The Potential for Sialic Acid and Sialylated Glycoconjugates as Feed Additives to Enhance Pig Health and Production
Simple Summary
Abstract
1. Introduction
2. Problems of the Pig Industry
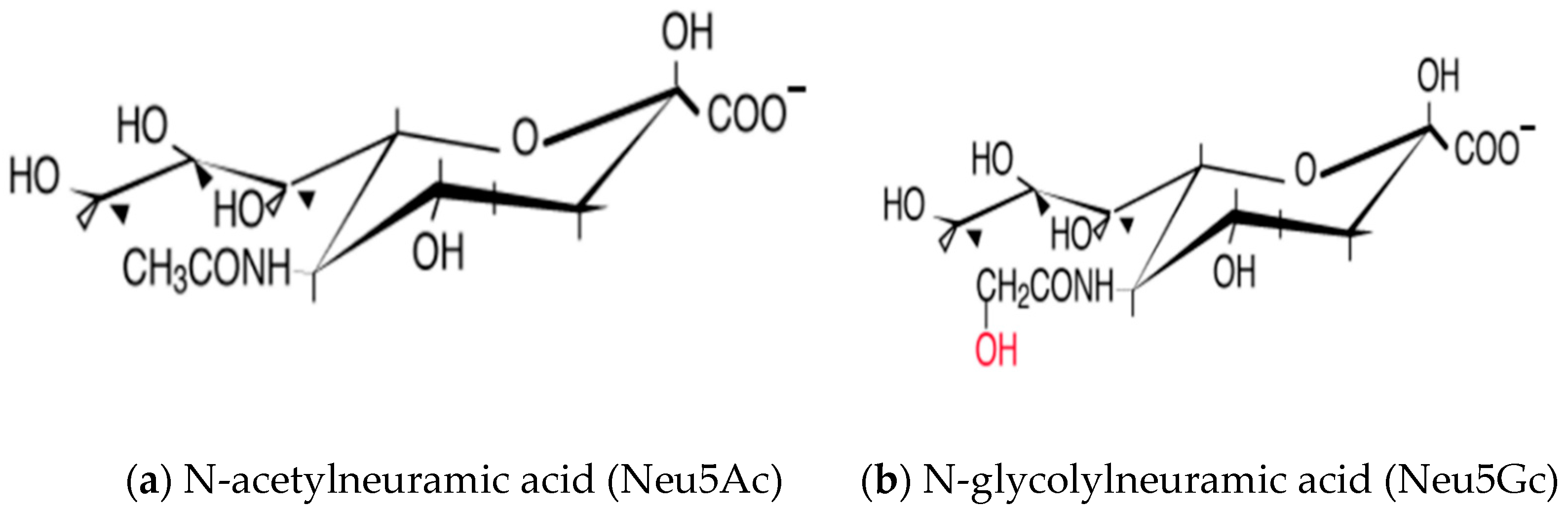
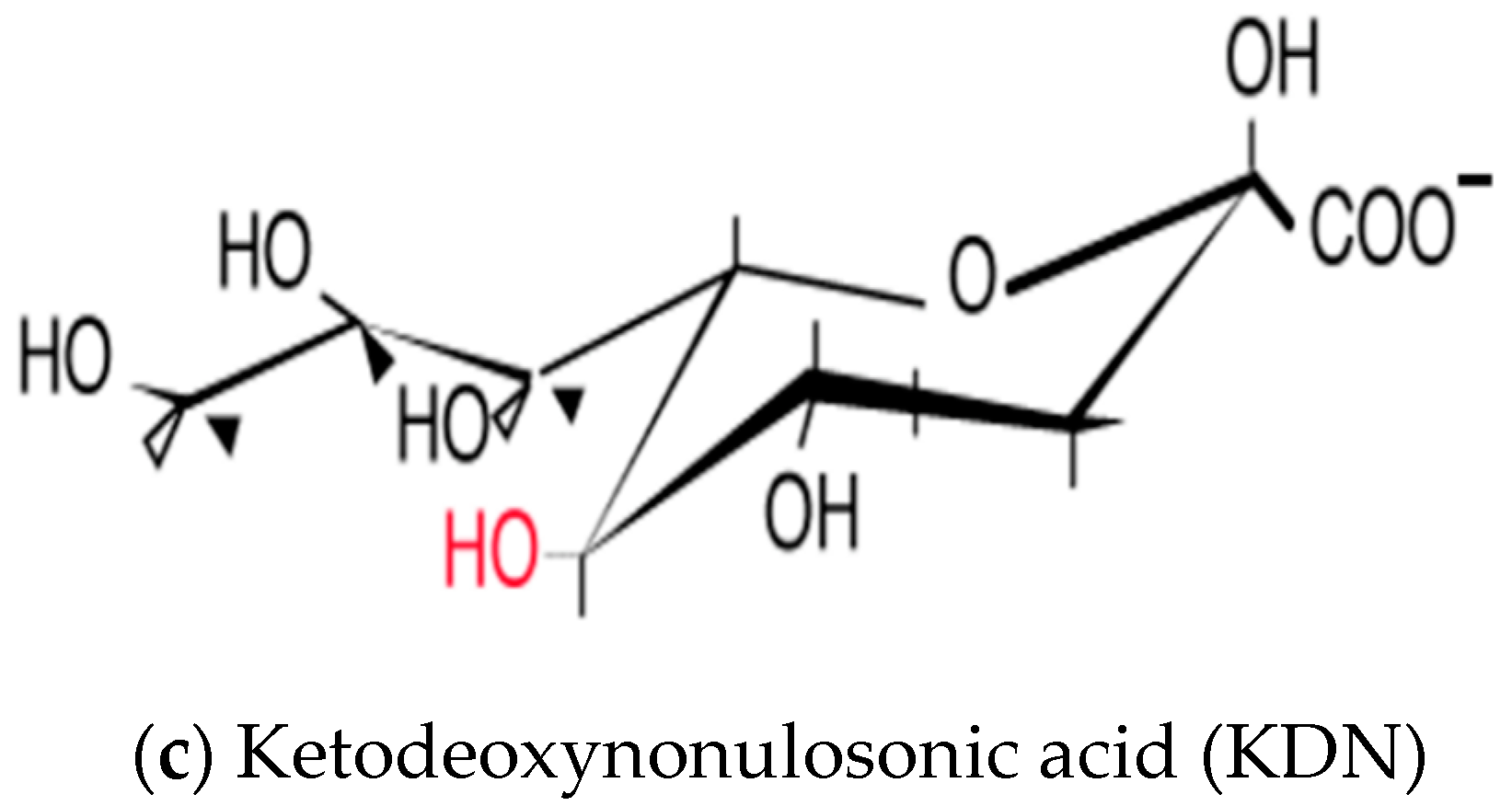
3. Sialic Acid and Its Diversity in Nature
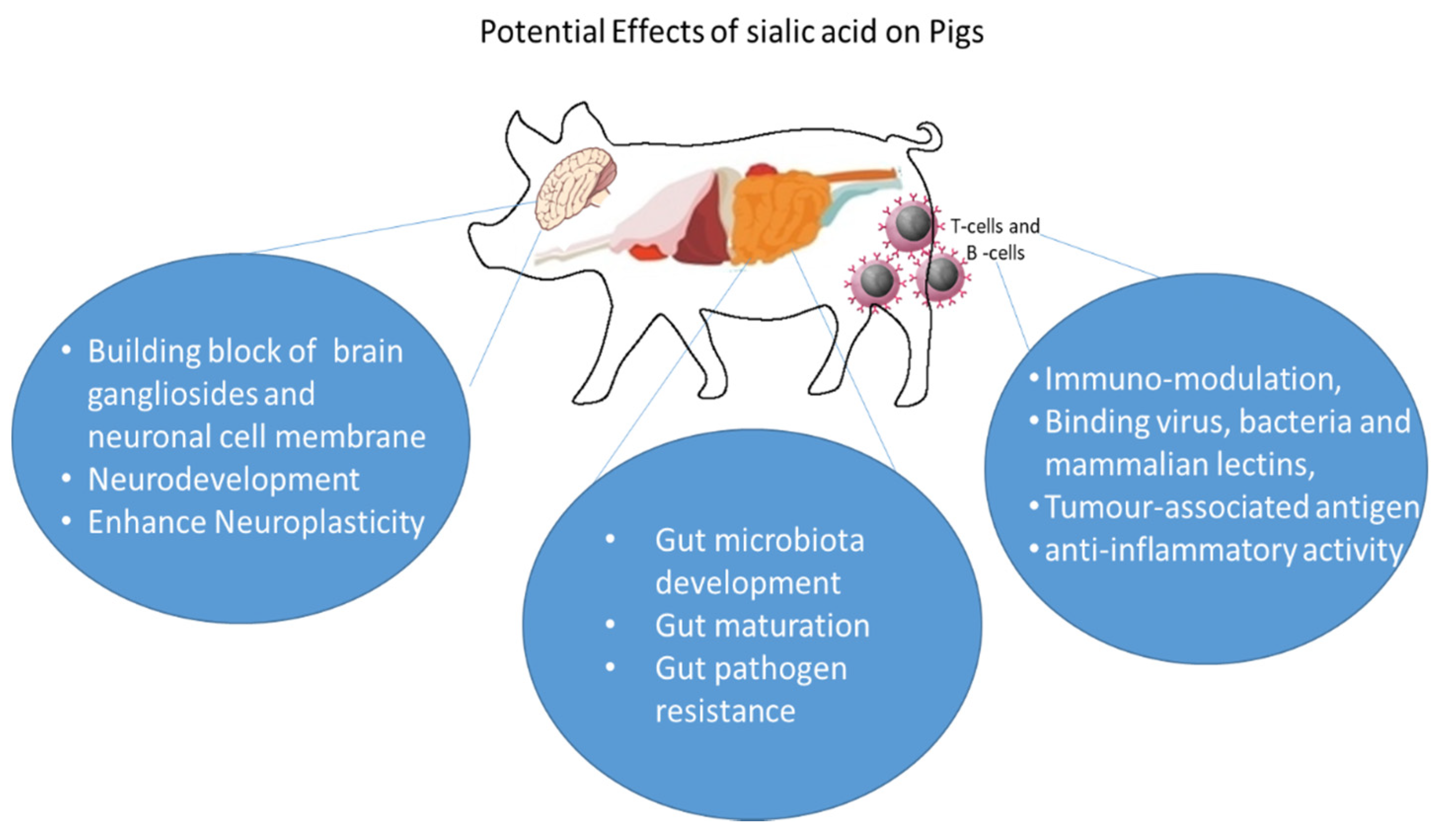
4. Biological Functions of Sialic Acids
4.1. Development of Gut Microbiota
4.2. Gut Maturation and Gut Pathogen Resistance
4.3. Immune Function and Inflammation
4.4. Brain Development and Cognition
5. Sialic Acid Concentration in Porcine Milk
6. Sialic Acid in Different Organs of Pig
7. Factors Affecting Sialic Acid Content of Milk
8. Sialylated Milk Oligosaccharides in Porcine Milk
9. Sialylated Glycoprotein Lactoferrin
10. Sialylated Glycolipid Ganglioside
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anderson, L.; Parker, R. Distribution and development of embryos in the pig. J. Reprod. Fertil. 1976, 46, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Bazer, F.W.; Wallace, J.M.; Spencer, T.E. Intrauterine growth retardation: Implications for the animal sciences. J. Anim. Sci. 2006, 84, 2316–2337. [Google Scholar] [CrossRef]
- Schoknecht, P.; Ebner, S.; Skottner, A.; Burrin, D.; Davis, T.; Ellis, K.; Pond, W. Exogenous insulin-like growth factor-I increases weight gain in intrauterine growth-retarded neonatal pigs. Pediatric Res. 1997, 42, 201–207. [Google Scholar] [CrossRef][Green Version]
- Décordé, K.; Teissèdre, P.L.; Sutra, T.; Ventura, E.; Cristol, J.P.; Rouanet, J.M. Chardonnay grape seed procyanidin extract supplementation prevents high-fat diet-induced obesity in hamsters by improving adipokine imbalance and oxidative stress markers. Mol. Nutr. Food Res. 2009, 53, 659–666. [Google Scholar] [CrossRef]
- Feillet-Coudray, C.; Sutra, T.; Fouret, G.; Ramos, J.; Wrutniak-Cabello, C.; Cabello, G.; Cristol, J.; Coudray, C. Oxidative stress in rats fed a high-fat high-sucrose diet and preventive effect of polyphenols: Involvement of mitochondrial and NAD (P) H oxidase systems. Free Radic. Biol. Med. 2009, 46, 624–632. [Google Scholar] [CrossRef]
- Luo, Z.; Fraser, W.; Julien, P.; Deal, C.; Audibert, F.; Smith, G.; Xiong, X.; Walker, M. Tracing the origins of “fetal origins” of adult diseases: Programming by oxidative stress? Med. Hypotheses 2006, 66, 38–44. [Google Scholar] [CrossRef]
- Muns, R.; Nuntapaitoon, M.; Tummaruk, P. Non-infectious causes of pre-weaning mortality in piglets. Livest. Sci. 2016, 184, 46–57. [Google Scholar] [CrossRef]
- VanderWaal, K.; Deen, J. Global trends in infectious diseases of swine. Proc. Natl. Acad. Sci. USA 2018, 115, 11495–11500. [Google Scholar] [CrossRef] [PubMed]
- McMillen, I.C.; Robinson, J.S. Developmental origins of the metabolic syndrome: Prediction, plasticity, and programming. Physiol. Rev. 2005, 85, 571–633. [Google Scholar] [CrossRef]
- Craig, J.R.; Collins, C.L.; Bunter, K.L.; Cottrell, J.J.; Dunshea, F.R.; Pluske, J.R. Poorer lifetime growth performance of gilt progeny compared with sow progeny is largely due to weight differences at birth and reduced growth in the preweaning period, and is not improved by progeny segregation after weaning. J. Anim. Sci. 2017, 95, 4904–4916. [Google Scholar] [CrossRef]
- Tummaruk, P.; Pearodwong, P. Postparturient disorders and backfat loss in tropical sows associated with parity, farrowing duration and type of antibiotic. Trop. Anim. Health Prod. 2015, 47, 1457–1464. [Google Scholar] [CrossRef]
- Blix, G. Über die kohlenhydratgruppen des submaxillarismucins. Biol. Chem. 1936, 240, 43–54. [Google Scholar] [CrossRef]
- Blix, G.; Svennerholm, L.; Werner, I. The isolation of chondrosamine from gangliosides and from submaxillary mucin. Acta Chem. Scand. 1952, 6, 358–362. [Google Scholar] [CrossRef]
- Wang, B. Sialic acid is an essential nutrient for brain development and cognition. Annu. Rev. Nutr. 2009, 29, 177–222. [Google Scholar] [CrossRef]
- Angata, T.; Varki, A. Chemical diversity in the sialic acids and related α-keto acids: An evolutionary perspective. Chem. Rev. 2002, 102, 439–470. [Google Scholar] [CrossRef]
- Varki, A. Glycan-based interactions involving vertebrate sialic-acid-recognizing proteins. Nature 2007, 446, 1023–1029. [Google Scholar] [CrossRef]
- Schauer, R. Achievements and challenges of sialic acid research. Glycoconj. J. 2000, 17, 485–499. [Google Scholar] [CrossRef]
- Chen, X.; Varki, A. Advances in the biology and chemistry of sialic acids. ACS Chem. Biol. 2010, 5, 163–176. [Google Scholar] [CrossRef]
- Iwasaki, M.; Inoue, S.; Troy, F. A new sialic acid analogue, 9-O-acetyl-deaminated neuraminic acid, and alpha-2, 8-linked O-acetylated poly (N-glycolylneuraminyl) chains in a novel polysialoglycoprotein from salmon eggs. J. Biol. Chem. 1990, 265, 2596–2602. [Google Scholar] [CrossRef]
