh, T.; Raveh, A.; Vilker, D.; Gabay, S. Magnitude integration in the Archerfish. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef]
- Gatto, E.; Lucon-Xiccato, T.; Bisazza, A.; Manabe, K.; Dadda, M. The devil is in the detail: Zebrafish learn to discriminate visual stimuli only if salient. Behav. Process. 2020, 179, 104215. [Google Scholar] [CrossRef] [PubMed]
- Agrillo, C.; Petrazzini, M.E.M.; Bisazza, A. Numerical acuity of fish is improved in the presence of moving targets, but only in the subitizing range. Anim. Cogn. 2013, 17, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Gatto, E.; Testolin, A.; Bisazza, A.; Zorzi, M.; Lucon-Xiccato, T. Poor numerical performance of guppies tested in a Skinner box. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bisazza, A.; Tagliapietra, C.; Bertolucci, C.; Foà, A.; Agrillo, C. Non-visual numerical discrimination in a blind cavefish (Phreatichthys andruzzii). J. Exp. Biol. 2014, 217, 1902–1909. [Google Scholar] [CrossRef] [Green Version]
- Jaakkola, K.; Fellner, W.; Erb, L.; Rodriguez, M.; Guarino, E. Understanding of the concept of numerically “less” by bottlenose dolphins (Tursiops truncatus). J. Comp. Psychol. 2005, 119, 296–303. [Google Scholar] [CrossRef]
- Rugani, R.; Vallortigara, G.; Regolin, L. The use of proportion by young domestic chicks (Gallus gallus). Anim. Cogn. 2014, 18, 605–616. [Google Scholar] [CrossRef]
- Rugani, R.; McCrink, K.; De Hevia, M.-D.; Vallortigara, G.; Regolin, L. Ratio abstraction over discrete magnitudes by newly hatched domestic chicks (Gallus gallus). Sci. Rep. 2016, 6, 30114. [Google Scholar] [CrossRef] [Green Version]
- Krusche, P.; Uller, C.; Dicke, U. Quantity discrimination in salamanders. J. Exp. Biol. 2010, 213, 1822–1828. [Google Scholar] [CrossRef] [Green Version]
- Bortot, M.; Agrillo, C.; Avarguès-Weber, A.; Bisazza, A.; Petrazzini, M.E.M.; Giurfa, M. Honeybees use absolute rather than relative numerosity in number discrimination. Biol. Lett. 2019, 15, 20190138. [Google Scholar] [CrossRef]
- Bortot, M.; Regolin, L.; Vallortigara, G. A sense of number in invertebrates. Biochem. Biophys. Res. Commun. 2020, 564, 37–42. [Google Scholar] [CrossRef]
- Bortot, M.; Stancher, G.; Vallortigara, G. Transfer from Number to Size Reveals Abstract Coding of Magnitude in Honeybees. iScience 2020, 23, 101122. [Google Scholar] [CrossRef] [PubMed]
- Tomonaga, M. Relative numerosity discrimination by chimpanzees (Pan troglodytes): Evidence for approximate numerical representations. Anim. Cogn. 2007, 11, 43–57. [Google Scholar] [CrossRef]
