Spatial Cognition in Teleost Fish: Strategies and Mechanisms
Abstract
Simple Summary
Abstract
1. Spatial Cognition in Teleost Fish
1.1. Teleost Fish Can Use Multiple, Parallel Spatial Strategies for Navigation
1.2. Map-Like Memories and Fish Navigation
2. Neural Mechanisms for Spatial Navigation
2.1. Neural Mechanisms for Egocentric Orientation in Teleost Fish
2.2. Teleost Fish Hippocampal Pallium and Map-Like Navigation
3. Hippocampal Pallium Mechanisms for Map-Like Spatial Navigation in Teleost Fish
3.1. Space-Related Cells in the Pallium of Teleost Fish
3.2. Spatial Memory Encoding and Retrieval in Teleost Fish
3.3. Hippocampal Map-Like Memory in Teleost Fish: More Than Space?
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dodson, S. The ecological role of chemical stimuli for the zooplankton: Predator-avoidance behavior in Daphnia. Limnol. Oceanogr. 1988, 33, 1431–1439. [Google Scholar] [CrossRef]
- Quinn, T.P. Fishes. In Animal Homing; Papi, F., Ed.; Chapman & Hall: London, UK, 1992; pp. 145–211. [Google Scholar]
- Hallacher, L.E. Relocation of original territories by displaced black-and-yellow rockfish, Sebastes chrysomelas, from Carmel Bay, California. Calif. Fish Game 1984, 7, 158–162. [Google Scholar]
- Kroon, F.J.; de Graaf, M.; Liley, N.R. Social organisation and competition for refuges and nest sites in Coryphopterus nicholsii (Gobiidae), a temperature protogynous reef fish. Environ. Biol. Fishes 2000, 57, 401–411. [Google Scholar] [CrossRef]
- Matthews, K.R. An experimental study of the habitat preferences and movement patterns of copper, quillback, and brown rockfishes (Sebastes spp.). Environ. Biol. Fishes 1990, 29, 161–178. [Google Scholar] [CrossRef]
- Warburton, K.; Hughes, R. Learning and foraging skills by fish. In Fish Cognition and Behavior, 2nd ed.; Brown, C., Laland, K., Krause, J., Eds.; Wiley-Blackwell: Chichester, UK, 2011; pp. 10–35. [Google Scholar]
- Carlson, H.R.; Haight, R.E. Evidence for a home site and homing of adult yellowtail rockfish, Sebastes flavidus. J. Fish. Board Can. 1972, 29, 1011–1014. [Google Scholar] [CrossRef]
- Green, J.M. High tide movements and homing behaviour of the tidepool sculping Oligocottus maculosus. J. Fish. Res. Board Can. 1971, 28, 383–389. [Google Scholar] [CrossRef]
- Griffiths, S.P. Homing behaviour of intertidal rockpool fishes in south-eastern New South Wales, Australia. Aust. J. Zool. 2003, 51, 387–398. [Google Scholar] [CrossRef]
- Markevich, A.I. Nature of territories and homing in the eastern sea-perch Sebastes taczanowski. J. Ichthyol. 1988, 28, 161–163. [Google Scholar]
- Matthews, K.R. A telemetric study of the home ranges and homing of cooper and quillback, and brown rockfishes on shallows rocky reefs. Can. J. Fish. Aquat. Sci. 1990, 68, 2243–2250. [Google Scholar]
- Lucas, M.; Baras, E.; Timothy, J.; Duncan, A.; Slavík, O. Migration of Freshwater Fishes; Blackwell Science: Oxford, UK, 2001. [Google Scholar]
- Morais, P.; Daverat, F. History of fish migration research. In An Introduction to Fish Migration; CRC Press: Boca Raton, FL, USA, 2016; pp. 3–13. [Google Scholar]
- Orlov, A.M.; Rabazanov, N.I.; Nikiforov, A.I. Transoceanic Migrations of Fishlike Animals and Fish: Norm or Exclusion? J. Ichthyol. 2020, 60, 242–262. [Google Scholar] [CrossRef]
- Hughes, R.N.; Blight, C.M. Two intertidal fish species use visual association learning to track the status of food patches in a radial maze. Anim. Behav. 2000, 59, 613–621. [Google Scholar] [CrossRef][Green Version]
- López, J.C.; Broglio, C.; Rodríguez, F.; Thinus-Blanc, C.; Salas, C. Multiple spatial learning strategies in goldfish (Carassius auratus). Anim. Cogn. 1999, 2, 109–120. [Google Scholar] [CrossRef]
- Rodríguez, F.; Durán, E.; Vargas, J.; Torres, B.; Salas, C. Performance of goldfish trained in allocentric and egocentric maze procedures suggests the presence of a cognitive mapping system in fishes. Anim. Learn. Behav. 1994, 22, 409–420. [Google Scholar] [CrossRef]
- Warburton, K. The use of local landmarks by foraging goldfish. Anim. Behav. 1990, 40, 500–505. [Google Scholar] [CrossRef]
- Dittman, A.H.; Quinn, T.P. Homing in pacific salmon: Mechanisms and ecological basis. J. Exp. Biol. 1996, 199, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Heard, W.R. Sequential imprinting in chinook salmon: Is it essential for homing fidelity? Bull. Natl. Res. Inst. Aquac. 1996, 2, 59–64. [Google Scholar]
- Simpson, S.D.; Meekan, M.G.; Montgomery, J.C.; McCauley, R.D.; Jeffs, A.G. Home ward sound. Science 2005, 308, 221. [Google Scholar] [CrossRef]
- Tolimieri, N.; Jeffs, A.; Montgomery, J.C. Ambient sound as a cue for navigation by the pelagic larvae of reef fishes. Mar Ecol. Prog. Ser. 2000, 207, 219–224. [Google Scholar] [CrossRef]
- Campenhausen, C.V.; Riess, I.; Weissert, R. Detection of stationary objects by the blind cave fish Anoptichtys jordani (Characidae). J. Comp. Physiol. 1981, 143, 369–374. [Google Scholar] [CrossRef]
- Sovrano, V.A.; Potrich, D.; Foà, A. Extra-Visual Systems in the Spatial Reorientation of Cavefish. Sci. Rep. 2018, 8, 17698. [Google Scholar] [CrossRef] [PubMed]
- Teyke, T. Collision and avoidance of obstacles in blind cave fish Anoptichthys jordani (Characidae). J. Comp. Physiol. A 1985, 157, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Cain, P. Navigation in familiar environments by the weakly electric fish, Gnathonemus petersii L. (Mormyriformes, Teleostei). Ethology 1995, 99, 332–349. [Google Scholar] [CrossRef]
- Cain, P.; Gerin, W.; Moller, P. Short-range navigation of the weakly electric fish Gnathonemus petersii L. (Mormyridae, Teleostei) in novel and familiar environments. Ethology 1994, 96, 33–45. [Google Scholar] [CrossRef]
- Fotowat, H.; Lee, C.; Jun, J.J.; Maler, L. Neural activity in a hippocampus-like region of the teleost pallium is associated with active sensing and navigation. eLife 2019, 8, e44119. [Google Scholar] [CrossRef] [PubMed]