- Varki, A. Diversity in the sialic acids. Glycobiology 1992, 2, 25–40. [Google Scholar] [CrossRef]
- Inoue, S.; Kitajima, K. KDN (deaminated neuraminic acid): Dreamful past and exciting future of the newest member of the sialic acid family. Glycoconj. J. 2006, 23, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Nadano, D.; Iwasaki, M.; Endo, S.; Kitajima, K.; Inoue, S.; Inoue, Y. A naturally occurring deaminated neuraminic acid, 3-deoxy-d-glycero-d-galacto-nonulosonic acid (KDN). Its unique occurrence at the nonreducing ends of oligosialyl chains in polysialoglycoprotein of rainbow trout eggs. J. Biol. Chem. 1986, 261, 11550–11557. [Google Scholar] [CrossRef]
- Schauer, R. Chemistry, metabolism, and biological functions of sialic acids. Adv. Carbohydr. Chem. Biochem. 1981, 40, 131–234. [Google Scholar]
- Maru, I.; Ohnishi, J.; Ohta, Y.; Tsukada, Y. Why is sialic acid attracting interest now? complete enzymatic synthesis of sialic acid with N-acylglucosamine 2-epimerase. J. Biosci. Bioeng. 2002, 93, 258–265. [Google Scholar] [CrossRef]
- Traving, C.; Schauer, R. Structure, function and metabolism of sialic acids. Cell. Mol. Life Sci. 1998, 54, 1330–1349. [Google Scholar] [CrossRef] [PubMed]
- Varki, A. Sialic acids. In Essentials of Glycobiology; Varki, A., Cummings, R., Esko, J., Freeze, H., Hart, G., Marth, J., Eds.; Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1999; pp. 195–209. [Google Scholar]
- Troy, F.A. Polysialylation: From bacteria to brains. Glycobiology 1992, 2, 5–23. [Google Scholar] [CrossRef] [PubMed]
- Knirel, Y.A.; Shashkov, A.S.; Tsvetkov, Y.E.; Jansson, P.-E.; Zahringer, U. 5,7-Diamino-3,5,7,9-tetradeoxynon-2-ulosonic acids in bacterial glycopolymers: Chemistry and biochemistry. Adv. Carbohydr. Chem. Biochem. 2003, 58, 371–418. [Google Scholar]
- Varki, A.; Angata, T. Siglecs—The major subfamily of I-type lectins. Glycobiology 2006, 16, 1R–27R. [Google Scholar] [CrossRef]
- Varki, A.; Diaz, S. The release and purification of sialic acids from glycoconjugates: Methods to minimize the loss and migration of O-acetyl groups. Anal. Biochem. 1984, 137, 236–247. [Google Scholar] [CrossRef]
- Cohen, M.; Hurtado-Ziola, N.; Varki, A. ABO blood group glycans modulate sialic acid recognition on erythrocytes. Blood 2009, 114, 3668–3676. [Google Scholar] [CrossRef]
- Schwarzkopf, M.; Knobeloch, K.-P.; Rohde, E.; Hinderlich, S.; Wiechens, N.; Lucka, L.; Horak, I.; Reutter, W.; Horstkorte, R. Sialylation is essential for early development in mice. Proc. Natl. Acad. Sci. USA 2002, 99, 5267–5270. [Google Scholar] [CrossRef] [PubMed]
- Schauer, R. Sialic acids as regulators of molecular and cellular interactions. Curr. Opin. Struct. Biol. 2009, 19, 507–514. [Google Scholar] [CrossRef]
- Varki, A. Sialic acids in human health and disease. Trends Mol. Med. 2008, 14, 351–360. [Google Scholar] [CrossRef]
- Kolling, G.; Wu, M.; Guerrant, R.L. Enteric pathogens through life stages. Front. Cell. Infect. Microbiol. 2012, 2, 3389. [Google Scholar] [CrossRef]
- Duncan, P.I.; Raymond, F.; Fuerholz, A.; Sprenger, N. Sialic acid utilisation and synthesis in the neonatal rat revisited. PLoS ONE 2009, 4, e8241. [Google Scholar] [CrossRef]
- Vester Boler, B.M.; Rossoni Serao, M.C.; Faber, T.A.; Bauer, L.L.; Chow, J.; Murphy, M.R.; Fahey, G.C., Jr. In vitro fermentation characteristics of select nondigestible oligosaccharides by infant fecal inocula. J. Agric. Food Chem. 2013, 61, 2109–2119. [Google Scholar] [CrossRef]
- Ruiz-Moyano, S.; Totten, S.M.; Garrido, D.A.; Smilowitz, J.T.; German, J.B.; Lebrilla, C.B.; Mills, D.A. Variation in consumption of human milk oligosaccharides by infant gut-associated strains of Bifidobacterium breve. Appl. Environ. Microbiol. 2013, 79, 6040–6049. [Google Scholar] [CrossRef]
- Servin, A.L. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol. Rev. 2004, 28, 405–440. [Google Scholar] [CrossRef]
- Lievin, V.; Peiffer, I.; Hudault, S.; Rochat, F.; Brassart, D.; Neeser, J.; Servin, A. Bifidobacterium strains from resident infant human gastrointestinal microflora exert antimicrobial activity. Gut 2000, 47, 646–652. [Google Scholar] [CrossRef]
- Kavanaugh, D.W.; O’Callaghan, J.; Buttó, L.F.; Slattery, H.; Lane, J.; Clyne, M.; Kane, M.; Joshi, L.; Hickey, R.M. Exposure of Bifidobacterium longum subsp. infantis to milk oligosaccharides increases adhesion to epithelial cells and induces a substantial transcriptional response. PLoS ONE 2013, 8, e67224. [Google Scholar] [CrossRef]
- Baker, H.M.; Baker, E.N. Lactoferrin and iron: Structural and dynamic aspects of binding and release. Biometals 2004, 17, 209–216. [Google Scholar] [CrossRef]