- Beran, M.J. Monkeys (Macaca mulatta and Cebus apella) track, enumerate, and compare multiple sets of moving items. J. Exp. Psychol. Anim. Behav. Process. 2008, 34, 63–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pepperberg, I.M. Ordinality and inferential abilities of a grey parrot (Psittacus erithacus). J. Comp. Psychol. 2006, 120, 205–216. [Google Scholar] [CrossRef] [Green Version]
- Bisazza, A.; Agrillo, C.; Lucon-Xiccato, T. Extensive training extends numerical abilities of guppies. Anim. Cogn. 2014, 17, 1413–1419. [Google Scholar] [CrossRef]
- DeLong, C.M.; Barbato, S.; O’Leary, T.; Wilcox, K. Small and large number discrimination in goldfish (Carassius auratus) with extensive training. Behav. Process. 2017, 141, 172–183. [Google Scholar] [CrossRef]
- Petrazzini, M.E.M.; Agrillo, C.; Izard, V.; Bisazza, A. Relative versus absolute numerical representation in fish: Can guppies represent “fourness”? Anim. Cogn. 2015, 18, 1007–1017. [Google Scholar] [CrossRef]
- Petrazzini, M.E.M.; Agrillo, C.; Izard, V.; Bisazza, A. Do humans (Homo sapiens) and fish (Pterophyllum scalare) make similar numerosity judgments? J. Comp. Psychol. 2016, 130, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Honig, W.K.; Stewart, K.E. Discrimination of relative numerosity by pigeons. Learn. Behav. 1989, 17, 134–146. [Google Scholar] [CrossRef] [Green Version]
- Boysen, S.T.; Berntson, G.G.; Shreyer, T.A.; Quigley, K.S. Processing of ordinality and transitivity by chimpanzees (Pan troglodytes). J. Comp. Psychol. 1993, 107, 208–215. [Google Scholar] [CrossRef]
- Judge, P.G.; Evans, T.A.; Vyas, D.K. Ordinal Representation of Numeric Quantities by Brown Capuchin Monkeys (Cebus apella). J. Exp. Psychol. Anim. Behav. Process. 2005, 31, 79–94. [Google Scholar] [CrossRef]
- Suzuki, K.; Kobayashi, T. Numerical competence in rats (Rattus norvegicus): Davis and Bradford (1986) extended. J. Comp. Psychol. 2000, 114, 73–85. [Google Scholar] [CrossRef]
- Pepperberg, I.M. Grey parrot numerical competence: A review. Anim. Cogn. 2006, 9, 377–391. [Google Scholar] [CrossRef]
- Pfuhl, G.; Biegler, R. Ordinality and novel sequence learning in jackdaws. Anim. Cogn. 2012, 15, 833–849. [Google Scholar] [CrossRef]
- Rugani, R.; Regolin, L.; Vallortigara, G. Rudimental numerical competence in 5-day-old domestic chicks (Gallus gallus): Identification of ordinal position. J. Exp. Psychol. Anim. Behav. Process. 2007, 33, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Chittka, L.; Geiger, K. Can honey bees count landmarks? Anim. Behav. 1995, 49, 159–164. [Google Scholar] [CrossRef] [Green Version]