- Jun, J.J.; Longtin, A.; Maler, L. Active sensing associated with spatial learning reveals memory-based attention in an electric fish. J. Neurophysiol. 2016, 115, 2577–2592. [Google Scholar] [CrossRef]
- Goodyear, C.P.; Bennett, D.H. Sun-compass orientation of inmature bluegill. Trans. Am. Fish. Soc. 1979, 108, 555–559. [Google Scholar] [CrossRef]
- Schwassmann, H.O.; Braemer, W. The effect of experimentally changed photoperiod on the sun orientation rhythm of fish. Physiol. Zool. 1961, 34, 273–326. [Google Scholar] [CrossRef]
- Davitz, M.A.; McKaye, K.R. Discrimination between vertically and horizontally polarized light by the cichlid fish Pseudotropheus macrophthalmus. Copeia 1978, 333–334. [Google Scholar] [CrossRef]
- Loyacano, H.A.; Chappell, J.A.; Gauthreaux, S.A. Sun-compass orientation of juvenile largemouth bass, Micropterus salmoides. Trans. Am. Fish. Soc. 1971, 106, 77–79. [Google Scholar] [CrossRef]
- Hawryshyn, C.W.; Arnold, M.G.; Bowering, E.; Cole, R.L. Spatial orientation of rainbow trout to plane-polarised light: The ontogeny of E-vector discrimination and spectral sensitivity characteristics. J. Comp. Physiol. 1990, 166, 565–574. [Google Scholar] [CrossRef]
- Kalmijn, A. Experimental evidence of geomagnetic orientation in elasmobranch fishes. In Animal Migration, Navigation and Homing; Schmidt-Koenig, K., Keeton, W.T., Eds.; Springer: Berlin, Germany, 1978. [Google Scholar]
- Jonsson, N.; Jonsson, B.; Skurdal, J.; Hansen, L.P. Differential response to water current in offspring of inlet- and outlet-spawning brown trout Salmo trutta. J. Fish Biol. 1994, 45, 356–359. [Google Scholar]
- Smith, R.J.F.; Smith, M.J. Rapid acquisition of directional preferences by migratory juveniles two amphidromous Hawaiian gobies, Awaous guamensis and Sicyopterus stimpsoni. Environ. Biol. Fishes 1998, 53, 275–282. [Google Scholar] [CrossRef]
- Tolman, E.C. Cognitive maps in rats and men. Psychol. Rev. 1948, 55, 189–208. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, J.; Nadel, L. The Hippocampus as a Cognitive Map; Clarendon Press: Oxford, UK, 1978. [Google Scholar]
- Burgess, N.; Donnett, J.G.; Jeffery, K.J.; O’Keefe, J. Robotic and neuronal simulation of the hippocampus and rat navigation. Philos. Trans. R. Soc. Lond. B 1997, 352, 1535–1543. [Google Scholar] [CrossRef][Green Version]
- Cartwright, B.A.; Collett, T.S. Landmark maps for honeybees. Biol. Cybernet. 1987, 57, 85–93. [Google Scholar] [CrossRef]
- Franz, M.O.; Mallot, H.A. Biomimetic robot navigation. Robot. Auton. Syst. 2000, 30, 133–153. [Google Scholar] [CrossRef]
- Gallistel, C.R. The Organization of Learning; MIT Press/Bradford Books: Cambridge, MA, USA, 1990. [Google Scholar]
- Levitt, T.S.; Lawton, D.T. Qualitative navigation for mobile robots. Artif. Intell. 1990, 44, 305–360. [Google Scholar] [CrossRef]
- Leonard, B.; McNaughton, B.L. Rat: Conceptual, behavioral, and neurophysiological perspectives. In Neurobiology of Comparative Cognition; Kesner, R.P., Olton, D.S., Eds.; Lawrence Erlbaum Associates: New York, NY, USA, 1990. [Google Scholar]
- McNaughton, B.L.; Barnes, C.A.; Gerrard, J.L.; Gothard, K.; Jung, M.W.; Knierim, J.J.; Weaver, K.L. Deciphering the hippocampal polyglot: The hippocampus as a path integration system. J. Exp. Biol. 1996, 199, 173–185. [Google Scholar] [CrossRef]
- Morris, R.G.M. Spatial localization does not require the presence of local cues. Learn. Mot. 1981, 12, 239–260. [Google Scholar] [CrossRef]
- Poucet, B. Spatial cognitive maps in animals: New hypotheses on their structure and neural mechanisms. Psychol. Rev. 1993, 100, 163–182. [Google Scholar] [CrossRef]
- Samsonovich, A.; McNaughton, B.L. Path integration and cognitive mapping in a continuous attractor neural network model. J. Neurosci. 1997, 17, 5900–5920. [Google Scholar] [CrossRef]
- Tolman, E.C.; Ritchie, B.F.; Kalish, D. Studies in spatial learning. I. Orientation and the short-cut. J. Exp. Psychol. 1946, 36, 13–24. [Google Scholar] [CrossRef]
- Trullier, O.; Wiener, S.I.; Berthoz, A.; Meyer, J.A. Biologically based artificial navigation systems: Review and prospects. Prog. Neurobiol. 1997, 51, 483–544. [Google Scholar] [CrossRef]
- Broglio, C.; Rodríguez, F.; Salas, C. Spatial cognition and its neural basis in teleost fishes. Fish Fish. 2003, 4, 247–255. [Google Scholar] [CrossRef]
- Salas, C.; Broglio, C.; Durán, E.; Ocaña, F.M.; Martín-Monzón, I.; Gómez, A.; Rodríguez, F. Spatial Learning and Its Neural Basis in Fish. In Learning and Memory: A Comprehensive Reference, 2nd ed.; Learning Theory and Behavior; Menzel, R., Ed.; Academic Press: Oxford, UK, 2017; Volume 1, pp. 347–373. [Google Scholar]
- López, J.C.; Bingman, V.P.; Rodríguez, F.; Gómez, Y.; Salas, C. Dissociation of place and cue learning by telencephalic ablation in goldfish. Behav. Neurosci. 2000, 114, 687–699. [Google Scholar] [CrossRef]
- Able, K.P. Common themes and variations in animal orientation systems. Am. Zool. 1991, 31, 157–167. [Google Scholar] [CrossRef]
- Brodbeck, D.R. Memory for spatial and local cues: A comparison of a storing and a nonstoring species. Anim. Learn. Behav. 1994, 22, 119–133. [Google Scholar] [CrossRef]
- Burgess, N. Spatial cognition and the brain. Ann. N. Y. Acad. Sci. 2008, 1124, 77–97. [Google Scholar] [CrossRef]
- Cheng, K. Some psychophysics of the pigeon’s use of landmarks. J. Comp. Physiol. A 1988, 162, 815–826. [Google Scholar] [CrossRef]
- Clayton, N.S.; Krebs, J.R. Memory for spatial and object-specific cues in food-storing and non-storing birds. J. Comp. Physiol. A 1994, 174, 371–379. [Google Scholar] [CrossRef]
- Gagliardo, A.; Mazzotto, M.; Bingman, V.P. Hippocampal lesion effects on learning strategies in homing pigeons. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1996, 263, 529–534. [Google Scholar]
- McDonald, R.J.; White, N.M. Parallel information processing in the water maze: Evidence for independent memory systems involving dorsal striatum and hippocampus. Behav. Neural Biol. 1994, 61, 260–270. [Google Scholar] [CrossRef]