- LoCascio, R.G.; Ninonuevo, M.R.; Freeman, S.L.; Sela, D.A.; Grimm, R.; Lebrilla, C.B.; Mills, D.A.; German, J.B. Glycoprofiling of bifidobacterial consumption of human milk oligosaccharides demonstrates strain specific, preferential consumption of small chain glycans secreted in early human lactation. J. Agric. Food Chem. 2007, 55, 8914–8919. [Google Scholar] [CrossRef]
- Wolin, M.J.; Miller, T.L.; Yerry, S.; Zhang, Y.; Bank, S.; Weaver, G.A. Changes of fermentation pathways of fecal microbial communities associated with a drug treatment that increases dietary starch in the human colon. Appl. Environ. Microbiol. 1999, 65, 2807–2812. [Google Scholar] [CrossRef]
- Bäckhed, F.; Ley, R.E.; Sonnenburg, J.L.; Peterson, D.A.; Gordon, J.I. Host-bacterial mutualism in the human intestine. Science 2005, 307, 1915–1920. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.M.; De Souza, R.; Kendall, C.W.; Emam, A.; Jenkins, D.J. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Gill, R.K.; Mahmood, S.; Nagpaul, J.P.; Mahmood, A. Functional role of sialic acid in IgG binding to microvillus membranes in neonatal rat intestine. Neonatology 1999, 76, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Dall’Olio, F.; Malagolini, N.; Di Stefano, G.; Ciambella, M.; Serafini-Cessi, F. Postnatal development of rat colon epithelial cells is associated with changes in the expression of the β1, 4-N-acetylgalactosaminyltransferase involved in the synthesis of Sda antigen of α2, 6-sialyltransferase activity towards N-acetyl-lactosamine. Biochem. J. 1990, 270, 519–524. [Google Scholar] [CrossRef]
- Donovan, S.M. Human milk oligosaccharides–the plot thickens. Br. J. Nutr. 2009, 101, 1267–1269. [Google Scholar] [CrossRef]
- Kawashima, N.; Yoon, S.-J.; Itoh, K.; Nakayama, K.-I. Tyrosine Kinase Activity of Epidermal Growth Factor Receptor Is Regulated by GM3 Binding through Carbohydrate to Carbohydrate Interactions. J. Biol. Chem. 2009, 284, 6147–6155. [Google Scholar] [CrossRef]
- Ten Bruggencate, S.J.M.; Bovee-Oudenhoven, I.M.J.; Feitsma, A.L.; van Hoffen, E.; Schoterman, M.H.C. Functional role and mechanisms of sialyllactose and other sialylated milk oligosaccharides. Nutr. Rev. 2014, 72, 377–389. [Google Scholar] [CrossRef]
- Sakarya, S.; Göktürk, C.; Öztürk, T.; Ertugrul, M.B. Sialic acid is required for nonspecific adherence of Salmonella enterica ssp. enterica serovar Typhi on Caco-2 cells. FEMS Immunol. Med. Microbiol. 2010, 58, 330–335. [Google Scholar] [CrossRef]
- Newburg, D.S.; Ruiz-Palacios, G.M.; Morrow, A.L. Human milk glycans protect infants against enteric pathogens. Annu. Rev. Nutr. 2005, 25, 37–58. [Google Scholar] [CrossRef] [PubMed]
- Craft, K.M.; Thomas, H.C.; Townsend, S.D. Sialylated variants of lacto-N-tetraose exhibit antimicrobial activity against Group B Streptococcus. Org. Biomol. Chem. 2019, 17, 1893–1900. [Google Scholar] [CrossRef] [PubMed]
- Salcedo, J.; Barbera, R.; Matencio, E.; Alegría, A.; Lagarda, M.J. Gangliosides and sialic acid effects upon newborn pathogenic bacteria adhesion: An in vitro study. Food Chem. 2013, 136, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Coppa, G.V.; Zampini, L.; Galeazzi, T.; Facinelli, B.; Ferrante, L.; Capretti, R.; Orazio, G. Human milk oligosaccharides inhibit the adhesion to Caco-2 cells of diarrheal pathogens: Escherichia coli, Vibrio cholerae, and Salmonella fyris. Pediatric Res. 2006, 59, 377–382. [Google Scholar] [CrossRef]
- Martín-Sosa, S.; Martín, M.-J.; Hueso, P. The sialylated fraction of milk oligosaccharides is partially responsible for binding to enterotoxigenic and uropathogenic Escherichia coli human strains. J. Nutr. 2002, 132, 3067–3072. [Google Scholar] [CrossRef]
- Angeloni, S.; Ridet, J.; Kusy, N.; Gao, H.; Crevoisier, F.; Guinchard, S.; Kochhar, S.; Sigrist, H.; Sprenger, N. Glycoprofiling with micro-arrays of glycoconjugates and lectins. Glycobiology 2005, 15, 31–41. [Google Scholar] [CrossRef]
- Burger, O.; Weiss, E.; Sharon, N.; Tabak, M.; Neeman, I.; Ofek, I. Inhibition of Helicobacter pylori adhesion to human gastric mucus by a high-molecular-weight constituent of cranberry juice. Crit. Rev. Food Sci. Nutr. 2002, 42, 279–284. [Google Scholar] [CrossRef]
- Simon, P.; Goode, P.; Mobasseri, A.; Zopf, D. Inhibition of Helicobacter pylori binding to gastrointestinal epithelial cells by sialic acid-containing oligosaccharides. Infect. Immun. 1997, 65, 750–757. [Google Scholar] [CrossRef]
- Unemo, M.; Aspholm-Hurtig, M.; Ilver, D.; Bergström, J.; Borén, T.; Danielsson, D.; Teneberg, S. The sialic acid binding SabA adhesin of Helicobacter pylori is essential for nonopsonic activation of human neutrophils. J. Med. Chem. 2005, 280, 15390–15397. [Google Scholar]