- Dacke, M.; Srinivasan, M.V. Evidence for counting in insects. Anim. Cogn. 2008, 11, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Potrich, D.; Rugani, R.; Sovrano, V.A.; Regolin, L.; Vallortigara, G. Use of numerical and spatial information in ordinal counting by zebrafish. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Cheng, R.-K.; Jesuthasan, S.; Penney, T.B. Time for Zebrafish. Front. Integr. Neurosci. 2011, 5, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerlai, R. Evolutionary conservation, translational relevance and cognitive function: The future of zebrafish in behavioral neuroscience. Neurosci. Biobehav. Rev. 2020, 116, 426–435. [Google Scholar] [CrossRef]
- Streisinger, G.; Walker, C.; Dower, N.; Knauber, D.; Singer, F. Production of clones of homozygous diploid zebra fish (Brachydanio rerio). Nature 1981, 291, 293–296. [Google Scholar] [CrossRef]
- Kimmel, C.B. Genetics and early development of zebrafish. Trends Genet. 1989, 5, 283–288. [Google Scholar] [CrossRef]
- Mullins, M.C.; Hammerschmidt, M.; Haffter, P.; Nüsslein-Volhard, C. Large-scale mutagenesis in the zebrafish: In search of genes controlling development in a vertebrate. Curr. Biol. 1994, 4, 189–202. [Google Scholar] [CrossRef]
- Amsterdam, A.; Burgess, S.; Golling, G.; Chen, W.; Sun, Z.; Townsend, K.; Farrington, S.; Haldi, M.; Hopkins, N. A large-scale insertional mutagenesis screen in zebrafish. Genes Dev. 1999, 13, 2713–2724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasevicius, A.; Ekker, S. Effective targeted gene ‘knockdown’ in zebrafish. Nat. Genet. 2000, 26, 216–220. [Google Scholar] [CrossRef]
- Golling, G.; Amsterdam, A.; Sun, Z.; Antonelli, M.; Maldonado, E.; Chen, W.; Burgess, S.; Haldi, M.; Artzt, K.; Farrington, S.; et al. Insertional mutagenesis in zebrafish rapidly identifies genes essential for early vertebrate development. Nat. Genet. 2002, 31, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Hwang, W.Y.; Fu, Y.; Reyon, D.; Maeder, M.L.; Kaini, P.; Sander, J.D.; Joung, J.K.; Peterson, R.T.; Yeh, J.-R.J. Heritable and Precise Zebrafish Genome Editing Using a CRISPR-Cas System. PLoS ONE 2013, 8, e68708. [Google Scholar] [CrossRef]
- Gagnon, J.A.; Valen, E.; Thyme, S.; Huang, P.; Ahkmetova, L.; Pauli, A.; Montague, T.G.; Zimmerman, S.; Richter, C.; Schier, A.F. Efficient Mutagenesis by Cas9 Protein-Mediated Oligonucleotide Insertion and Large-Scale Assessment of Single-Guide RNAs. PLoS ONE 2014, 9, e98186. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, J.; Marques, J.; Grama, A.; Hildebrand, D.G.C.; Gu, W.; Li, J.M.; Robson, D.N. Pan-neuronal calcium imaging with cellular resolution in freely swimming zebrafish. Nat. Methods 2017, 14, 1107–1114. [Google Scholar] [CrossRef]
- Chen, X.; Mu, Y.; Hu, Y.; Kuan, A.T.; Nikitchenko, M.; Randlett, O.; Chen, A.; Gavornik, J.P.; Sompolinsky, H.; Engert, F.; et al. Brain-wide Organization of Neuronal Activity and Convergent Sensorimotor Transformations in Larval Zebrafish. Neuron 2018, 100, 876–890.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loring, M.D.; Thomson, E.E.; Naumann, E.A. Whole-brain interactions underlying zebrafish behavior. Curr. Opin. Neurobiol. 2020, 65, 88–99. [Google Scholar] [CrossRef]