- Packard, M.G.; McGaugh, J.L. Double dissociation of fornix and caudate nucleus lesions on acquisition of two water maze tasks: Further evidence for multiple memory systems. Behav. Neurosci. 1992, 106, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Reese, E.S. Orientation behavior of butterflyfishes (family Chaetodontidae) on coral reefs: Spatial learning of route specific landmarks and cognitive maps. Environ. Biol. Fishes 1989, 25, 79–86. [Google Scholar] [CrossRef]
- Roitblat, H.L.; Tham, W.; Golub, L. Performance of Betta splendens in a radial arm maze. Anim. Learn. Behav. 1982, 10, 108–114. [Google Scholar] [CrossRef]
- Schenk, F.; Morris, R.G.M. Dissociation between components of spatial memory in rats after recovery from the effects of the retrohippocampal lesions. Exp. Brain Res. 1985, 58, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Strasser, R.; Bingman, V.P. Goal recognition and hippocampal formation in the homing pigeon (Columba livia). Behav. Neurosci. 1997, 111, 1245–1256. [Google Scholar] [CrossRef]
- Whishaw, I.Q. Dissociating performance and learning deficits on spatial navigation tasks in rats subjected to cholinergic muscarinic blockade. Brain Res. Bull. 1989, 23, 347–358. [Google Scholar] [CrossRef]
- Whishaw, I.Q.; Mittleman, G. Visits to starts, routes and places by rats (Rattus norvegicus) in swimming pool navigation tasks. J. Comp. Psychol. 1986, 100, 422–431. [Google Scholar] [CrossRef]
- Durán, E.; Ocaña, F.M.; Gómez, A.; Jiménez-Moya, F.; Broglio, C.; Rodríguez, F.; Salas, C. Telencephalon ablation impairs goldfish allocentric spatial learning in a “hole-board” task. Acta Neurobiol. Exp. 2008, 68, 519–525. [Google Scholar]
- Salas, C.; Broglio, C.; Rodríguez, F.; López, J.C.; Portavella, M.; Torres, B. Telencephalic ablation in goldfish impairs performance in a spatial constancy problem but not in a cued one. Behav. Brain Res. 1996, 79, 193–200. [Google Scholar] [CrossRef]
- Salas, C.; Rodríguez, F.; Vargas, J.P.; Durán, E.; Torres, B. Spatial learning and memory deficits after telencephalic ablation in goldfish trained in place and turn maze procedures. Behav. Neurosci. 1996, 110, 965–980. [Google Scholar] [CrossRef] [PubMed]
- Hartley, T.; Lever, C.; Burgess, N.; O’Keefe, J. Space in the brain: How the hippocampal formation supports spatial cognition. Philos. Trans. R. Soc. B 2014, 369, 20120510. [Google Scholar] [CrossRef] [PubMed]
- Knierim, J.J.; Hamilton, D.A. Framing spatial cognition: Neural representations of proximal and distal frames of reference and their roles in navigation. Physiol. Rev. 2011, 91, 1245–1279. [Google Scholar] [CrossRef] [PubMed]
- Moser, E.I.; Kropff, E.; Moser, M.-B. Place cells, grid cells, and the brain’s spatial representation system. Ann. Rev. Neurosci. 2008, 31, 69–89. [Google Scholar] [CrossRef]
- Bird, C.M.; Burgess, N. The hippocampus and memory: Insights from spatial processing. Nat. Rev. Neurosci. 2008, 9, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Burgess, N.; Maguire, E.A.; O’Keefe, J. The human hippocampus and spatial and episodic memory. Neuron 2002, 35, 625–641. [Google Scholar] [CrossRef]
- Guzowski, J.F.; Knierim, J.J.; Moser, E.I. Ensemble dynamics of hippocampal regions CA3 and CA1. Neuron 2004, 44, 581–584. [Google Scholar] [CrossRef]
- Leutgeb, S.; Leutgeb, J.K. Pattern separation, pattern completion, and new neuronal codes within a continuous CA3 map. Learn. Mem. 2007, 14, 745–757. [Google Scholar] [CrossRef]
- Kesner, R.P.; Rolls, E.T. A computational theory of hippocampal function, and tests of the theory: New developments. Neurosci. Biobehav. Rev. 2015, 48, 92–147. [Google Scholar] [CrossRef]
- Rolls, E. The mechanisms for pattern completion and pattern separation in the hippocampus. Front. Syst. Neurosci. 2013, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- Rolls, E.T. Pattern separation, completion, and categorisation in the hippocampus and neocortex. Neurobiol. Learn. Mem. 2016, 129, 4–28. [Google Scholar] [CrossRef] [PubMed]
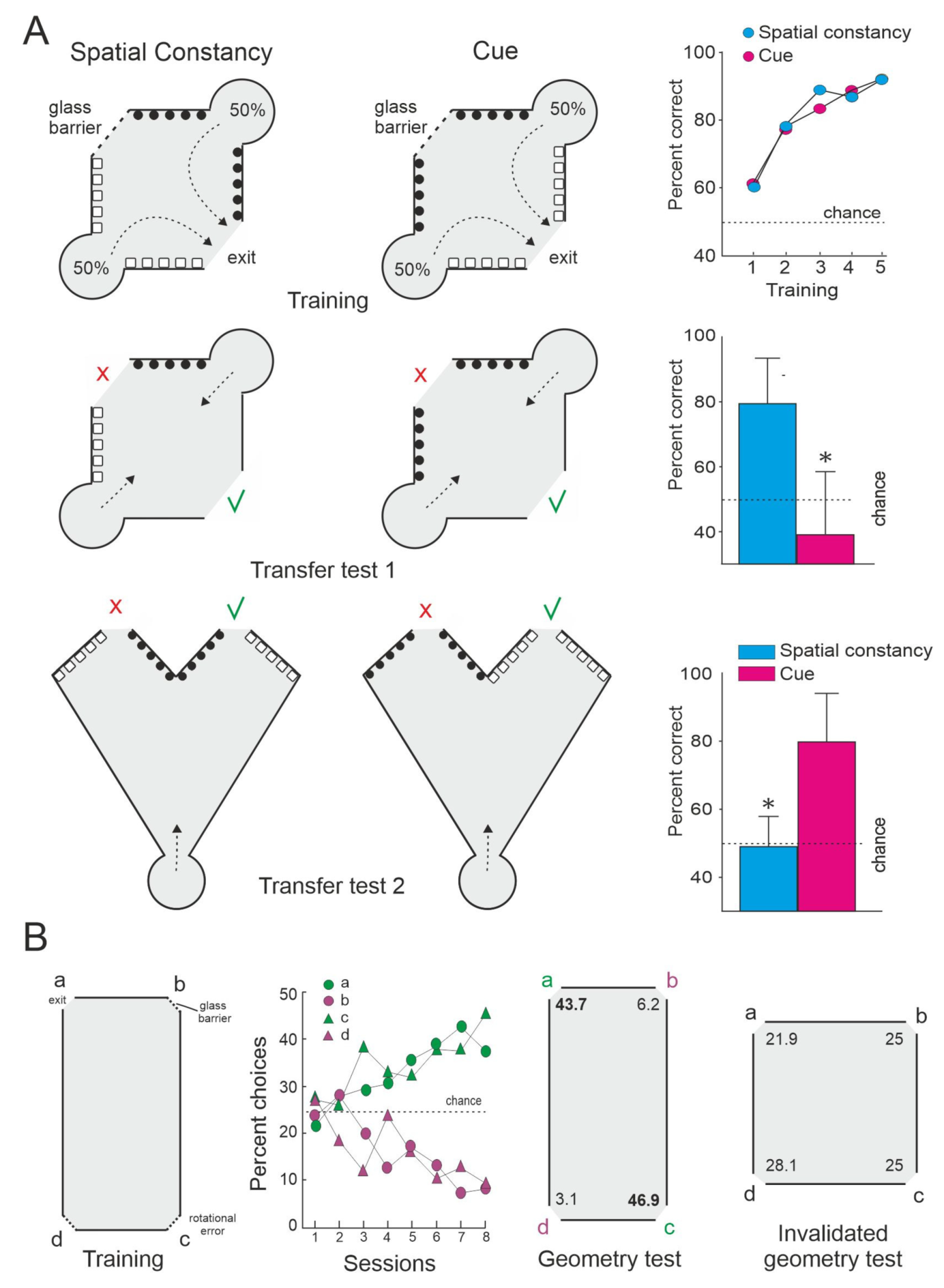
- Baratti, G.; Potrich, D.; Sovrano, V.A. The environmental geometry in spatial learning by zebrafish (Danio rerio). Zebrafish 2020, 17, 131–138. [Google Scholar] [CrossRef]
- Broglio, C.; Gómez, Y.; López, J.C.; Rodríguez, F.; Salas, C.; Vargas, J.P. Encoding of geometric and featural properties of a spatial environment in teleostean fish (Carassius auratus). Int. J. Psychol. 2000, 35, 195. [Google Scholar]
- Lee, S.A.; Vallortigara, G.; Flore, M.; Spelke, E.S.; Sovrano, V.A. Navigation by environmental geometry: The use of zebrafish as a model. J. Exp. Biol. 2013, 216, 3693–3699. [Google Scholar] [CrossRef]
- Sovrano, V.A.; Bisazza, A.; Vallortigara, G. Modularity as a fish (Xenotoca eiseni) views it: Conjoining geometric and nongeometric information for spatial reorientation. J. Exp. Psychol. Anim. Behav. Process. 2003, 29, 199–210. [Google Scholar] [CrossRef]
- Sovrano, V.A.; Bisazza, A.; Vallortigara, G. How fish do geometry in large and in small spaces. Anim. Cogn. 2007, 10, 47–54. [Google Scholar] [CrossRef]
- Vargas, J.P.; López, J.C.; Salas, C.; Thinus-Blanc, C. Encoding of geometric and featural spatial information by Goldfish (Carassius auratus). J. Comp. Psychol. 2004, 118, 206–216. [Google Scholar] [CrossRef]
- Yashina, K.; Tejero-Cantero, A.; Herz, A.; Baier, H. Zebrafish Exploit Visual Cues and Geometric Relationships to Form a Spatial Memory. iScience 2019, 19, 119–134. [Google Scholar] [CrossRef]
- Blum, K.I.; Abbott, L.F. A model of spatial map formation in the hippocampus of the rat. Neural Comp. 1996, 8, 85–93. [Google Scholar] [CrossRef]
- Burgess, N.; Recce, M.; O’Keefe, J. A model of hippocampal function. Neural Netw. 1994, 7, 1065–1081. [Google Scholar] [CrossRef]
- Eichenbaum, H.B. Thinking about cell assemblies. Science 1993, 261, 993–994. [Google Scholar] [CrossRef]