- Koketsu, M.; Nitoda, T.; Sugino, H.; Juneja, L.R.; Kim, M.; Yamamoto, T.; Abe, N.; Kajimoto, T.; Wong, C.-H. Synthesis of a novel sialic acid derivative (sialylphospholipid) as an antirotaviral agent. J. Med. Chem. 1997, 40, 3332–3335. [Google Scholar] [CrossRef]
- Takahashi, K.; Ohashi, K.; Abe, Y.; Mori, S.; Taniguchi, K.; Ebina, T.; Nakagomi, O.; Terada, M.; Shigeta, S. Protective efficacy of a sulfated sialyl lipid (NMSO3) against human rotavirus-induced diarrhea in a mouse model. Antimicrob. Agents Chemother. 2002, 46, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-C.; Shun, C.-T.; Chien, C.-T.; Wang, T.-H. Effective prevention and treatment of Helicobacter pylori infection using a combination of catechins and sialic acid in AGS cells and BALB/c mice. J. Nutr. 2008, 138, 2084–2090. [Google Scholar] [CrossRef]
- Idota, T.; Kawakami, H.; Murakami, Y.; Sugawara, M. Inhibition of cholera toxin by human milk fractions and sialyllactose. Biosci. Biotechnol. Biochem. 1995, 59, 417–419. [Google Scholar] [CrossRef]
- Mysore, J.V.; Wigginton, T.; Simon, P.M.; Zopf, D.; Heman-Ackah, L.M.; Dubois, A. Treatment of Helicobacter pylori infection in rhesus monkeys using a novel antiadhesion compound. Gastroenterology 1999, 117, 1316–1325. [Google Scholar] [CrossRef]
- Newburg, D.S.; Peterson, J.A.; Ruiz-Palacios, G.M.; Matson, D.O.; Morrow, A.L.; Shults, J.; de Lourdes Guerrero, M.; Chaturvedi, P.; Newburg, S.O.; Scallan, C.D. Role of human-milk lactadherin in protectoin against symptomatic rotavirus infection. Lancet 1998, 351, 1160–1164. [Google Scholar] [CrossRef]
- Hester, S.N.; Chen, X.; Li, M.; Monaco, M.H.; Comstock, S.S.; Kuhlenschmidt, T.B.; Kuhlenschmidt, M.S.; Donovan, S.M. Human milk oligosaccharides inhibit rotavirus infectivity in vitro and in acutely infected piglets. Br. J. Nutr. 2013, 110, 1233–1242. [Google Scholar] [CrossRef]
- Videira, P.A.; Amado, I.F.; Crespo, H.J.; Algueró, M.C.; Dall’Olio, F.; Cabral, M.G.; Trindade, H. Surface α2-3-and α2-6-sialylation of human monocytes and derived dendritic cells and its influence on endocytosis. Glycoconj. J. 2008, 25, 259–268. [Google Scholar] [CrossRef]
- Cabral, M.G.; Silva, Z.; Ligeiro, D.; Seixas, E.; Crespo, H.; Carrascal, M.A.; Silva, M.; Piteira, A.R.; Paixão, P.; Lau, J.T. The phagocytic capacity and immunological potency of human dendritic cells is improved by α2, 6-sialic acid deficiency. Immunology 2013, 138, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Redelinghuys, P.; Antonopoulos, A.; Liu, Y.; Campanero-Rhodes, M.A.; McKenzie, E.; Haslam, S.M.; Dell, A.; Feizi, T.; Crocker, P.R. Early murine T-lymphocyte activation is accompanied by a switch from N-Glycolyl-to N-acetyl-neuraminic acid and generation of ligands for siglec-E. J. Biol. Chem. 2011, 286, 34522–34532. [Google Scholar] [CrossRef]
- Crocker, P.R.; Paulson, J.C.; Varki, A. Siglecs and their roles in the immune system. Nat. Rev. Immunol. 2007, 7, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.; Netravali, I.A.; Cariappa, A.; Mattoo, H. Siglecs and immune regulation. Annu. Rev. Immunol. 2012, 30, 357. [Google Scholar] [CrossRef] [PubMed]
- Böhm, S.; Schwab, I.; Lux, A.; Nimmerjahn, F. The role of sialic acid as a modulator of the anti-inflammatory activity of IgG. Semin. Immunopathol. 2012, 34, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Zenhom, M.; Hyder, A.; Kraus-Stojanowic, I.; Auinger, A.; Roeder, T.; Schrezenmeir, J. PPARγ-dependent peptidoglycan recognition protein 3 (PGlyRP3) expression regulates proinflammatory cytokines by microbial and dietary fatty acids. Immunobiology 2011, 216, 715–724. [Google Scholar] [CrossRef]
- Jantscher-Krenn, E.; Bode, L. Human milk oligosaccharides and their potential benefits for the breast-fed neonate. Minerva Pediatrica 2012, 64, 83–99. [Google Scholar]
- Schauer, R. Sialic Acids, Chemistry, Metabolism, and Function; Springer: Berlin/Heidelberg, Germany, 1982; p. xvii. [Google Scholar]
- Miller, J.B.; McVeagh, P. Human milk oligosaccharides: 130 reasons to breast-feed. Br. J. Nutr. 1999, 82, 333–335. [Google Scholar] [CrossRef]
- Wang, B.; McVeagh, P.; Petocz, P.; Brand-Miller, J. Brain ganglioside and glycoprotein sialic acid in breastfed compared with formula-fed infants. Am. J. Clin. Nutr. 2003, 78, 1024–1029. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yu, B.; Karim, M.; Hu, H.; Sun, Y.; McGreevy, P.; Petocz, P.; Held, S.; Brand-Miller, J. Dietary sialic acid supplementation improves learning and memory in piglets. Am. J. Clin. Nutr. 2007, 85, 561–569. [Google Scholar] [CrossRef]
