In Vitro Antibacterial Effect of the Methanolic Extract of the Korean Soybean Fermented Product Doenjang against Staphylococcus aureus
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Methanol Extraction of Isoflavones and Soyasaponins from the Fermented Soybean Paste Doenjang
2.2. Ultra-High Performance Liquid Chromatography/Mass Spectrometry of Isoflavones and Soyasaponins in the MED
2.3. Bacterial Strains and Their Maintenance
2.4. Testing the Antibacterial Effects of the MED
2.5. Determination of the Minimum Inhibitory Concentration of Methanolic Extract Obtained from Doenjang
2.6. Evaluation of Bacterial Growth under Various Concentrations of the MED
3. Results
3.1. Qualitative and Quantitative Profile of Isoflavones and Soyasaponins Detected in the MED
3.2. Antistaphylococcal Action of MED
3.3. Growth of Staphylococcus Aureus Strains with Various Concentrations of MED
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lakhundi, S.; Kunyan, Z. Methicillin-resistant Staphylococcus aureus: Molecular characterization, evolution, and epidemiology. Clin. Microbiol. Rev. 2018, 31, e00020-18. [Google Scholar] [CrossRef]
- Tong, S.Y.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [PubMed]
- Anjum, M.F.; Marco-Jimenez, F.; Duncan, D.; Marín, C.; Smith, R.P.; Evans, S. Livestock-associated methicillin-resistant Staphylococcus aureus from animals and animal products in the UK. Front. Microbiol. 2019, 10, 2136. [Google Scholar] [CrossRef] [PubMed]
- Huijsdens, X.W.; Van Dijke, B.J.; Spalburg, E.; van Santen-Verheuvel, M.G.; Heck, M.E.; Pluister, G.N.; Voss, A.; Wannet, W.J.B.; De Neeling, A.J. Community-acquired MRSA and pig-farming. Ann. Clin. Microbiol. Antimicrob. 2006, 5, 26. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kadariya, J.; Smith, T.C.; Thapaliya, D. Staphylococcus aureus and staphylococcal food-borne disease: An ongoing challenge in public health. Biomed. Res. Int. 2014, 2014, 827965. [Google Scholar] [CrossRef]
- Nemati, M.; Hermans, K.; Lipinska, U.; Denis, O.; Deplano, A.; Struelens, M.; Devriese, L.A.; Pasmans, F.; Haesebrouck, F. Antimicrobial resistance of old and recent Staphylococcus aureus isolates from poultry: First detection of livestock-associated methicillin-resistant strain ST398. Antimicrob. Agents Chemother. 2008, 52, 3817–3819. [Google Scholar] [CrossRef]
- Fluit, A.C. Livestock-associated Staphylococcus aureus. Clin. Microbiol. Infect. 2012, 18, 735–744. [Google Scholar] [CrossRef]