- Muller, R.U.; Stead, M.; Pach, J. The hippocampus as a cognitive graph. J. Gen. Physiol. 1996, 107, 663–694. [Google Scholar] [CrossRef]
- Schmajuk, N.A.; Thieme, A.D. Purposive behavior and cognitive mapping: A neural network model. Biol. Cyb. 1992, 67, 165–174. [Google Scholar] [CrossRef]
- Wan, H.S.; Touretzky, D.S.; Redish, A.D. Towards a computational theory of rat navigation. In Proceedings of the 1993 Connectionist Models Summer School; Mozer, M., Smolensky, P., Touretzky, D., Elman, J., Weigend, A., Eds.; Lawrence Erlbaum Associates: East Sussex, UK, 1994; pp. 11–19. [Google Scholar]
- Worden, R. Navigation by fragment fitting: A theory of hippocampal function. Hippocampus 1992, 2, 165–188. [Google Scholar] [CrossRef] [PubMed]
- Wiener, J.; Shettleworth, S.J.; Bingman, V.P.; Cheng, K.; Healy, S.D.; Jacobs, L.F.; Jeffery, K.J.; Mallot, H.A.; Menzel, R.; Newcombe, N.S. Animal navigation synthesis. In Animal Thinking; Menzel, R., Fisher, J., Eds.; MIT Press: Cambridge, MA, USA, 2011; pp. 1–33. [Google Scholar]
- Bingman, V.P.; Riters, L.V.; Strasser, R.; Gagliardo, A. Neuroethology of avian navigation. In Animal Cognition in Nature; Balda, R., Pepperberg, I., Kamil, A., Eds.; Academic Press: New York, NY, USA, 1998; pp. 201–226. [Google Scholar]
- Herold, C.; Coppola, V.J.; Bingman, V.P. The maturation of research into the avian hippocampal formation: Recent discoveries from one of the nature’s foremost navigators. Hippocampus 2015, 25, 1193–1211. [Google Scholar] [CrossRef]
- Sherry, D.F.; Duff, S.J. Behavioral and neural bases of orientation in food storing birds. J. Exp. Biol. 1996, 199, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Sherry, D.F.; Vaccarino, A.L. Hippocampus and memory for food caches in black-capped chickadees. Behav. Neurosci. 1989, 103, 308–318. [Google Scholar] [CrossRef]
- López, J.C.; Rodríguez, F.; Gómez, Y.; Vargas, J.P.; Broglio, C.; Salas, C. Place and cue learning in turtles. Anim. Learn. Behav. 2000, 28, 360–372. [Google Scholar] [CrossRef]
- López, J.C.; Gómez, Y.; Rodríguez, F.; Broglio, C.; Vargas, J.P.; Salas, C. Spatial learning in turtles. Anim. Cogn. 2001, 4, 49–59. [Google Scholar]
- Fuss, T.; Bleckmann, H.; Schluessel, V. The shark Chiloscyllium griseum can orient using turn responses before and after partial telencephalon ablation. J. Comp. Physiol. A 2014, 200, 19–35. [Google Scholar] [CrossRef]
- Fuss, T.; Bleckmann, H.; Schluessel, V. Place learning prior to and after telencephalon ablation in bamboo and coral cat sharks (Chiloscyllium griseum and Atelomycterus marmoratus). J. Comp. Physiol. A 2014, 200, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Schluessel, V.; Bleckmann, H. Spatial memory and orientation strategies in the elasmobranch Potamotrygon motoro. J. Comp. Physiol. A 2005, 191, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Antinucci, P.; Folgueira, M.; Bianco, I.H. Pretectal neurons control hunting behaviour. eLife 2019, 8, e48114. [Google Scholar] [CrossRef]
- Bahl, A.; Engert, F. Neural circuits for evidence accumulation and decision making in larval zebrafish. Nat. Neurosci. 2020, 23, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Bianco, I.H.; Engert, F. Visuomotor transformations underlying hunting behavior in zebrafish. Curr. Biol. 2015, 25, 831–846. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Mu, Y.; Hu, Y.; Kuan, A.T.; Nikitchenko, M.; Randlett, O.; Chen, A.B.; Gavornik, J.P.; Sompolinsky, H.; Engert, F.; et al. Brain-wide Organization of Neuronal Activity and Convergent Sensorimotor Transformations in Larval Zebrafish. Neuron 2018, 100, 876–890.e5. [Google Scholar] [CrossRef] [PubMed]
- Helmbrecht, T.O.; Dal Maschio, M.; Donovan, J.C.; Koutsouli, S.; Baier, H. Topography of a Visuomotor Transformation. Neuron 2018, 100, 1429–1445. [Google Scholar] [CrossRef]
- Luque, M.A.; Pérez-Pérez, M.P.; Herrero, L.; Torres, B. Involvement of the optic tectum and mesencephalic reticular formation in the generation of saccadic eye movements in goldfish. Brain Res. Rev. 2005, 49, 388–397. [Google Scholar] [CrossRef]
- Salas, C.; Broglio, C.; Rodríguez, F. Evolution of forebrain and spatial cognition in vertebrates: Conservation across diversity. Brain Behav. Evol. 2003, 62, 72–82. [Google Scholar] [CrossRef]
- Kasumyan, A.O. The vestibular system and sense of equilibrium in fish. J. Appl. Ichthyol. 2004, 44, 224–268. [Google Scholar]
- Simpson, J.I.; Graf, W. The selection of reference frames by nature and its investigators. In Adaptive Mechanisms in Gaze Control. Facts, and Theories; Berthoz, A., Jones, G.M., Eds.; Elsevier: Amsterdam, The Netherlands, 1985; pp. 3–16. [Google Scholar]
- Straka, H.; Baker, R. Vestibular blueprint in early vertebrates. Front. Neural Circuits 2013, 7, 182. [Google Scholar] [CrossRef] [PubMed]
- Straka, H.; Zwergal, A.; Cullen, K.E. Vestibular animal models: Contributions to understanding physiology and disease. J. Neurol. 2016, 263, 10–23. [Google Scholar] [CrossRef]
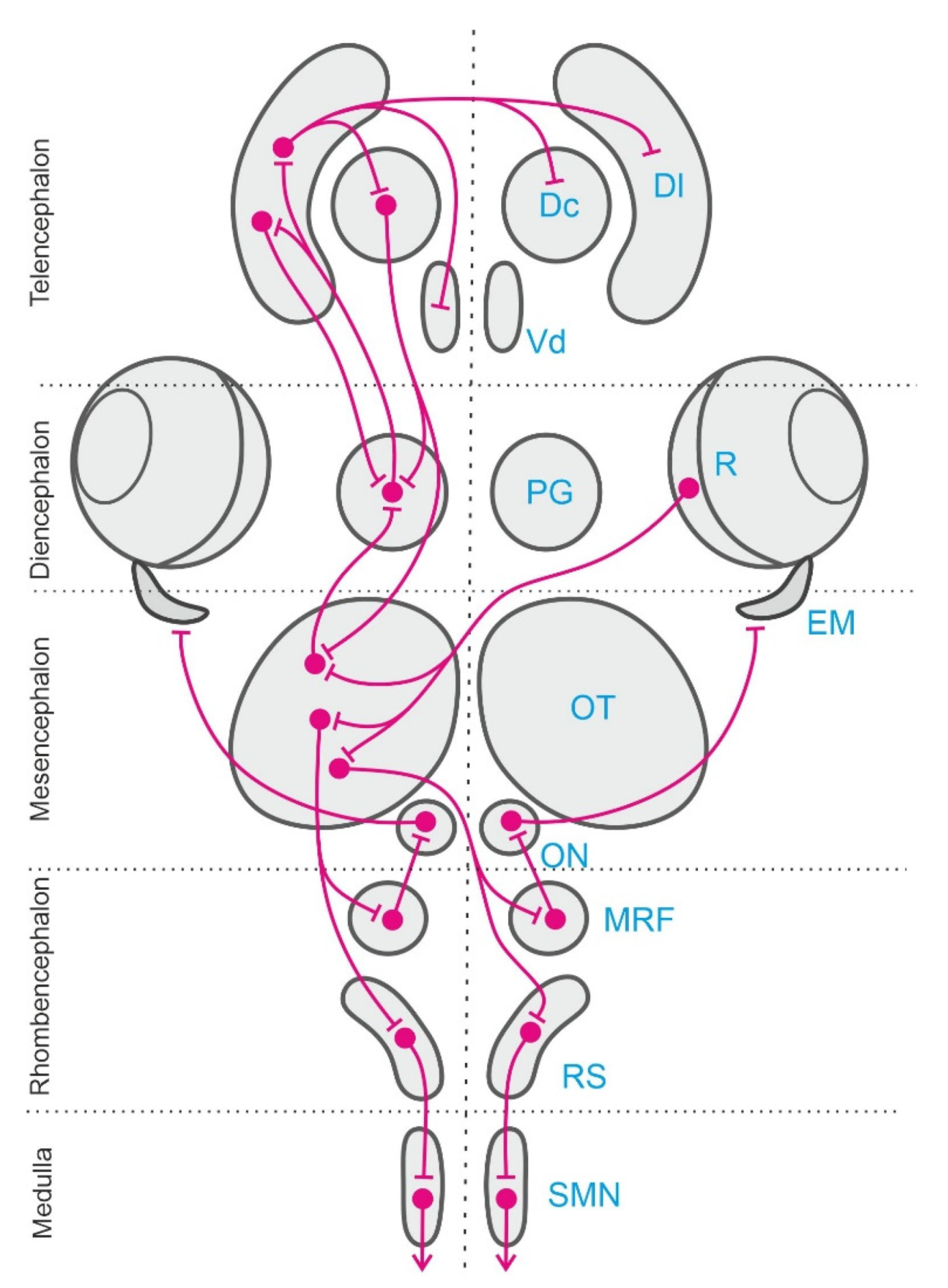
- Northmore, D. Optic tectum. In Encyclopedia of Fish Physiology: From Genome to Environment; Academic Press: London, UK, 2011; Volume 1, pp. 131–142. [Google Scholar]
- Isa, T.; Marquez-Legorreta, E.; Grillner, S.; Scott, E.K. The tectum/superior colliculus as the vertebrate solution for spatial sensory integration and action. Curr. Biol. 2021, 31, 741–762. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, V.; Sanguinetti-Scheck, J.I.; Künzel, S.; Geurten, B.; Gómez-Sena, L.; Engelmann, J. Sensory flow shaped by active sensing: Sensorimotor strategies in electric fish. J. Exp. Biol. 2013, 216, 2487–2500. [Google Scholar] [CrossRef] [PubMed]
- Vanegas, H. Organization and physiology of the teleostean optic tectum. In Fish Neurobiology, Higher Brain Areas and Functions; Davis, R.E., Northcutt, R.G., Eds.; The University of Michigan Press: Ann Arbor, WA, USA, 1983; Volume 2. [Google Scholar]