- Scholtz, S.A.; Gottipati, B.S.; Gajewski, B.J.; Carlson, S.E. Dietary sialic acid and cholesterol influence cortical composition in developing rats. J. Nutr. 2013, 143, 132–135. [Google Scholar] [CrossRef]
- Sprenger, N.; Julita, M.; Donnicola, D.; Jann, A. Sialic acid feeding aged rats rejuvenates stimulated salivation and colon enteric neuron chemotypes. Glycobiology 2009, 19, 1492–1502. [Google Scholar] [CrossRef]
- Sakai, F.; Ikeuchi, Y.; Urashima, T.; Fujihara, M.; Ohtsuki, K.; Yanahira, S. Effects of feeding sialyllactose and galactosylated N-acetylneuraminic acid on swimming learning ability and brain lipid composition in adult rats. J. Appl. Glycosci. 2006, 53, 249–254. [Google Scholar] [CrossRef]
- Hiratsuka, S.; Honma, H.; Saitoh, Y.; Yasuda, Y.; Yokogoshi, H. Effects of dietary sialic acid in n-3 fatty acid-deficient dams during pregnancy and lactation on the learning abilities of their pups after weaning. J. Nutr. Sci. Vitaminol. 2013, 59, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Carlson, S.E.; House, S.G. Oral and intraperitoneal administration of N-acetylneuraminic acid: Effect on rat cerebral and cerebellar N-acetylneuraminic acid. J. Nutr. 1986, 116, 881–886. [Google Scholar] [CrossRef]
- Wang, B.; Brand-Miller, J.; McVeagh, P.; Petocz, P. Concentration and distribution of sialic acid in human milk and infant formulas. Am. J. Clin. Nutr. 2001, 74, 510–515. [Google Scholar] [CrossRef]
- Jahan, M.; Wynn, P.; Wang, B. Molecular characterization of the level of sialic acids N-acetylneuraminic acid, N-glycolylneuraminic acid, and ketodeoxynonulosonic acid in porcine milk during lactation. J. Dairy Sci. 2016, 99, 8431–8442. [Google Scholar] [CrossRef]
- Quesnel, H.; Brossard, L.; Valancogne, A.; Quiniou, N. Influence of some sow characteristics on within-litter variation of piglet birth weight. Animal 2008, 2, 1842–1849. [Google Scholar] [CrossRef] [PubMed]
- Mudd, A.T.; Salcedo, J.; Alexander, L.S.; Johnson, S.K.; Getty, C.M.; Chichlowski, M.; Berg, B.M.; Barile, D.; Dilger, R.N. Porcine milk oligosaccharides and sialic acid concentrations vary throughout lactation. Front. Nutr. 2016, 3, 39. [Google Scholar] [CrossRef]
- Ji, S.; Wang, F.; Chen, Y.; Yang, C.; Zhang, P.; Zhang, X.; Troy, F.A.; Wang, B. Developmental changes in the level of free and conjugated sialic acids, Neu5Ac, Neu5Gc and KDN in different organs of pig: A LC-MS/MS quantitative analyses. Glycoconj. J. 2017, 34, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Jahan, M.; Thomson, P.C.; Wynn, P.C.; Wang, B. The non-human glycan, N-glycolylneuraminic acid (Neu5Gc), is not expressed in all organs and skeletal muscles of nine animal species. Food Chem. 2020, 343, 128439. [Google Scholar] [CrossRef]
- Altheide, T.K.; Hayakawa, T.; Mikkelsen, T.S.; Diaz, S.; Varki, N.; Varki, A. System-wide genomic and biochemical comparisons of sialic acid biology among primates and rodents: Evidence for two models of rapid evolution. J. Biol. Chem. 2006, 281, 25689–25702. [Google Scholar] [CrossRef] [PubMed]
- Arai, A.; Tanaka, K.; Ikeuchi, T.; Igarashi, S.; Kobayashi, H.; Asaka, T.; Saito, M.; Tanaka, H.; Kawasaki, S.; Uyama, E. A novel mutation in the GNE gene and a linkage disequilibrium in Japanese pedigrees. Ann. Neurol. 2002, 52, 516–519. [Google Scholar] [CrossRef]
- Huizing, M.; Rakocevic, G.; Sparks, S.E.; Mamali, I.; Shatunov, A.; Goldfarb, L.; Krasnewich, D.; Gahl, W.A.; Dalakas, M.C. Hypoglycosylation of α-dystroglycan in patients with hereditary IBM due to GNE mutations. Mol. Genet. Metab. 2004, 81, 196–202. [Google Scholar] [CrossRef]
- Martin-Sosa, S.; Martín, M.-J.; García-Pardo, L.-A.; Hueso, P. Sialyloligosaccharides in human and bovine milk and in infant formulas: Variations with the progression of lactation. J. Dairy Sci. 2003, 86, 52–59. [Google Scholar] [CrossRef]
- Penner, J.; Mantey, L.R.; Elgavish, S.; Ghaderi, D.; Cirak, S.; Berger, M.; Krause, S.; Lucka, L.; Voit, T.; Mitrani-Rosenbaum, S. Influence of UDP-GlcNAc 2-epimerase/ManNAc kinase mutant proteins on hereditary inclusion body myopathy. Biochemistry 2006, 45, 2968–2977. [Google Scholar] [CrossRef] [PubMed]
- Martín-Sosa, S.; Martín, M.-J.; García-Pardo, L.A.; Hueso, P. Distribution of sialic acids in the milk of Spanish mothers of full term infants during lactation. J. Pediatr. Gastroenterol. Nutr. 2004, 39, 499–503. [Google Scholar] [CrossRef]
- Nakamura, T.; Kawase, H.; Kimura, K.; Watanabe, Y.; Ohtani, M.; Arai, I.; Urashima, T. Concentrations of sialyloligosaccharides in bovine colostrum and milk during the prepartum and early lactation. J. Dairy Sci. 2003, 86, 1315–1320. [Google Scholar] [CrossRef]
- de Sousa, Y.R.F.; da Silva Vasconcelos, M.A.; Costa, R.G.; de Azevedo Filho, C.A.; de Paiva, E.P.; Queiroga, R.d.C.R.d.E. Sialic acid content of goat milk during lactation. Livest. Sci. 2015, 177, 175–180. [Google Scholar] [CrossRef]