- Simmich, J.; Staykov, E.; Scott, E. Zebrafish as an appealing model for optogenetic studies. Progr. Brain Res. 2012, 196, 145–162. [Google Scholar] [CrossRef]
- Huang, K.-H.; Rupprecht, P.; Frank, T.; Kawakami, K.; Bouwmeester, T.; Friedrich, R.W. A virtual reality system to analyze neural activity and behavior in adult zebrafish. Nat. Methods 2020, 17, 343–351. [Google Scholar] [CrossRef]
- Stuermer, C.A. Retinotopic organization of the developing retinotectal projection in the zebrafish embryo. J. Neurosci. 1988, 8, 4513–4530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Easter, S.S.; Nicola, G.N. The development of vision in the zebrafish (Danio rerio). Dev. Biol. 1996, 180, 646–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bilotta, J.; Saszik, S. The zebrafish as a model visual system. Int. J. Dev. Neurosci. 2001, 19, 621–629. [Google Scholar] [CrossRef]
- Niell, C.M.; Meyer, M.; Smith, S.J. In vivo imaging of synapse formation on a growing dendritic arbor. Nat. Neurosci. 2004, 7, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Niell, C.M.; Smith, S.J. Functional Imaging Reveals Rapid Development of Visual Response Properties in the Zebrafish Tectum. Neuron 2005, 45, 941–951. [Google Scholar] [CrossRef] [Green Version]
- Preuss, S.J.; Trivedi, C.; Berg, C.V.; Ryu, S.; Bollmann, J.H. Classification of Object Size in Retinotectal Microcircuits. Curr. Biol. 2014, 24, 2376–2385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barker, A.; Baier, H. Sensorimotor Decision Making in the Zebrafish Tectum. Curr. Biol. 2015, 25, 2804–2814. [Google Scholar] [CrossRef] [Green Version]
- Messina, A.; Potrich, D.; Schiona, I.; Sovrano, V.A.; Fraser, S.; Brennan, C.H.; Vallortigara, G. Response to change in the number of visual stimuli in zebrafish:A behavioural and molecular study. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Messina, A.; Potrich, D.; Schiona, I.; Sovrano, V.A.; Fraser, S.E.; Brennan, C.H.; Vallortigara, G. Neurons in the Dorso-Central Division of Zebrafish Pallium Respond to Change in Visual Numerosity. Cereb. Cortex 2021. [Google Scholar] [CrossRef] [PubMed]
- Levick, W.R. Receptive fields and trigger features of ganglion cells in the visual streak of the rabbit’s retina. J. Physiol. 1967, 188, 285–307. [Google Scholar] [CrossRef] [Green Version]
- Ölveczky, B.P.; Baccus, S.A.; Meister, M. Segregation of object and background motion in the retina. Nature 2003, 423, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Bianco, I.H.; Kampff, A.R.; Engert, F. Prey Capture Behavior Evoked by Simple Visual Stimuli in Larval Zebrafish. Front. Syst. Neurosci. 2011, 5, 101. [Google Scholar] [CrossRef] [Green Version]