- Ewert, J.P. Behavioral selectivity based on thalamotectal interactions: Ontogenetic and phylogenetic aspects in amphibians. Behav. Brain Sci. 1984, 7, 337–338. [Google Scholar] [CrossRef]
- Ingle, D.J. Brain mechanisms of visual localization by frogs and toads. In Advances in Vertebrate Neuroethology; Springer: Boston, MA, USA, 1983; pp. 177–226. [Google Scholar]
- Masino, T.; Grobstein, P. The organization of descending tectofugal pathways underlying orienting in the frog, Rana pipiens. Exp. Brain Res. 1989, 75, 245–264. [Google Scholar] [CrossRef]
- Masino, T.; Grobstein, P. The organization of descending tectofugal pathways underlying orienting in the frog, Rana pipiens. I. Lateralization, parcellation, and an intermediate spatial representation. Exp. Brain Res. 1989, 75, 227–244. [Google Scholar] [CrossRef]
- Vanegas, H. Comparative Neurology of the Optic Tectum; Plenum Press: New York, NY, USA, 1984. [Google Scholar]
- Al-Akel, A.S.; Guthrie, D.M.; Banks, J.R. Motor responses to localized electrical stimulation of the tectum in the freshwater perch (Perca fluviatilis). Neuroscience 1986, 19, 1381–1391. [Google Scholar] [CrossRef]
- Barker, A.J.; Baier, H. Sensorimotor decision making in the zebrafish tectum. Curr. Biol. 2015, 25, 2804–2814. [Google Scholar] [CrossRef] [PubMed]
- Dunn, T.W.; Gebhardt, C.; Naumann, E.A.; Riegler, C.; Ahrens, M.B.; Engert, F.; Del Bene, F. Neural circuits underlying visually evoked escapes in larval zebrafish. Neuron 2016, 89, 613–628. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, O.; Zhu, P.; Friedrich, R.W. Control of a specific motor program by a small brain area in zebrafish. Front. Neural Circuits 2013, 7, 67. [Google Scholar] [CrossRef] [PubMed]
- Förster, D.; Helmbrecht, T.O.; Mearns, D.S.; Jordan, L.; Mokayes, N.; Baier, H. Retinotectal circuitry of larval zebrafish is adapted to detection and pursuit of prey. eLife 2020, 9, e58596. [Google Scholar] [CrossRef] [PubMed]
- Herrero, L.; Rodríguez, F.; Salas, C.; Torres, B. Tail and eye movements evoked by electrical microstimulation of the optic tectum in goldfish. Exp. Brain Res. 1998, 120, 291–305. [Google Scholar] [CrossRef]
- Salas, C.; Herrero, L.; Rodríguez, F.; Torres, B. On the role of goldfish optic tectum in the generation of eye movements. In Information Processing Underlying Gaze Control; Delgado-García, J.M., Godaux, E., Vidal, P.P., Eds.; Pergamon: Oxford, UK, 1995; pp. 87–95. [Google Scholar]
- Salas, C.; Herrero, L.; Rodrıguez, F.; Torres, B. Tectal codification of eye movements in goldfish studied by electrical microstimulation. Neuroscience 1997, 78, 271–288. [Google Scholar] [CrossRef]
- Temizer, I.; Donovan, J.C.; Baier, H.; Semmelhack, J.L. A Visual Pathway for Looming-Evoked Escape in Larval Zebrafish. Curr. Biol. 2015, 25, 1823–1834. [Google Scholar] [CrossRef]
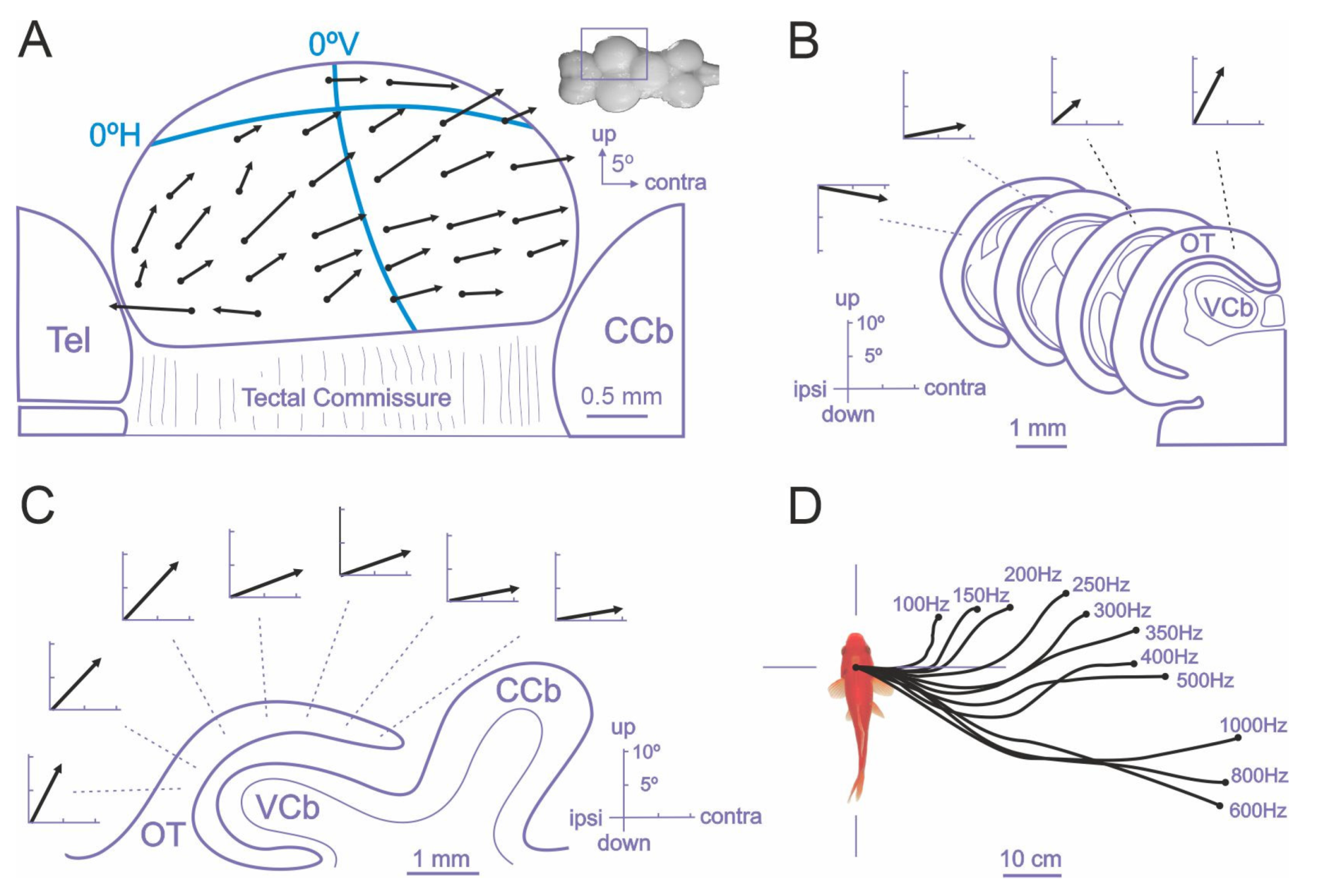
- Avitan, L.; Pujic, Z.; Hughes, N.J.; Scott, E.K.; Goodhill, G.J. Limitations of Neural Map Topography for Decoding Spatial Information. J. Neurosci. 2016, 36, 5385–5396. [Google Scholar] [CrossRef]
- Muto, A.; Ohkura, M.; Abe, G.; Nakai, J.; Kawakami, K. Real-time visualization of neuronal activity during perception. Curr. Biol. 2013, 23, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Schwassmann, H.O.; Kruger, L. Organization of the visual projection upon the optic tectum of some freshwater fish. J. Comp. Neurol. 1965, 124, 113–126. [Google Scholar] [CrossRef]
- Thompson, A.W.; Vanwalleghem, G.C.; Heap, L.A.; Scott, E.K. Functional Profiles of Visual-, Auditory-, and Water Flow-Responsive Neurons in the Zebrafish Tectum. Curr. Biol. 2016, 26, 743–754. [Google Scholar] [CrossRef]
- Knudsen, E.I.; Brainard, M.S. Visual instruction of the neural map of auditory space in the developing optic tectum. Science 1991, 253, 85–87. [Google Scholar] [CrossRef][Green Version]
- Stein, B.E.; Meredith, M.A. The Merging of the Senses; The MIT Press: Cambridge, MA, USA, 1993. [Google Scholar]
- Dragomir, E.I.; Štih, V.; Portugues, R. Evidence accumulation during a sensorimotor decision task revealed by whole-brain imaging. Nat. Neurosci. 2020, 23, 85–93. [Google Scholar] [CrossRef]
- Gahtan, E.; Tanger, P.; Baier, H. Visual prey capture in larval zebrafish is controlled by identified reticulospinal neurons downstream of the tectum. J. Neurosci. 2005, 25, 9294–9303. [Google Scholar] [CrossRef] [PubMed]
- Demski, L.S. Behavioral effects of electrical stimulation of the brain. In Fish Neurobiology, Higher Brain Areas and Functions; Davis, R.E., Northcutt, R.G., Eds.; The Michigan University Press: Ann Arbor, WA, USA, 1983; Volume 2, pp. 317–359. [Google Scholar]
- Meyer, D.L.; Schott, D.; Schaefer, K.P. Brain stimulation in the tectum opticum of freely swimming cods (Gadus morrhua L.). Pflugers Arch Eur. J. Physiol. 1970, 314, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Isa, T.; Sasaki, S. Brainstem control of head movements during orienting; organization of the premotor circuits. Prog. Neurobiol. 2002, 66, 205–224. [Google Scholar] [CrossRef]
- Torres, B.; Pérez-Pérez, M.P.; Herrero, L.; Ligero, M.; Núñez-Abades, P.A. Neural substrate underlying tectal eye movement codification in goldfish. Brain Res. Bull. 2002, 57, 345–348. [Google Scholar] [CrossRef]
- Torres, B.; Luque, M.A.; Pérez-Pérez, M.P.; Herrero, L. Visual orienting response in goldfish: A multidisciplinary study. Brain Res. Bull. 2005, 66, 376–380. [Google Scholar] [CrossRef]
- Salas, C.; Broglio, C.; Durán, E.; Gómez, A.; Ocana, F.M.; Jimenez-Moya, F.; Rodriguez, F. Neuropsychology of learning and memory in teleost fish. Zebrafish 2006, 3, 157–171. [Google Scholar] [CrossRef]
- Morris, R.G.M. Elements of a neurobiological theory of hippocampal function: The role of synaptic plasticity, synaptic tagging and schemas. Eur. J. Neurosci. 2006, 23, 2829–2846. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Broadbent, N. Is the avian hippocampus a functional homologue of the mammalian hippocampus? Neurosci. Biobehav. Rev. 2000, 24, 465–484. [Google Scholar] [CrossRef]