- Asakuma, S.; Ueda, Y.; Akiyama, F.; Uemura, Y.; Miyaji, M.; Nakamura, M.; Murai, M.; Urashima, T. Short communication: Effect of grazing on the concentrations of total sialic acid and hexose in bovine milk. J. Dairy Sci. 2010, 93, 4850–4854. [Google Scholar] [CrossRef] [PubMed]
- Puente, R.; Garcia-Pardo, L.-A.; Hueso, P. Gangliosides in bovine milk. Changes in content and distribution of individual ganglioside levels during lactation. Biol. Chem. 1992, 373, 283–288. [Google Scholar] [CrossRef]
- Puente, R.; Garcia-Pardo, L.A.; Rueda, R.; Gil, A.; Hueso, P. Changes in ganglioside and sialic acid contents of goat milk during lactation. J. Dairy Sci. 1994, 77, 39–44. [Google Scholar] [CrossRef]
- Wei, J.; Wang, Z.A.; Wang, B.; Jahan, M.; Wang, Z.; Wynn, P.C.; Du, Y. Characterization of porcine milk oligosaccharides over lactation between primiparous and multiparous female pigs. Sci. Rep. 2018, 8, 4688. [Google Scholar] [CrossRef]
- Difilippo, E.; Pan, F.; Logtenberg, M.; Willems, R.; Braber, S.; Fink-Gremmels, J.; Schols, H.A.; Gruppen, H. Milk oligosaccharide variation in sow milk and milk oligosaccharide fermentation in piglet intestine. J. Agric. Food Chem. 2016, 64, 2087–2093. [Google Scholar] [CrossRef]
- Tao, N.; Ochonicky, K.L.; German, J.B.; Donovan, S.M.; Lebrilla, C.B. Structural determination and daily variations of porcine milk oligosaccharides. J. Agric. Food Chem. 2010, 58, 4653–4659. [Google Scholar] [CrossRef]
- Johanson, B. Isolation of an iron-containing red protein from human milk. Acta Chem. Scand. 1960, 14, 510–512. [Google Scholar] [CrossRef]
- Wolfson, D.R.; Robbins, J.B. Heterogeneity of human lactoferrin due to differences in sialic acid content. Pediatric Res. 1971, 5, 514–517. [Google Scholar] [CrossRef]
- Yu, T.; Guo, C.; Wang, J.; Hao, P.; Sui, S.; Chen, X.; Zhang, R.; Wang, P.; Yu, G.; Zhang, L. Comprehensive characterization of the site-specific N-glycosylation of wild-type and recombinant human lactoferrin expressed in the milk of transgenic cloned cattle. Glycobiology 2011, 21, 206–224. [Google Scholar] [CrossRef] [PubMed]
- Van der Strate, B.; Beljaars, L.; Molema, G.; Harmsen, M.; Meijer, D. Antiviral activities of lactoferrin. Antivir. Res. 2001, 52, 225–239. [Google Scholar] [CrossRef]
- Pierce, A.; Legrand, D.; Mazurier, J. Lactoferrin: A multifunctional protein. Med. Sci. 2009, 25, 361–369. [Google Scholar]
- Yang, C.; Zhu, X.; Liu, N.; Chen, Y.; Gan, H.; Troy II, F.A.; Wang, B. Lactoferrin up-regulates intestinal gene expression of brain-derived neurotrophic factors BDNF, UCHL1 and alkaline phosphatase activity to alleviate early weaning diarrhea in postnatal piglets. J. Nutr. Biochem. 2014, 25, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Conesa, C.; Sánchez, L.; Rota, C.; Pérez, M.-D.; Calvo, M.; Farnaud, S.; Evans, R.W. Isolation of lactoferrin from milk of different species: Calorimetric and antimicrobial studies. Co Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2008, 150, 131–139. [Google Scholar] [CrossRef]
- Elliot, J.; Senft, B.; Erhardt, G.; Fraser, D. Isolation of lactoferrin and its concentration in sows’ colostrum and milk during a 21-day lactation. J. Anim. Sci. 1984, 59, 1080–1084. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jahan, M.; Francis, N.; Wang, B. Milk lactoferrin concentration of primiparous and multiparous sows during lactation. J. Dairy Sci. 2020, 103, 7521–7530. [Google Scholar] [CrossRef]
- Kell, D.B.; Heyden, E.L.; Pretorius, E. The biology of lactoferrin, an iron-binding protein that can help defend against viruses and bacteria. Front. Immunol. 2020, 11, 1221. [Google Scholar] [CrossRef] [PubMed]
- Telang, S. Lactoferrin: A critical player in neonatal host defense. Nutrients 2018, 10, 1228. [Google Scholar] [CrossRef]
- Yamauchi, K.; Wakabayashi, H.; Shin, K.; Takase, M. Bovine lactoferrin: Benefits and mechanism of action against infections. Biochem. Cell Biol. 2006, 84, 291–296. [Google Scholar] [CrossRef]
- Pammi, M.; Suresh, G. Enteral lactoferrin supplementation for prevention of sepsis and necrotizing enterocolitis in preterm infants. Cochrane Database Syst. Rev. 2020. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, B.; Yang, C.; Shi, Y.; Dong, Z.; Troy, F.A. Functional Correlates and Impact of Dietary Lactoferrin Intervention and Its Concentration-Dependence on Neurodevelopment and Cognition in Neonatal Piglets. Mol. Nutr. Food Res. 2021, 65, 2001099. [Google Scholar] [CrossRef] [PubMed]