- Trivedi, C.A.; Bollmann, J.H. Visually driven chaining of elementary swim patterns into a goal-directed motor sequence: A virtual reality study of zebrafish prey capture. Front. Neural Circuits 2013, 7, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbas, F.; Meyer, M.P. Fish vision: Size selectivity in the zebrafish retinotectal pathway. Curr. Biol. 2014, 24, R1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helmbrecht, T.O.; Maschio, M.D.; Donovan, J.C.; Koutsouli, S.; Baier, H. Topography of a Visuomotor Transformation. Neuron 2018, 100, 1429–1445.e4. [Google Scholar] [CrossRef] [Green Version]
- Kovas, Y.; Giampietro, V.; Viding, E.; Ng, V.; Brammer, M.; Barker, G.; Happé, F.G.E.; Plomin, R. Brain Correlates of Non-Symbolic Numerosity Estimation in Low and High Mathematical Ability Children. PLoS ONE 2009, 4, e4587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piazza, M.; Izard, V.; Pinel, P.; Le Bihan, D.; Dehaene, S. Tuning Curves for Approximate Numerosity in the Human Intraparietal Sulcus. Neuron 2004, 44, 547–555. [Google Scholar] [CrossRef]
- Nieder, A. Coding of abstract quantity by ‘number neurons’ of the primate brain. J. Comp. Physiol. A 2012, 199, 1–16. [Google Scholar] [CrossRef]
- Nieder, A. Evolution of cognitive and neural solutions enabling numerosity judgements: Lessons from primates and corvids. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20160514. [Google Scholar] [CrossRef] [PubMed]
- Wagener, L.; Loconsole, M.; Ditz, H.M.; Nieder, A. Neurons in the Endbrain of Numerically Naive Crows Spontaneously Encode Visual Numerosity. Curr. Biol. 2018, 28, 1090–1094.e4. [Google Scholar] [CrossRef] [Green Version]
- Lorenzi, E.; Perrino, M.; Vallortigara, G. Numerosities and Other Magnitudes in the Brains: A Comparative View. Front. Psychol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Ito, H. Fiber connections of the anterior preglomerular nucleus in cyprinids with notes on telencephalic connections of the preglomerular complex. J. Comp. Neurol. 2005, 491, 212–233. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Yamamoto, N.; Yoshimoto, M.; Yasuda, T.; Maruyama, K.; Kage, T.; Takeda, H.; Ito, H. Developmental Origin of Diencephalic Sensory Relay Nuclei in Teleosts. Brain Behav. Evol. 2007, 69, 87–95. [Google Scholar] [CrossRef]