- Coppola, V.J.; Spencer, J.M.; Peterson, R.M.; Bingman, V.P. Hippocampal lesions in homing pigeons do not impair feature-quality or feature-quantity discrimination. Behav. Brain Res. 2014, 260, 83–91. [Google Scholar] [CrossRef]
- Fremouw, T.; Jackson-Smith, P.; Kesner, R.P. Impaired place learning and unimpaired cue learning in hippocampal-lesioned pigeons. Behav. Neurosci. 1997, 111, 963–975. [Google Scholar] [CrossRef]
- Good, M.; Macphail, E.M. The avian hippocampus and short-term memory for spatial and non-spatial information. Q. J. Exp. Psychol. B 1994, 47, 293–317. [Google Scholar]
- Holding, M.L.; Frazier, J.A.; Taylor, E.N.; Strand, C.R. Experimentally altered navigational demands induce changes in the cortical forebrain of free-ranging Northern Pacific rattlesnakes (Crotalus o. oreganus). Brain Behav. Evol. 2012, 79, 144–154. [Google Scholar] [CrossRef] [PubMed]
- López, J.C.; Gómez, Y.; Vargas, J.P.; Salas, C. Spatial reversal learning deficit after medial cortex lesion in turtles. Neurosci. Lett. 2003, 341, 197–200. [Google Scholar] [CrossRef]
- López, J.C.; Vargas, J.P.; Gómez, Y.; Salas, C. Spatial and non-spatial learning in turtles: The role of medial cortex. Behav. Brain Res. 2003, 143, 109–120. [Google Scholar] [CrossRef]
- Rodríguez, F.; López, J.C.; Vargas, J.P.; Gómez, Y.; Broglio, C.; Salas, C. Conservation of spatial memory function in the pallial forebrain of amniotes and ray-finned fishes. J. Neurosci. 2002, 22, 2894–2903. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.B.; Hodos, W. Comparative Vertebrate Neuroanatomy: Evolution and Adaptation; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Striedter, G.F.; Northcutt, R.G. Brains through Time: A Natural History of Vertebrates; Oxford University Press: Oxford, UK, 2020. [Google Scholar]
- Folgueira, M.; Bayley, P.; Navratilova, P.; Becker, T.S.; Wilson, S.W.; Clarke, J.D. Morphogenesis underlying the development of the everted teleost telencephalon. Neural Dev. 2012, 7, 212. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuys, R. The comparative anatomy of the actinopterygian forebrain. J. Hirnforsch. 1963, 7, 171–192. [Google Scholar]
- Nieuwenhuys, R. The development and general morphology of the telencephalon of actinopterygian fishes: Synopsis, documentation and commentary. Brain Struct. Funct. 2011, 215, 141–157. [Google Scholar] [CrossRef]
- Striedter, G.F.; Northcutt, R.G. Head size constrains forebrain development and evolution in ray-finned fishes. Evol. Dev. 2006, 8, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Northcutt, R.G. Connections of the lateral and medial divisions of the goldfish telencephalic pallium. J. Comp. Neurol. 2006, 494, 903–943. [Google Scholar] [CrossRef] [PubMed]
- Wullimann, M.F.; Mueller, T. Teleostean and mammalian forebrains contrasted: Evidence from genes to behavior. J. Comp. Neurol. 2004, 475, 143–162. [Google Scholar] [CrossRef] [PubMed]
- Ganz, J.; Kroehne, V.; Freudenreich, D.; Machate, A.; Geffarth, M.; Braasch, I.; Kaslin, J.; Brand, M. Subdivisions of the adult zebrafish pallium based on molecular marker analysis. F1000Research 2015, 3, 308. [Google Scholar] [CrossRef]
- Northcutt, R.G. Forebrain evolution in bony fishes. Brain Res. Bull. 2008, 75, 191–205. [Google Scholar] [CrossRef]
- Porter, B.A.; Mueller, T. The Zebrafish Amygdaloid Complex–Functional Ground Plan, Molecular Delineation, and Everted Topology. Front. Neurosci. 2020, 14, 608. [Google Scholar] [CrossRef]
- Yamamoto, N.; Ishikawa, Y.; Yoshimoto, M.; Xue, H.G.; Bahaxar, N.; Sawai, N.; Yang, C.Y.; Ozawa, H.; Ito, H. A new interpretation on the homology of the teleostean telencephalon based on hodology and a new eversion model. Brain Behav. Evol. 2007, 69, 96–104. [Google Scholar] [CrossRef]
- Butler, A.B. Topography and topology of the teleost telencephalon: A paradox resolved. Neurosci. Lett. 2000, 293, 95–98. [Google Scholar] [CrossRef]
- Nieuwenhuys, R.; Meek, J. The telencephalon of actinopterygian fishes. In Comparative Structure and Evolution of Cerebral Cortex, Part I; Springer: Boston, MA, USA, 1990; pp. 31–73. [Google Scholar]
- Northcutt, R.G. The forebrain of gnathostomes: In search of a morphotype. Brain Behav. Evol. 1995, 46, 275–318. [Google Scholar] [CrossRef]
- Northcutt, R.G.; Braford, M.R. New observations on the organization and evolution of the telencephalon in actinopterygian fishes. In Comparative Neurology of the Telencephalon; Ebbesson, S.O.E., Ed.; Plenum Press: New York, NY, USA, 1980; pp. 41–98. [Google Scholar]
- Dirian, L.; Galant, S.; Coolen, M.; Chen, W.; Bedu, S.; Houart, C.; Bally-Cuif, L.; Foucher, I. Spatial regionalization and heterochrony in the formation of adult pallial neural stem cells. Dev. Cell 2014, 30, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Harvey-Girard, E.; Giassi, A.C.; Maler, L. The organization of the gymnotiform fish pallium in relation to learning and memory: IV. Expression of conserved transcription factors and implications for the evolution of dorsal telencephalon. J. Comp. Neurol. 2012, 520, 3395–3413. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, Y.; Yamamoto, N.; Yoshimoto, M.; Yasuda, T.; Maruyama, K.; Kage, T.; Takeda, H.; Ito, H. Developmental origin of diencephalic sensory relay nuclei in teleosts. Brain Behav. Evol. 2007, 69, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Vernier, P.; Wullimann, M.F. Evolution of the posterior tuberculum and preglomerular nuclear complex. In Encyclopedia of Neuroscience; Binder, M.D., Hirokawa, N., Windhorst, U., Eds.; Springer: Berlin, Germany, 2009; Volume 2, pp. 1404–1413. [Google Scholar]
- Yamamoto, N.; Ito, H. Fiber connections of the anterior preglomerular nucleus in cyprinids with notes on telencephalic connections of the preglomerular complex. J. Comp. Neurol. 2005, 491, 212–233. [Google Scholar] [CrossRef]