- Haag, A.F.; Fitzgerald, J.R.; Penadés, J.R. Staphylococcus aureus in animals. Microbiol. Spectr. 2019, 7, GPP3-0060-2019. [Google Scholar] [CrossRef] [PubMed]
- ECDC. Staphylococcus aureus . In Antimicrobial Resistance in the EU/EEA (EARS-Net)—Annual Epidemiological Report 2019, 1st ed.; European Centre for Disease Prevention and Control, Ed.; ECDC: Stockholm, Sweden, 2020; Volume 1, pp. 21–22. [Google Scholar]
- Cassini, A.; Hogberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef]
- Harkins, C.P.; Pichon, B.; Doumith, M.; Parkhill, J.; Westh, H.; Tomasz, A.; de Lencastre, H.; Bentley, S.D.; Kearns, A.M.; Holden, M.T.G. Methicillin-resistant Staphylococcus aureus emerged long before the introduction of methicillin into clinical practice. Genome Biol. 2017, 18, 130. [Google Scholar] [CrossRef]
- Malachowa, N.; DeLeo, F.R. Mobile genetic elements of Staphylococcus aureus. Cell. Mol. Life Sci. 2010, 67, 3057–3071. [Google Scholar] [CrossRef]
- Mwangi, M.M.; Wu, S.W.; Zhou, Y.; Sieradzki, K.; de Lencastre, H.; Richardson, P.; Bruce, D.; Rubin, E.; Myers, E.; Siggia, E.D.; et al. Tracking the in vivo evolution of multidrug resistance in Staphylococcus aureus by whole-genome sequencing. Proc. Natl. Acad. Sci. USA 2007, 104, 9451–9456. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.A.; Sharma-Kuinkel, B.K.; Maskarinec, S.A.; Eichenberger, E.M.; Shah, P.P.; Carugati, M.; Holland, T.L.; Fowler, V.G. Methicillin-resistant Staphylococcus aureus: An overview of basic and clinical research. Nat. Rev. Microbiol. 2019, 17, 203–218. [Google Scholar] [CrossRef]
- Clauss, M.; Tafin, U.F.; Bizzini, A.; Trampuz, A.; Ilchmann, T. Biofilm formation by staphylococci on fresh, fresh-frozen and processed human and bovine bone grafts. Eur. Cell Mater. 2013, 25, 159–166. [Google Scholar] [CrossRef]
- Millet, S.; Maertens, L. The European ban on antibiotic growth promoters in animal feed: From challenges to opportunities. Vet. J. 2011, 187, 143–144. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Saleem, M.; Nazir, M.; Ali, M.S.; Hussain, H.; Lee, Y.S.; Riaz, N.; Jabbar, A. Antimicrobial natural products: An update on future antibiotic drug candidates. Nat. Prod. Rep. 2010, 27, 238–254. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Han, R.; Xu, Y.; Li, N.; Wang, J.; Dan, W. Recent progress of antibacterial natural products: Future antibiotics candidates. Bioorg. Chem. 2020, 101, 103922. [Google Scholar] [CrossRef]