- Harvey-Girard, E.; Giassi, A.C.; Ellis, W.; Maler, L. Organization of the gymnotiform fish pallium in relation to learning and memory: IV. Expression of conserved transcription factors and implications for the evolution of dorsal telencephalon. J. Comp. Neurol. 2012, 520, 3395–3413. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Yamamoto, N. Non-laminar cerebral cortex in teleost fishes? Biol. Lett. 2008, 5, 117–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez, F.; Quintero, B.; Amores, L.; Madrid, D.; Salas-Peña, C.; Salas, C. Spatial Cognition in Teleost Fish: Strategies and Mechanisms. Animals 2021, 11, 2271. [Google Scholar] [CrossRef]
- Agrillo, C.; Bisazza, A. Understanding the origin of number sense: A review of fish studies. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20160511. [Google Scholar] [CrossRef] [Green Version]
- Messina, A.; Boiti, A.; Vallortigara, G. Asymmetric distribution of pallial-expressed genes in zebrafish (Danio rerio). Eur. J. Neurosci. 2020, 53, 362–375. [Google Scholar] [CrossRef]
- Langova, V.; Vales, K.; Horka, P.; Horacek, J. The Role of Zebrafish and Laboratory Rodents in Schizophrenia Research. Front. Psychiatry 2020, 11. [Google Scholar] [CrossRef]
- Rea, V.; Van Raay, T.J. Using Zebrafish to Model Autism Spectrum Disorder: A Comparison of ASD Risk Genes Between Zebrafish and Their Mammalian Counterparts. Front. Mol. Neurosci. 2020, 13. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.-H.; Locke, D.; Greally, J.; Knoll, J.; Ohta, T.; Dunai, J.; Yavor, A.; Eichler, E.; Nicholls, R. Identification of Four Highly Conserved Genes between Breakpoint Hotspots BP1 and BP2 of the Prader-Willi/Angelman Syndromes Deletion Region That Have Undergone Evolutionary Transposition Mediated by Flanking Duplicons. Am. J. Hum. Genet. 2003, 73, 898–925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tassabehji, M. Williams-Beuren syndrome: A challenge for genotype-phenotype correlations. Hum. Mol. Genet. 2003, 12, R229–R237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Docherty, S.J.; Kovas, Y.; Petrill, S.A.; Plomin, R. Generalist genes analysis of DNA markers associated with mathematical ability and disability reveals shared influence across ages and abilities. BMC Genet. 2010, 11, 61. [Google Scholar] [CrossRef] [Green Version]
- Maver, A.; Čuturilo, G.; Kovanda, A.; Miletić, A.; Peterlin, B. Rare missense TUBGCP5 gene variant in a patient with primary microcephaly. Eur. J. Med. Genet. 2018, 62, 103598. [Google Scholar] [CrossRef]
- Rogers, L.J. Asymmetry of brain and behavior in animals: Its development, function, and human relevance. Genesis 2014, 52, 555–571. [Google Scholar] [CrossRef]
- Petrazzini, M.E.M.; Sovrano, V.A.; Vallortigara, G.; Messina, A. Brain and Behavioral Asymmetry: A Lesson From Fish. Front. Neuroanat. 2020, 14. [Google Scholar] [CrossRef]
- Shalev, R.; Manor, O.; Amir, N.; Wertman-Elad, R.; Gross-Tsur, V. Developmental Dyscalculia and Brain Laterality. Cortex 1995, 31, 357–365. [Google Scholar] [CrossRef]