- Yamamoto, N.; Ito, H. Visual, lateral line, and auditory ascending pathways to the dorsal telencephalic area through the rostrolateral region of the lateral preglomerular nucleus in cyprinids. J. Comp. Neurol. 2008, 508, 615–647. [Google Scholar] [CrossRef] [PubMed]
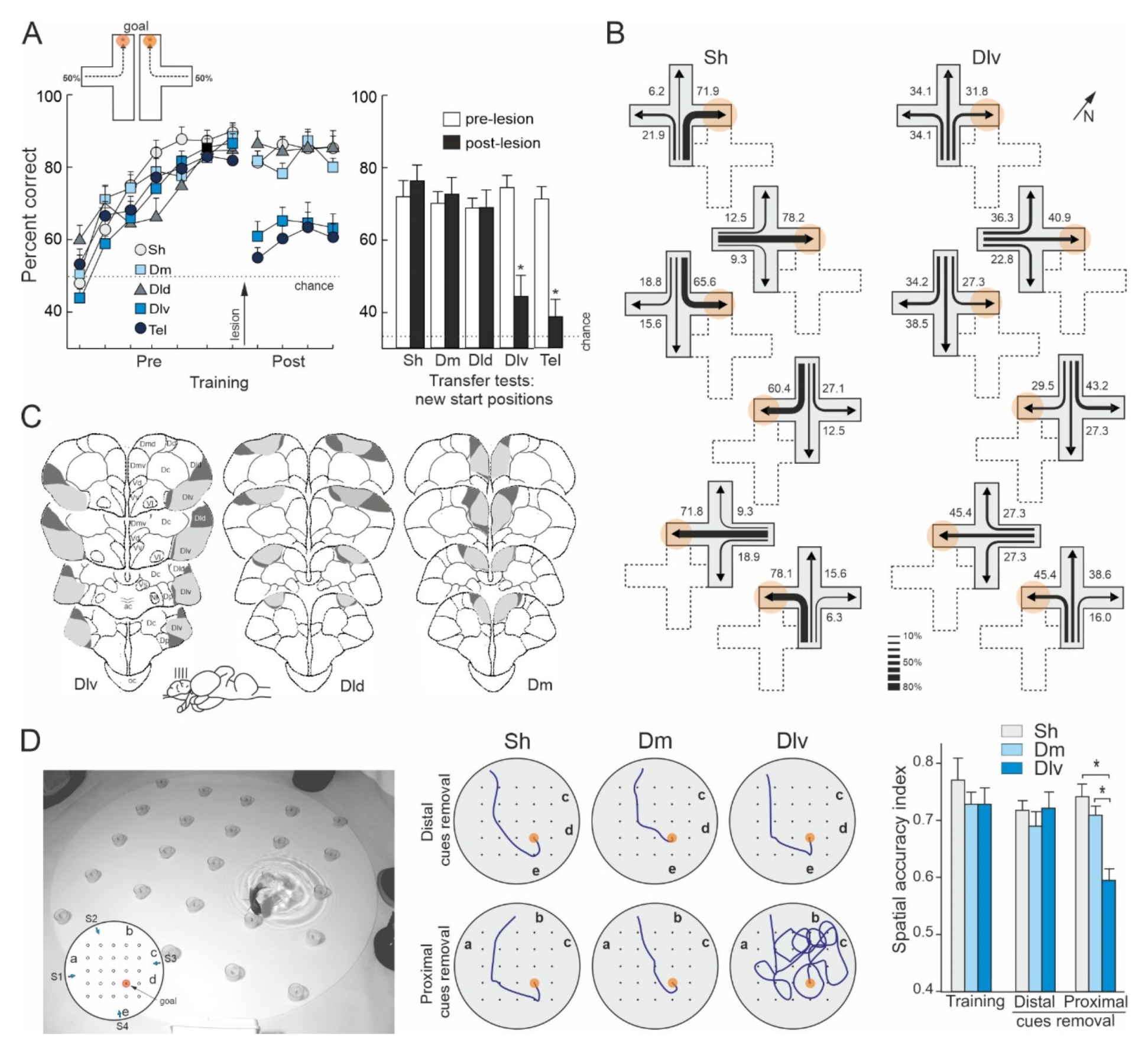
- Broglio, C.; Gómez, A.; Durán, E.; Ocaña, F.M.; Jiménez-Moya, F.; Rodríguez, F.; Salas, C. Hallmarks of a common forebrain vertebrate plan: Specialized pallial areas for spatial, temporal and emotional memory in actinopterygian fish. Brain Res. Bull. 2005, 66, 277–281. [Google Scholar] [CrossRef]
- Broglio, C.; Rodríguez, F.; Gómez, A.; Arias, J.L.; Salas, C. Selective involvement of the goldfish lateral pallium in spatial memory. Behav. Brain Res. 2010, 210, 191–201. [Google Scholar] [CrossRef]
- Durán, E.; Ocaña, F.M.; Broglio, C.; Rodríguez, F.; Salas, C. Lateral but not medial telencephalic pallium ablation impairs the use of goldfish spatial allocentric strategies in a hole-board task. Behav. Brain Res. 2010, 214, 480–487. [Google Scholar] [CrossRef]
- Ocaña, F.M.; Uceda, S.; Arias, J.L.; Salas, C.; Rodríguez, F. Dynamics of goldfish subregional hippocampal pallium activity throughout spatial memory formation. Brain Behav. Evol. 2017, 90, 154–170. [Google Scholar] [CrossRef]
- Uceda, S.; Ocaña, F.M.; Martín-Monzón, I.; Rodríguez-Expósito, B.; Durán, E.; Rodríguez, F. Spatial learning-related changes in metabolic brain activity contribute to the delimitation of the hippocampal pallium in goldfish. Behav. Brain Res. 2015, 292, 403–408. [Google Scholar] [CrossRef]
- Vargas, J.P.; Rodríguez, F.; López, J.C.; Arias, J.L.; Salas, C. Spatial learning-induced increase in the argyrophilic nucleolar organizer region of dorsolateral telencephalic neurons in goldfish. Brain Res. 2000, 865, 77–84. [Google Scholar] [CrossRef]
- Vinepinsky, E.; Cohen, L.; Perchik, S.; Ben-Shahar, O.; Donchin, O.; Segev, R. Representation of edges, head direction, and swimming kinematics in the brain of freely-navigating fish. Sci. Rep. 2020, 10, 14762. [Google Scholar] [CrossRef]
- Wood, L.S.; Desjardins, J.K.; Fernald, R.D. Effects of stress and motivation on performing a spatial task. Neurobiol. Learn. Mem. 2011, 95, 277–285. [Google Scholar] [CrossRef][Green Version]
- Buzsáki, G.; Moser, E.I. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat. Neurosci. 2013, 16, 130–138. [Google Scholar] [CrossRef]
- Eichenbaum, H.; Sauvage, M.; Fortin, N.; Komorowski, R.; Lipton, P. Towards a functional organization of episodic memory in the medial temporal lobe. Neurosci. Biobehav. Rev. 2012, 36, 1597–1608. [Google Scholar] [CrossRef]
- McClelland, J.L.; McNaughton, B.L.; O’Reilly, R.C. Why there are complementary learning systems in the hippocampus and neocortex: Insights from the successes and failures of connectionist models of learning and memory. Psychol. Rev. 1995, 102, 419. [Google Scholar] [CrossRef]
- Moscovitch, M.; Cabeza, R.; Winocur, G.; Nadel, L. Episodic memory and beyond: The hippocampus and neocortex in transformation. Annu. Rev. Psychol. 2016, 67, 105–134. [Google Scholar] [CrossRef] [PubMed]
- Squire, L.R.; Stark, C.E.; Clark, R.E. The medial temporal lobe. Ann. Rev. Neurosci. 2004, 27, 279–306. [Google Scholar] [CrossRef]
- Hafting, T.; Fyhn, M.; Molden, S.; Moser, M.B.; Moser, E.I. Microstructure of a spatial map in the entorhinal cortex. Nature 2005, 436, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Kropff, E.; Carmichael, J.E.; Moser, M.B.; Moser, E.I. Speed cells in the medial entorhinal cortex. Nature 2015, 523, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Savelli, F.; Yoganarasimha, D.; Knierim, J.J. Influence of boundary removal on the spatial representations of the medial entorhinal cortex. Hippocampus 2008, 18, 1270–1282. [Google Scholar] [CrossRef]
- Taube, J.S.; Muller, R.U.; Ranck, J.B., Jr. Head-direction cells recorded from the postsubiculum in freely moving rats. I. Description and quantitative analysis. J. Neurosci. 1990, 10, 420–435. [Google Scholar] [CrossRef] [PubMed]
- McNaughton, B.L.; Battaglia, F.P.; Jensen, O.; Moser, E.I.; Moser, M.B. Path integration and the neural basis of the ‘cognitive map’. Nat. Rev. Neurosci. 2006, 7, 663–678. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, J.; Dostrovsky, J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely mnving rat. Brain Res. 1971, 34, 171–175. [Google Scholar] [CrossRef]
- Demski, L.S. The pallium and mind/behavior relationships in teleost fishes. Brain Behav. Evol. 2013, 82, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Prechtl, J.C.; von der Emde, G.; Wolfart, J.; Karamursel, S.; Akoev, G.N.; Andrianov, Y.N.; Bullock, T.H. Sensory processing in the pallium of a mormyrid fish. J. Neurosci. 1998, 18, 7381–7393. [Google Scholar] [CrossRef]
- Saidel, W.M.; Marquez-Houston, K.; Butler, A.B. Identification of visual pallial telencephalon in the goldfish, Carassius auratus: A combined cytochrome oxidase and electrophysiological study. Brain Res. 2001, 919, 82–93. [Google Scholar] [CrossRef]
- Canfield, J.G.; Mizumori, S.J. Methods for chronic neural recording in the telencephalon of freely behaving fish. J. Neurosci. Methods 2004, 133, 127–134. [Google Scholar] [CrossRef]
- Takahashi, S.; Hombe, T.; Takahashi, R.; Ide, K.; Okamoto, S.; Yoda, K.; Makiguchi, Y. Wireless logging of extracellular neuronal activity in the telencephalon of free-swimming salmonids. Anim. Biotelemetry 2021, 9, 9. [Google Scholar] [CrossRef]
- Trinh, A.T.; Clarke, S.E.; Harvey-Girard, E.; Maler, L. Cellular and network mechanisms may generate sparse coding of sequential object encounters in hippocampal-like circuits. eNeuro 2019, 6. [Google Scholar] [CrossRef]
- Wallach, A.; Harvey-Girard, E.; Jun, J.J.; Longtin, A.; Maler, L. A time-stamp mechanism may provide temporal information necessary for egocentric to allocentric spatial transformations. eLife 2018, 7, e36769. [Google Scholar] [CrossRef]