- Gohel, V.; Singh, A.; Vimal, M.; Ashwini, P.; Chhatpar, H.S. Bioprospecting and antifungal potential of chitinolytic microorganisms. Afr. J. Biotechnol. 2006, 5, 54–72. [Google Scholar]
- Toghyani, M.; Toghyani, M.; Gheisari, A.; Ghalamkari, G.; Eghbalsaied, S. Evaluation of cinnamon and garlic as antibiotic growth promoter substitutions on performance, immune responses, serum biochemical and haematological parameters in broiler chicks. Livest. Sci. 2011, 138, 167–173. [Google Scholar] [CrossRef]
- Jarriyawattanachaikul, W.; Chaveerach, P.; Chokesajjawatee, N. Antimicrobial activity of Thai-herbal plants against food-borne pathogens E. coli, S. aureus and C. jejuni. Agric. Agric. Sci. Procedia 2016, 11, 20–24. [Google Scholar] [CrossRef]
- Ghasemi, H.A.; Kasani, N.; Taherpour, K. Effects of black cumin seed (Nigella sativa L.), a probiotic, a prebiotic and a synbiotic on growth performance, immune response and blood characteristics of male broilers. Livest. Sci. 2014, 164, 128–134. [Google Scholar] [CrossRef]
- Peng, Q.Y.; Li, J.D.; Li, Z.; Duan, Z.Y.; Wu, Y.P. Effects of dietary supplementation with oregano essential oil on growth performance, carcass traits and jejunal morphology in broiler chickens. Anim. Feed Sci. Technol. 2016, 214, 148–153. [Google Scholar] [CrossRef]
- Emami, N.K.; Samie, A.; Rahmani, H.R.; Ruiz-Feria, C.A. The effect of peppermint essential oil and fructooligosaccharides, as alternatives to virginiamycin, on growth performance, digestibility, gut morphology and immune response of male broilers. Anim. Feed Sci. Technol. 2012, 175, 57–64. [Google Scholar] [CrossRef]
- Marinho, M.; Lordelo, M.; Cunha, L.; Freire, J. Microbial activity in the gut of piglets: I. Effect of prebiotic and probiotic supplementation. Livest. Sci. 2007, 108, 236–239. [Google Scholar] [CrossRef]
- Bomba, A.; Jonecova, Z.; Koscova, J.; Nemcova, R.; Gancarikova, S.; Mudronova, D.; Scirankova, L.; Buleca, V.; Lazar, G.; Posivak, J.; et al. The improvement of probiotics efficacy by synergistically acting components of natural origin: A review. Biologia 2006, 61, 729–734. [Google Scholar] [CrossRef]
- Lemke, S.L.; Mayura, K.; Reeves, W.R.; Wang, N.; Fickey, C.; Phillips, T.D. Investigation of organophilic montmorillonite clay inclusion in zearalenonecontaminated diets using the mouse uterine weight bioassay. J. Toxicol. Environ. Health 2001, 62, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.F.; Riis, A.L.; Bresson, S.; Højbjerg, O.; Jensen, B.B. Feeding organic acids enhances the barrier function against pathogenic bacteria of the piglet stomach. Livest. Sci. 2007, 108, 206–209. [Google Scholar] [CrossRef]