- Jahan, M.; Kracht, S.; Ho, Y.; Haque, Z.; Bhattachatyya, B.N.; Wynn, P.C.; Wang, B. Dietary lactoferrin supplementation to gilts during gestation and lactation improves pig production and immunity. PLoS ONE 2017, 12, e0185817. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, Z.; Zhu, X.; Shi, Y.; Tian, D.; Zhao, F.; Liu, N.; Hüppi, P.S.; Troy, F.A., II; Wang, B. Lactoferrin promotes early neurodevelopment and cognition in postnatal piglets by upregulating the bdnf signaling pathway and polysialylation. Mol. Neurobiol. 2014, 52, 256–269. [Google Scholar] [CrossRef]
- Reznikov, E.A.; Comstock, S.S.; Yi, C.; Contractor, N.; Donovan, S.M. Dietary bovine lactoferrin increases intestinal cell proliferation in neonatal piglets. J. Nutr. 2014, 144, 1401–1408. [Google Scholar] [CrossRef]
- Tang, X.-S.; Shao, H.; Li, T.-J.; Tang, Z.-R.; Huang, R.-L.; Wang, S.-P.; Kong, X.-F.; Wu, X.; Yin, Y.-L. Dietary supplementation with bovine lactoferrampin–lactoferricin produced by Pichia pastoris fed-batch fermentation affects intestinal microflora in weaned piglets. Appl. Biochem. Biotechnol. 2012, 168, 887–898. [Google Scholar] [CrossRef]
- Tang, Z.; Yin, Y.; Zhang, Y.; Huang, R.; Sun, Z.; Li, T.; Chu, W.; Kong, X.; Li, L.; Geng, M. Effects of dietary supplementation with an expressed fusion peptide bovine lactoferricin–lactoferrampin on performance, immune function and intestinal mucosal morphology in piglets weaned at age 21 d. Br. J. Nutr. 2009, 101, 998–1005. [Google Scholar] [CrossRef] [PubMed]
- Shan, T.; Wang, Y.; Wang, Y.; Liu, J.; Xu, Z. Effect of dietart lactoferrin on the immune functions and serum iron level of weanling piglets. J. Anim. Sci. 2007, 85, 2140–2146. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, C.; An, Z.; Liu, J.; Feng, J. Effect of dietary bovine lactoferrin on performance and antioxidant status of piglets. Anim. Feed Sci. Technol. 2008, 140, 326–336. [Google Scholar] [CrossRef]
- Wang, Y.; Shan, T.; Xu, Z.; Liu, J.; Feng, J. Effect of lactoferrin on the growth performance, intestinal morphology, and expression of PR-39 and protegrin-1 genes in weaned piglets. J. Anim. Sci. 2006, 84, 2636–2641. [Google Scholar] [CrossRef]
- Wang, Y.-Z.; Shan, T.-Z.; Xu, Z.-R.; Feng, J.; Wang, Z.-Q. Effects of the lactoferrin (LF) on the growth performance, intestinal microflora and morphology of weanling pigs. Anim. Feed Sci. Technol. 2007, 135, 263–272. [Google Scholar] [CrossRef]
- Giuffrida, F.; Elmelegy, I.M.; Thakkar, S.K.; Marmet, C.; Destaillats, F. Longitudinal evolution of the concentration of gangliosides GM3 and GD3 in human milk. Lipids 2014, 49, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Ledeen, R. The chemistry of gangliosides: A review. J. Am. Oil Chem. Soc. 1966, 43, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Lacomba, R.; Salcedo, J.; Alegría, A.; Lagarda, M.J.; Barberá, R.; Matencio, E. Determination of sialic acid and gangliosides in biological samples and dairy products: A review. J. Pharm. Biomed. Anal. 2010, 51, 346–357. [Google Scholar] [CrossRef] [PubMed]
- McJarrow, P.; Schnell, N.; Jumpsen, J.; Clandinin, T. Influence of dietary gangliosides on neonatal brain development. Nutr. Rev. 2009, 67, 451–463. [Google Scholar] [CrossRef]
- Rueda, R.; Puente, R.; Hueso, P.; Maldonado, J.; Gil, A. New data on content and distribution of gangliosides in human milk. Biol. Chem. Hoppe-Seyler 1995, 376, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.L.; Izumi, T. Variation of the ganglioside compositions of human milk, cow’s milk and infant formulas. Early Hum. Dev. 2000, 57, 25–31. [Google Scholar] [CrossRef]
- Ma, L.; Liu, X.; MacGibbon, A.K.; Rowan, A.; McJarrow, P.; Fong, B.Y. Lactational changes in concentration and distribution of ganglioside molecular species in human breast milk from Chinese mothers. Lipids 2015, 50, 1145–1154. [Google Scholar] [CrossRef]
- Rueda, R. The role of dietary gangliosides on immunity and the prevention of infection. Br. J. Nutr. 2007, 98, S68–S73. [Google Scholar] [CrossRef] [PubMed]
- Sonnino, S.; Mauri, L.; Ciampa, M.G.; Prinetti, A. Gangliosides as regulators of cell signaling: Ganglioside-protein interactions or ganglioside-driven membrane organization? J. Neurochem. 2013, 124, 432–435. [Google Scholar] [CrossRef]
- Mendez-Otero, R.; Pimentel-Coelho, P.M.; Ukraintsev, S.; McJarrow, P. Role of gangliosides in neurological development and the influence of dietary sources. In Nutrition in Infancy; Watson, J.L., Grimble, G., Preedy, R., Zibadi, S., Eds.; Springer: London, UK, 2013; pp. 105–118. [Google Scholar]
- Gurnida, D.A.; Rowan, A.M.; Idjradinata, P.; Muchtadi, D.; Sekarwana, N. Association of complex lipids containing gangliosides with cognitive development of 6-month-old infants. Early Hum. Dev. 2012, 88, 595–601. [Google Scholar] [CrossRef] [PubMed]
| Study Model | Pathogen | Reference |