- McNaughton, B.L.; Morris, R.G. Hippocampal synaptic enhancement and information storage within a distributed memory system. Trends Neurosci. 1987, 10, 408–415. [Google Scholar] [CrossRef]
- Treves, A.; Rolls, E.T. Computational analysis of the role of the hippocampus in memory. Hippocampus 1994, 4, 374–391. [Google Scholar] [CrossRef] [PubMed]
- Leutgeb, J.K.; Leutgeb, S.; Moser, M.B.; Moser, E.I. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science 2007, 315, 961–966. [Google Scholar] [CrossRef] [PubMed]
- Bontempi, B.; Laurent-Demir, C.; Destrade, C.; Jaffard, R. Time-dependent reorganization of brain circuitry underlying long-term memory storage. Nature 1999, 400, 671–675. [Google Scholar] [CrossRef]
- Churchwell, J.C.; Morris, A.M.; Musso, N.D.; Kesner, R.P. Prefrontal and hippocampal contributions to encoding and retrieval of spatial memory. Neurobiol. Learn. Mem. 2010, 93, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Lacy, J.W.; Yassa, M.A.; Stark, S.M.; Muftuler, L.T.; Stark, C.E. Distinct pattern separation related transfer functions in human CA3/dentate and CA1 revealed using high-resolution fMRI and variable mnemonic similarity. Learn. Mem. 2011, 18, 15–18. [Google Scholar] [CrossRef]
- Poirier, G.L.; Amin, E.; Aggleton, J.P. Qualitatively different hippocampal subfield engagement emerges with mastery of a spatial memory task by rats. J. Neurosci. 2008, 28, 1034–1045. [Google Scholar] [CrossRef] [PubMed]
- Vazdarjanova, A.; Guzowski, J.F. Differences in hippocampal neuronal population responses to modifications of an environmental context: Evidence for distinct, yet complementary, functions of CA3 and CA1 ensembles. J. Neurosci. 2004, 24, 6489–6496. [Google Scholar] [CrossRef]
- Elliott, S.B.; Harvey-Girard, E.; Giassi, A.C.; Maler, L. Hippocampal-like circuitry in the pallium of an electric fish: Possible substrates for recursive pattern separation and completion. J. Comp. Neurol. 2017, 525, 8–46. [Google Scholar] [CrossRef]
- Trinh, A.T.; Harvey-Girard, E.; Teixeira, F.; Maler, L. Cryptic laminar and columnar organization in the dorsolateral pallium of a weakly electric fish. J. Comp. Neurol. 2016, 524, 408–428. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.E.; Squire, L.R. Classical conditioning and brain systems: The role of awareness. Science 1998, 280, 77–81. [Google Scholar] [CrossRef]
- Cohen, N.J.; Eichenbaum, H. Memory, Amnesia, and the Hippocampal System; The MIT Press: Cambridge, MA, USA, 1993. [Google Scholar]
- Eichenbaum, H. The role of the hippocampus in navigation is memory. J. Neurophysiol. 2017, 117, 1785–1796. [Google Scholar] [CrossRef] [PubMed]
- Rawlins, J.N.P. Associations across time: The hippocampus as a temporary memory store. Behav Brain Sci 1985, 8, 479–497. [Google Scholar] [CrossRef]
- Portavella, M.; Torres, B.; Salas, C. Avoidance response in goldfish: Emotional and temporal involvement of medial and lateral telencephalic pallium. J. Neurosci. 2004, 24, 2335–2342. [Google Scholar] [CrossRef]
- Kim, J.J.; Fanselow, M.S. Modality-specific retrograde amnesia of fear. Science 1992, 256, 675–677. [Google Scholar] [CrossRef] [PubMed]
- Maren, S.; Quirk, G.J. Neuronal signalling of fear memory. Nat. Rev. Neurosci. 2004, 5, 844–852. [Google Scholar] [CrossRef]
- McGaugh, J.L. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu. Rev. Neurosci. 2004, 27, 1–28. [Google Scholar] [CrossRef]
- Phillips, R.G.; LeDoux, J.E. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav. Neurosci. 1992, 106, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Expósito, B.; Gómez, A.; Martín-Monzón, I.; Reiriz, M.; Rodríguez, F.; Salas, C. Goldfish hippocampal pallium is essential to associate temporally discontiguous events. Neurobiol. Learn. Mem. 2017, 139, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, T.; Macdonald, C.J.; Tonegawa, S. Entorhinal–hippocampal neuronal circuits bridge temporally discontiguous events. Learn. Mem. 2015, 22, 438–443. [Google Scholar] [CrossRef]
- MacDonald, C.J.; Lepage, K.Q.; Eden, U.T.; Eichenbaum, H. Hippocampal ‘‘time cells” bridge the gap in memory for discontiguous events. Neuron 2011, 71, 737–749. [Google Scholar] [CrossRef] [PubMed]
- McEchron, M.D.; Bouwmeester, H.; Tseng, W.; Weiss, C.; Disterhoft, J.F. Hippocampectomy disrupts auditory trace fear conditioning and contextual fear conditioning in the rat. Hippocampus 1998, 8, 638–646. [Google Scholar] [CrossRef]
- Moyer, J.R.; Deyo, R.A.; Disterhoft, J.F. Hippocampectomy disrupts trace eye-blink conditioning in rabbits. Behav. Neurosci. 1990, 104, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Solomon, P.R.; Vander Schaaf, E.R.; Thompson, R.F.; Weisz, D.J. Hippocampus and trace conditioning of the rabbit’s classically conditioned nictitating membrane response. Behav. Neurosci. 1986, 100, 729–744. [Google Scholar] [CrossRef]
- Staresina, B.P.; Davachi, L. Mind the gap: Binding experiences across space and time in the human hippocampus. Neuron 2009, 63, 267–276. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez, F.; Quintero, B.; Amores, L.; Madrid, D.; Salas-Peña, C.; Salas, C. Spatial Cognition in Teleost Fish: Strategies and Mechanisms. Animals 2021, 11, 2271. https://doi.org/10.3390/ani11082271
Rodríguez F, Quintero B, Amores L, Madrid D, Salas-Peña C, Salas C. Spatial Cognition in Teleost Fish: Strategies and Mechanisms. Animals. 2021; 11(8):2271. https://doi.org/10.3390/ani11082271
Chicago/Turabian StyleRodríguez, Fernando, Blanca Quintero, Lucas Amores, David Madrid, Carmen Salas-Peña, and Cosme Salas. 2021. "Spatial Cognition in Teleost Fish: Strategies and Mechanisms" Animals 11, no. 8: 2271. https://doi.org/10.3390/ani11082271
APA StyleRodríguez, F., Quintero, B., Amores, L., Madrid, D., Salas-Peña, C., & Salas, C. (2021). Spatial Cognition in Teleost Fish: Strategies and Mechanisms. Animals, 11(8), 2271. https://doi.org/10.3390/ani11082271