- Singh, B.P.; Yadav, D.; Vij, S. Soybean Bioactive Molecules: Current Trend and Future Prospective. In Bioactive Molecules in Food, 1st ed.; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer: Cham, Switzerland, 2017; pp. 1–29. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, H.; Xie, M. Antibacterial mechanism of soybean isoflavone on Staphylococcus aureus. Arch. Microbiol. 2010, 192, 893–898. [Google Scholar] [CrossRef]
- Jang, H.H.; Noh, H.; Kim, H.W.; Cho, S.Y.; Kim, H.J.; Lee, S.H.; Lee, S.H.; Gunter, M.J.; Ferrari, P.; Scalbert, A.; et al. Metabolic tracking of isoflavones in soybean products and biosamples from healthy adults after fermented soybean consumption. Food Chem. 2020, 330, 127317. [Google Scholar] [CrossRef]
- Teekachunhatean, S.; Hanprasertpong, N.; Teekachunhatean, T. Factors affecting isoflavone content in soybean seeds grown in Thailand. Int. J. Agron. 2013, 2013, 163573. [Google Scholar] [CrossRef]
- Surh, J.; Kim, Y.K.L.; Kwon, H. Korean Fermented Foods: Kimchi and Doenjang. In Handbook of Fermented Functional Foods, 2nd ed.; Farnworth, E.R., Ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 333–351. [Google Scholar]
- Park, K.Y.; Jung, K.O.; Rhee, S.H.; Choi, Y.H. Antimutagenic effects of doenjang (Korean fermented soypaste) and its active compounds. Mutat. Res. 2003, 523, 43–53. [Google Scholar] [CrossRef]
- Huang, Q.; Zhang, H.; Xue, D. Enhancement of antioxidant activity of Radix Puerariae and red yeast rice by mixed fermentation with Monascus purpureus. Food Chem. 2017, 226, 89–94. [Google Scholar] [CrossRef]
- Křížová, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef]
- Verdrengh, M.; Collins, L.V.; Bergin, P.; Tarkowski, A. Phytoestrogen genistein as an anti-staphylococcal agent. Microbes Infect. 2004, 6, 86–92. [Google Scholar] [CrossRef]
- Hong, H.; Landauer, M.R.; Foriska, M.A.; Ledney, G.D. Antibacterial activity of the soy isoflavone genistein. J. Basic Microbiol. 2006, 46, 329–335. [Google Scholar] [CrossRef]