|---|---|---|
| In vitro | Group B Streptococcus | [54] |
| In vitro | Salmonella | [55,56] |
| Various E. coli strains (S-fimbriated strains in particular) | [55,56,57,58] | |
| Vibrio cholera | [55] | |
| Helicobacter pylori | [55,59,60,61] | |
| Campylobacter Jejuni | [55] | |
| Rotavirus | [62,63] | |
| Mice | H. pylori | [64] |
| Rotavirus | [53,63,65] | |
| cholera toxin (specific for SL) | [65] | |
| Rhesus monkey | H. pylori | [66] |
| Human infant | Rotavirus | [67] |
| Pig | Rotavirus | [68] |
| Animal | Dose of Sia | Duration of Treatment | Effect | References |
|---|---|---|---|---|
| Piglet: 3 days old, male (average weight 1.5–2.4 kg) | Sia @40, 85, 180 or 240 mg/Kg body wt/day | 35 days | Increased learning and memory function when exposed to eight arm radial maze learning tests, increased brain sialylated glycoprotein and also increased levels of mRNA expression of uridine-diphospho-N-acetlylglucosamine-2-epimerase, a key enzyme in the biosynthetic pathway of Sia, in the brain and liver. | [80] |
| Rat: 17 days old | Sia @20, 40 or 60 and 240 mg/Kg body wt/day | 16 days | Increased cortical ganglioside Sia content. | [81] |
| Rat: 2 years old | Sia @ 0.8 g/100 g diet | 3 weeks | Sia feeding in aged rats normalized brain gangliosides Sia levels to the levels measured in young rats. | [82] |
| Rat: 9 weeks old (average weight 300 g) | Galactosylated Sia or SL @ 1% of diet | 2 weeks | Improved learning ability in a swimming learning test, which was associated with increased Sia and ganglioside content of the brain. | [83] |
| Rat: 12 weeks old pregnant rat (fed with n-3 fatty acid-deficient diet for 3 weeks before mating) | Sia @ 1% of water ad libitum | Throughout pregnancy and lactation | Improved the recognition index of novel object recognition test in rat pups after weaning. Increased the level of dimethylacetal (from plasmalogen) in the cerebral cortex of weaned pups. There was no effect in the total Sia content in the brain cortex or hippocampus of rat pups. | [84] |
| Rat: 14 days | Sia @ 20 mg/Kg body wt/day | 8 days | Intraperitoneally or orally administered Sia in rat pups resulted in increased cerebral and cerebellar ganglioside concentration. | [85] |
| Age of Piglet | Type and Dose of LF | Duration of Treatment | Effect | References |
|---|---|---|---|---|
| Postnatal piglet (3 days) | bLF @ 155 mg/Kg body wt/day and 385 mg/Kg body wt/day | 35 days |
| Chen et al. [119] |
| Primiparous sows (gilts) (average weight 151.8 kg ± 15.1 kg) | LF top dressed @1 g/day | Day 1 post mating until 21 days post lactation period (total ~135 days) |
| Jahan et al. [120] |
| Postnatal piglet (3 days) | bLF @ 155 mg/Kg body wt/day and 385 mg/Kg body wt/day | 35 days |
| Yang et al. [111] |
| Postnatal piglet (3 days) | bLF @ 155 mg/Kg body wt/day | 35 days |
| Chen et al. [121] |
| Newborn piglet from day 1 of age | bLF @ 155 mg/Kg body wt/day and 385 mg/Kg body wt/day | 14 days |
| Reznikov et al. [122] |
| Weaned piglet (21 days) | Recombinant bovine lactoferrampin-lactoferricin (LFA-LFC-Lactoferrin derivative) @ 100 mg/Kg, 3 times/day | 21 days | Increases the intestinal microbiota by increasing the Lactobacillus and Bifidobacterium in the chime of GIT. | Tang et al. [123] |
| Weaned piglet (21 days) | Lactoferrin derivative LFA-LFC @ 100 mg/Kg, 3 times/day | 21 days |
| Tang et al. [124] |
| Weaned female piglet (28 days) | bLF @ 1 g//Kg body wt/day | 30 days |
| Shan et al. [125] |
| Female piglet (35 days) | bLF @ 1250 or 2500 mg/Kg body wt/day | 30 days |
| Wang et al. [126] |
| Weaned piglet | 1 g/kg body wt/day | 15 days |
| Wang et al. [127] |
| Weaned piglet | 1 g/kg body wt/day | 30 days |
| Wang et al. [128] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jahan, M.; Francis, N.; Wynn, P.; Wang, B. The Potential for Sialic Acid and Sialylated Glycoconjugates as Feed Additives to Enhance Pig Health and Production. Animals 2021, 11, 2318. https://doi.org/10.3390/ani11082318
Jahan M, Francis N, Wynn P, Wang B. The Potential for Sialic Acid and Sialylated Glycoconjugates as Feed Additives to Enhance Pig Health and Production. Animals. 2021; 11(8):2318. https://doi.org/10.3390/ani11082318
Chicago/Turabian StyleJahan, Marefa, Nidhish Francis, Peter Wynn, and Bing Wang. 2021. "The Potential for Sialic Acid and Sialylated Glycoconjugates as Feed Additives to Enhance Pig Health and Production" Animals 11, no. 8: 2318. https://doi.org/10.3390/ani11082318
APA StyleJahan, M., Francis, N., Wynn, P., & Wang, B. (2021). The Potential for Sialic Acid and Sialylated Glycoconjugates as Feed Additives to Enhance Pig Health and Production. Animals, 11(8), 2318. https://doi.org/10.3390/ani11082318