- Shalev, R.S. Developmental Dyscalculia. J. Child Neurol. 2004, 19, 765–771. [Google Scholar] [CrossRef] [PubMed]
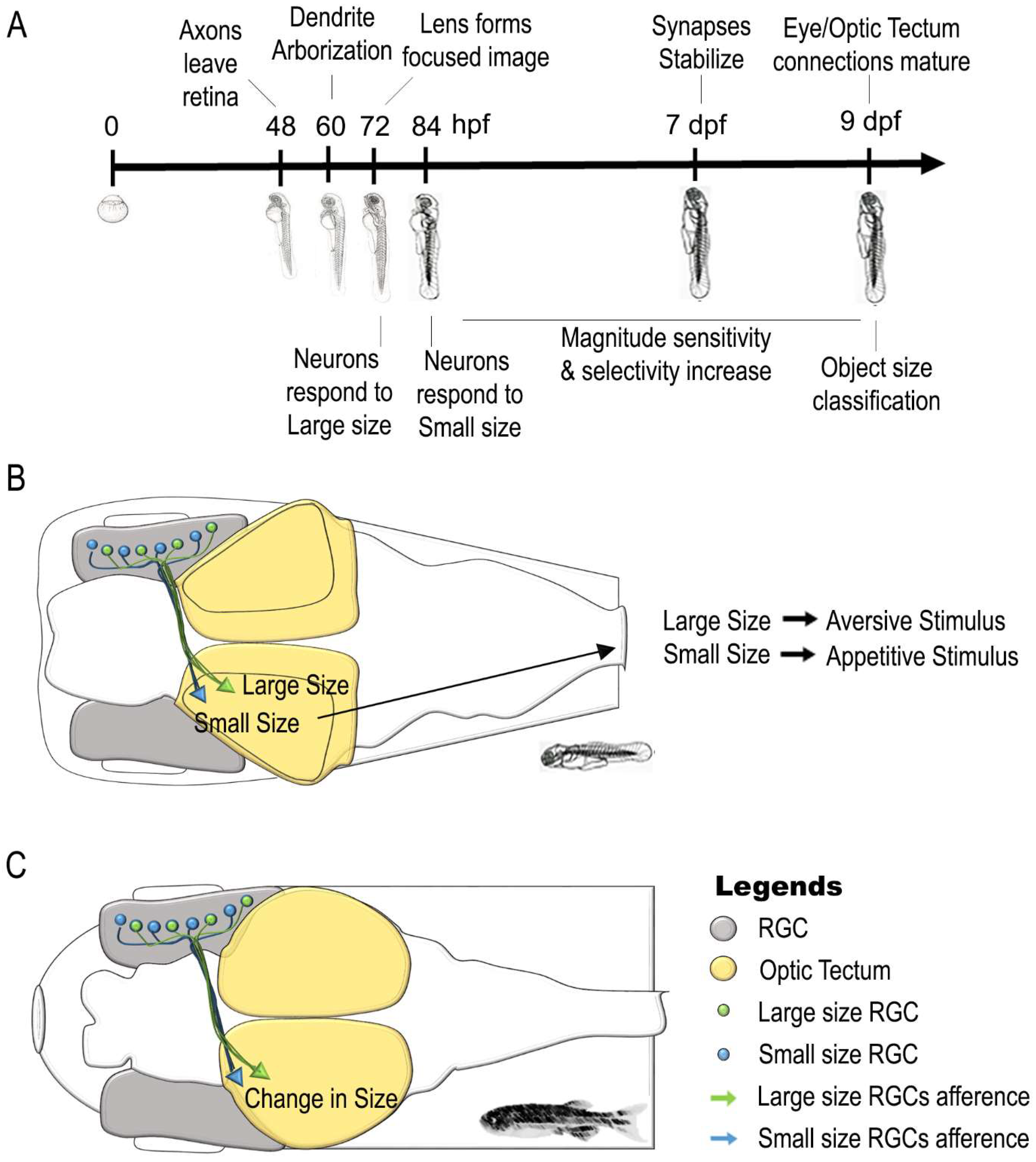
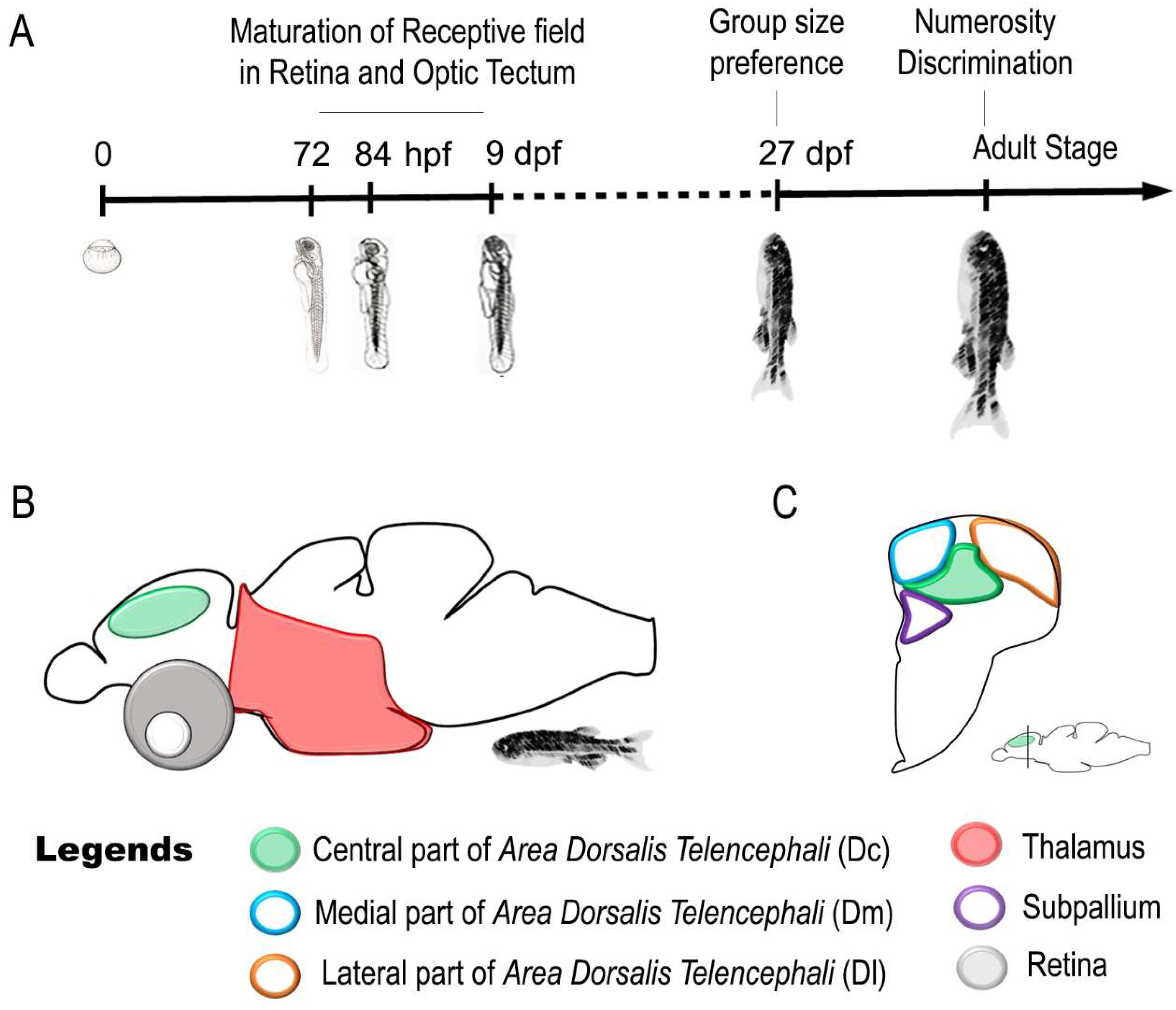
| Stage | Findings | Literature Data | |
|---|---|---|---|
| Sense of Magnitude | 72 hpf | Retinal Ganglion Cells (RGCs) respond to Large Size Object | [131,134] |
| 84 hpf | Retinal Ganglion Cells (RGCs) respond to Small Size Object | [133,134] | |
| 5–8 dpf | Optic tectum contains different population of neurons involved in large and small size object discrimination | [135] | |
| Retinal Ganglion Cells (RGCs) afferents synapt with Deeper layer of Optic Tectum for Large Size Object | |||
| Retinal Ganglion Cells (RGCs) afferents synapt with Superficial layer of Optic Tectum for Small Size Object | |||
| 5–8 dpf | Size-based categorization of visual targets and involvement of Optic tectum in approach/avoidance behaviors | [136,143] | |
| 5–7 dpf | Receptive field outputs and visuo-motor response in relation to object size changes | [144] | |
| 9 dpf | Size-based categorization of visual targets similar to adult life | [134] | |
| Adult | Retina responds to change in size of a visual Stimulus | [137] | |
| Optic Tectum responds to change in size of a visual Stimulus | |||
| Sense of Number | Adult | Thalamus responds to change in numerosity of a visual Stimulus | [137] |
| Telencephalon responds to change in numerosity of a visual Stimulus | |||
| Adult | The caudal region of the central part of area dorsalis telencephali (Dc) responds to change in numerosity of a visual Stimulus | [138] | |
| Numerosity-based categorization of a visual Stimulus and involvement of Dc in approach/avoidance behaviors |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Messina, A.; Potrich, D.; Schiona, I.; Sovrano, V.A.; Vallortigara, G. The Sense of Number in Fish, with Particular Reference to Its Neurobiological Bases. Animals 2021, 11, 3072. https://doi.org/10.3390/ani11113072
Messina A, Potrich D, Schiona I, Sovrano VA, Vallortigara G. The Sense of Number in Fish, with Particular Reference to Its Neurobiological Bases. Animals. 2021; 11(11):3072. https://doi.org/10.3390/ani11113072
Chicago/Turabian StyleMessina, Andrea, Davide Potrich, Ilaria Schiona, Valeria Anna Sovrano, and Giorgio Vallortigara. 2021. "The Sense of Number in Fish, with Particular Reference to Its Neurobiological Bases" Animals 11, no. 11: 3072. https://doi.org/10.3390/ani11113072
APA StyleMessina, A., Potrich, D., Schiona, I., Sovrano, V. A., & Vallortigara, G. (2021). The Sense of Number in Fish, with Particular Reference to Its Neurobiological Bases. Animals, 11(11), 3072. https://doi.org/10.3390/ani11113072