- Hummelova, J.; Rondevaldova, J.; Balastikova, A.; Lapcik, O.; Kokoska, L. The relationship between structure and in vitro antibacterial activity of selected isoflavones and their metabolites with special focus on antistaphylococcal effect of demethyltexasin. Lett. Appl. Microbiol. 2015, 60, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Ulanowska, K.; Tkaczyk, A.; Konopa, G.; Wegrzyn, G. Differential antibacterial activity of genistein arising from global inhibition of DNA, RNA and protein synthesis in some bacterial strains. Arch. Microbiol. 2006, 184, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Gayibova, S.; Ivanišová, E.; Árvay, J.; Hŕstková, M.; Slávik, M.; Petrová, J.; Hleba, L.; Tóth, T.; Kačániová, M.; Aripov, T. In vitro screening of antioxidant and antimicrobial activities of medicinal plants growing in Slovakia. J. Microbiol. Biotechnol. Food Sci. 2019, 8, 1281–1289. [Google Scholar] [CrossRef]
- Hassan, S.M.; Byrd, J.A.; Cartwright, A.L.; Bailey, C.A. Hemolytic and antimicrobial activities differ among saponin-rich extracts from guar, quillaja, yucca, and soybean. Appl. Biochem. Biotechnol. 2010, 162, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Cha, Y.S.; Yang, J.; Back, H.I.; Kim, S.R.; Kim, M.G.; Jung, S.J.; Song, W.O.; Chae, S.W. Visceral fat and body weight are reduced in overweight adults by the supplementation of Doenjang, a fermented soybean paste. Nutr. Res. Pract. 2012, 6, 520–526. [Google Scholar] [CrossRef]
- Park, N.Y.; Rico, C.W.; Lee, S.C.; Kang, M.Y. Comparative effects of doenjang prepared from soybean and brown rice on the body weight and lipid metabolism in high fat-fed mice. J. Clin. Biochem. Nutr. 2012, 51, 12–24. [Google Scholar] [CrossRef][Green Version]
- Mun, E.G.; Park, J.E.; Cha, Y.S. Effects of Doenjang, a traditional Korean soybean paste, with high-salt diet on blood pressure in Sprague–Dawley rats. Nutrients 2019, 11, 2745. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, H.; Kim, J.C. Anti-inflammatory effect of water extracts obtained from doenjang in LPS-stimulated RAW 264.7 cells. Food Sci. Technol. 2019, 39, 947–954. [Google Scholar] [CrossRef]
- Nam, Y.R.; Won, S.B.; Chung, Y.S.; Kwak, C.S.; Kwon, Y.H. Inhibitory effects of Doenjang, Korean traditional fermented soybean paste, on oxidative stress and inflammation in adipose tissue of mice fed a high-fat diet. Nutr. Res. Pract. 2015, 9, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Koh, E.; Surh, J.; Kim, Y.K.L.; Kwon, H. Distribution of isoflavones and coumestrol in legumes and their products consumed in Korea. Food Sci. Biotechnol. 2003, 12, 278–284. [Google Scholar]
- Wadhwani, T.; Desai, K.; Patel, D.; Lawani, D.; Bahaley, P.; Joshi, P.; Kothari, V. Effect of various solvents on bacterial growth in context of determining MIC of various antimicrobials. Internet J. Microbiol. 2009, 7, 1–13. [Google Scholar]
- Rondevaldova, J.; Hummelova, J.; Tauchen, J.; Kokoska, L. In vitro antistaphylococcal synergistic effect of isoflavone metabolite demethyltexasin with amoxicillin and oxacillin. Microb. Drug Resist. 2018, 24, 24–29. [Google Scholar] [CrossRef]
- Rondevaldova, J.; Novy, P.; Urban, J.; Kokoska, L. Determination of anti-staphylococcal activity of thymoquinone in combinations with antibiotics by checkerboard method using EVA capmat as a vapor barrier. Arab. J. Chem. 2017, 10, 566–572. [Google Scholar] [CrossRef]
- Frankova, A.; Vistejnova, L.; Merinas-Amoc, T.; Leheckova, Z.; Doskocil, I.; Wong Soon, J.; Kudera, T.; Laupua, F.; Alonso-Moraga, A.; Kokoska, L. In vitro antibacterial activity of extracts from samoan medicinal plants and their effect on proliferation and migration of human fibroblasts. J. Ethnopharmacol. 2021, 264, 113220. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard M07, 11th ed.; CLSI: Wayne, PA, USA, 2018; p. 91. [Google Scholar]
- Cos, P.; Vlietinck, A.J.; Berghe, D.V.; Maes, L. Anti-infective potential of natural products: How to develop a stronger in vitro ‘proof-of-concept’. J. Ethnopharmacol. 2006, 106, 290–302. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Methods for Determining Bactericidal Activity of Antimicrobial Agents; Approved Guideline M26-A, 1st ed.; CLSI: Wayne, PA, USA, 1999; p. 32. [Google Scholar]
- Landers, T.F.; Cohen, B.; Wittum, T.E.; Larson, E.L. A review of antibiotic use in food animals: Perspective, policy, and potential. Public Health Rep. 2012, 127, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef]
- Goerge, T.; Lorenz, M.B.; van Alen, S.; Hübner, N.O.; Becker, K.; Köck, R. MRSA colonization and infection among persons with occupational livestock exposure in Europe: Prevalence, preventive options and evidence. Vet. Microbiol. 2017, 200, 6–12. [Google Scholar] [CrossRef]
- Voss, A.; Loeffen, F.; Bakker, J.; Klaassen, C.; Wulf, M. Methicillin-resistant Staphylococcus aureus in swine farming. Emerg. Infect. Dis. 2005, 11, 1965–1966. [Google Scholar] [CrossRef]
- Mackenzie, J.S.; Jeggo, M. The one health approach—Why is it so important? Trop. Med. Infect. Dis. 2019, 4, 88. [Google Scholar] [CrossRef]
- Kirchhelle, C. Pharming animals: A global history of antibiotics in food production (1935–2017). Palgrave Commun. 2018, 4, 1–13. [Google Scholar] [CrossRef]
- European Commission. Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition. Off. J. Eur. Union 2003, 46, 29–43. [Google Scholar]
- Rossiter, S.E.; Fletcher, M.H.; Wuest, W.M. Natural products as platforms to overcome antibiotic resistance. Chem. Rev. 2017, 117, 12415–12474. [Google Scholar] [CrossRef]
- Kim, Y.K.; Jang, Y.Y.; Kim, D.H.; Ko, H.H.; Han, E.S.; Lee, C.S. Differential regulation of protein tyrosine kinase on free radical production, granule enzyme release, and cytokine synthesis by activated murine peritoneal macrophages. Biochem. Pharmacol. 2001, 61, 87–96. [Google Scholar] [CrossRef]
- Bernard, F.X.; Sable, S.; Cameron, B.; Provost, J.; Desnottes, J.F.; Crouzet, J.; Blanche, F. Glycosylated flavones as selective inhibitors of topoisomerase IV. Antimicrob. Agents Chemother. 1997, 41, 992–998. [Google Scholar] [CrossRef]
- Abreu, A.C.; Coqueiro, A.; Sultan, A.R.; Lemmens, N.; Kim, H.K.; Verpoorte, R.; van Wamel, W.J.B.; Simões, M.; Choi, Y.H. Looking to nature for a new concept in antimicrobial treatments: Isoflavonoids from Cytisus striatus as antibiotic adjuvants against MRSA. Sci. Rep. 2017, 7, 3777. [Google Scholar] [CrossRef]
- Dhayakaran, R.P.A.; Neethirajan, S.; Xue, J.; Shi, J. Characterization of antimicrobial efficacy of soy isoflavones against pathogenic biofilms. LWT Food Sci. Tech. 2015, 63, 859–865. [Google Scholar] [CrossRef]
- Birt, D.F.; Hendrich, S.; Wang, W. Dietary agents in cancer prevention: Flavonoids and isoflavonoids. Pharmacol. Ther. 2001, 90, 157–177. [Google Scholar] [CrossRef]
- Xu, M.L.; Liu, J.; Zhu, C.; Gao, Y.; Zhao, S.; Liu, W.; Zhang, Y. Interactions between soy isoflavones and other bioactive compounds: A review of their potentially beneficial health effects. Phytochem. Rev. 2015, 14, 459–467. [Google Scholar] [CrossRef]
- Peluso, M.R.; Winters, T.A.; Shanahan, M.F.; Banz, W.J. A cooperative interaction between soy protein and its isoflavone-enriched fraction lowers hepatic lipids in male obese Zucker rats and reduces blood platelet sensitivity in male Sprague-Dawley rats. J. Nutr. 2000, 130, 2333–2342. [Google Scholar] [CrossRef] [PubMed]
- Cline, J.M.; Wood, C.E. Estrogen/isoflavone interactions in cynomolgus macaques (Macaca fascicularis). Am. J. Primatol. 2009, 71, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Tsuboy, M.S.; Marcarini, J.C.; de Souza, A.O.; de Paula, N.A.; Dorta, D.J.; Mantovani, M.S.; Ribeiro, L.R. Genistein at maximal physiologic serum levels induces G0/G1 arrest in MCF-7 and HB4a cells, but not apoptosis. J. Med. Food 2014, 17, 218–225. [Google Scholar] [CrossRef]
- Han, B.J.; Li, W.; Jiang, G.B.; Lai, S.H.; Zhang, C.; Zeng, C.C.; Liu, Y.J. Effects of daidzein in regards to cytotoxicity in vitro, apoptosis, reactive oxygen species level, cell cycle arrest and the expression of caspase and Bcl-2 family proteins. Oncol. Rep. 2015, 34, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.Q.; Feng, Y.Y.; Luo, Y.H.; Zhai, Y.Q.; Ju, X.Y.; Feng, Y.C.; Wang, J.R.; Yu, C.Q.; Jin, C.H. Glycitein induces reactive oxygen species-dependent apoptosis and G0/G1 cell cycle arrest through the MAPK/STAT3/NF-κB pathway in human gastric cancer cells. Drug Dev. Res. 2019, 805, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Díaz, I.M.; Altuntas, E.G.; Juneja, V.K. Microbial fermentation in food preservation. In Microbial Control and Food Preservation, 1st ed.; Juneja, V.K., Dwivedi, H.P., Sofos, J.N., Eds.; Springer: New York, NY, USA, 2017; pp. 281–298. [Google Scholar] [CrossRef]
- Wocławek-Potocka, I.; Mannelli, C.; Boruszewska, D.; Kowalczyk-Zieba, I.; Waśniewski, T.; Skarżyński, D.J. Diverse effects of phytoestrogens on the reproductive performance: Cow as a model. Int. J. Endocrinol. 2013, 650984. [Google Scholar] [CrossRef] [PubMed]
- Kaminska, B.; Ciereszko, R.; Kiezun, M.; Dusza, L. In vitro effects of genistein and daidzein on the activity of adrenocortical steroidogenic enzymes in mature female pigs. J. Physiol. Pharmacol. 2013, 64, 103–108. [Google Scholar] [PubMed]
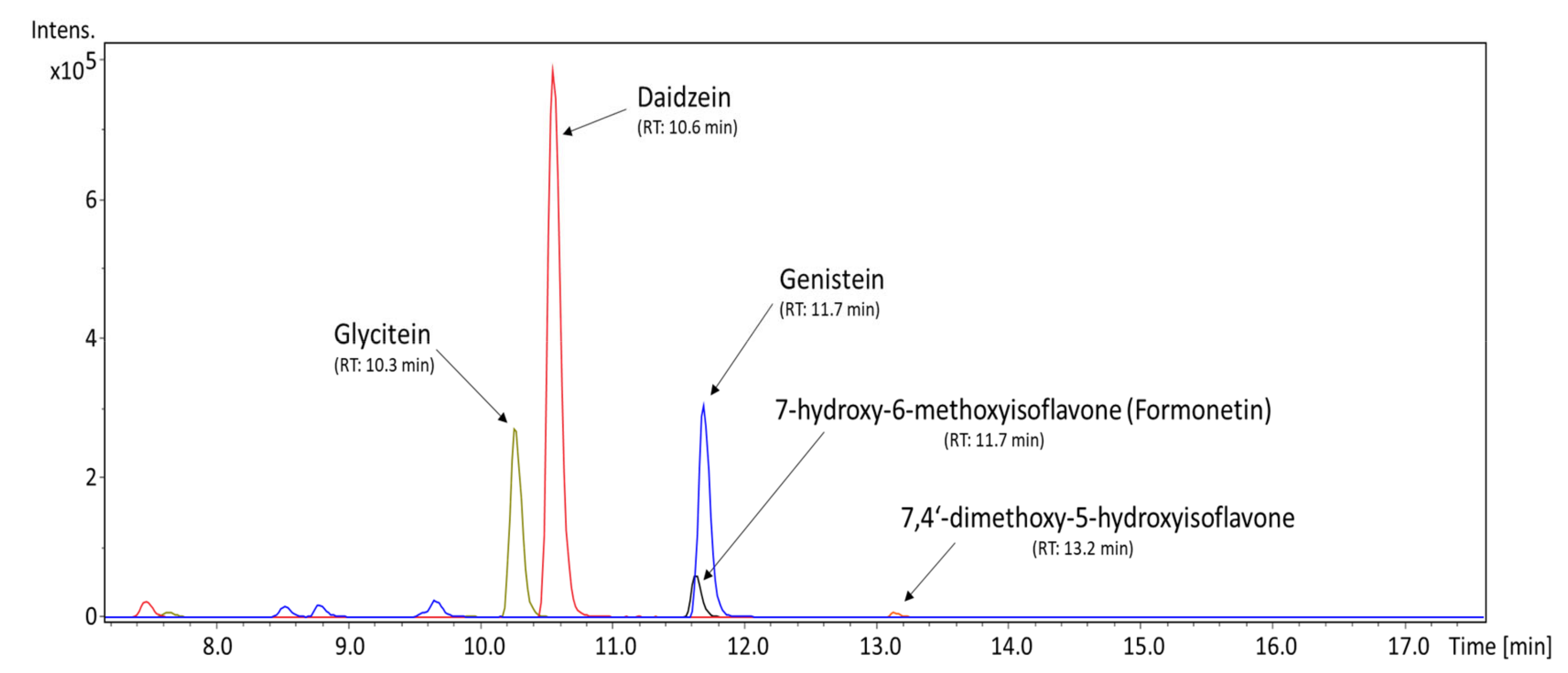

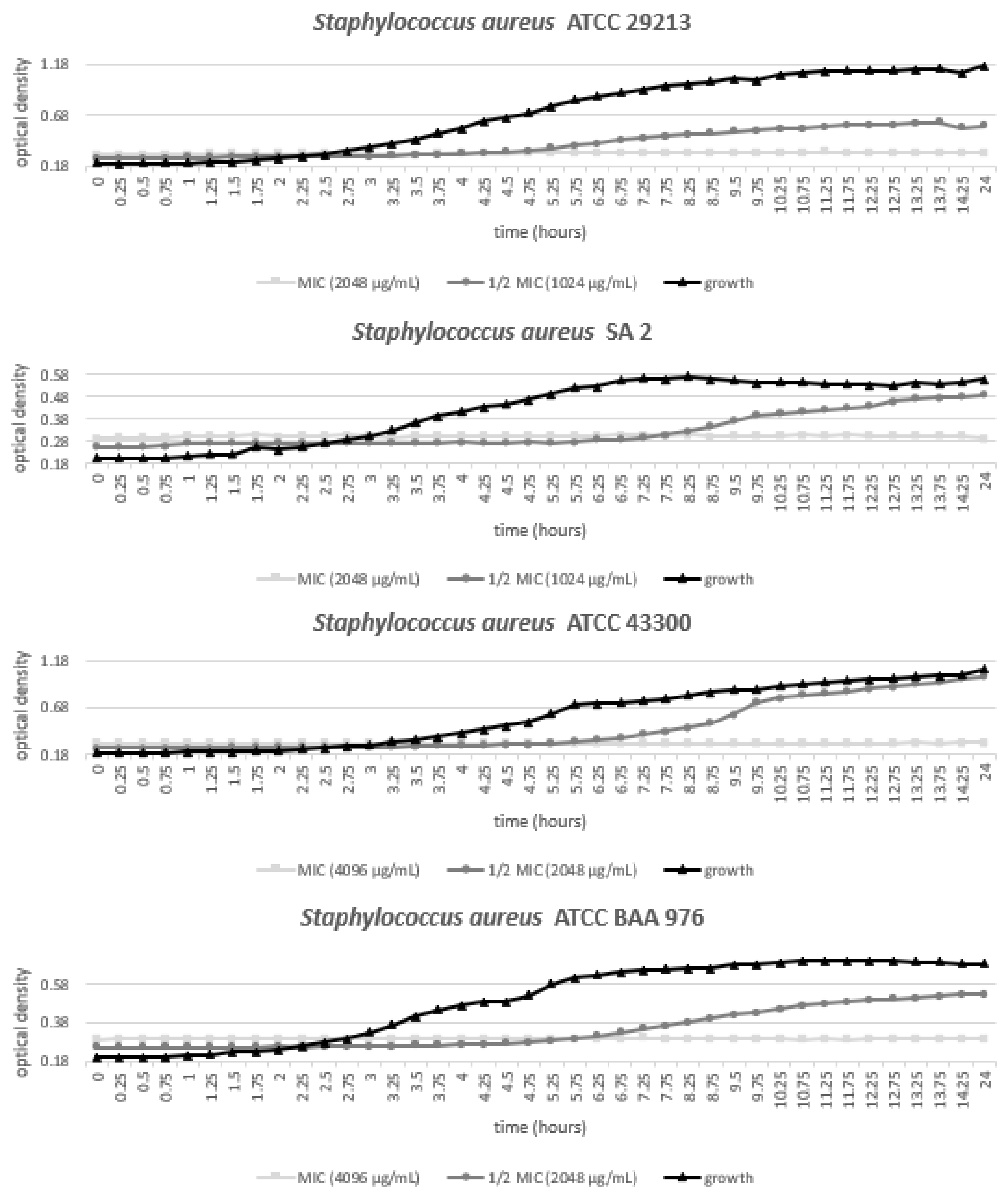
| Bacterium | Strain | MSSA/MRSA | Specification |
|---|---|---|---|
| Staphylococcus aureus subsp. aureus | ATCC 29213 | MSSA | Standard strain for CLSI antimicrobial susceptibility testing |
| ATCC 33591 | MRSA | SCCmec: Type III spa type Ridom: t037 spa type Kreiswirth: WGKAOMQ pvl gene amplification: negative | |
| ATCC 43300 | MRSA | Oxacillin-resistant SCCmec: Type II spa type Ridom: t007 spa type Kreiswirth: WGKKKKAOM pvl gene amplification: negative | |
| ATCC BAA 976 | MRSA | Tracheal aspirate Clinical specimen Isolated March 2003 | |
| CCM 4442 | MSSA | Bovine mastitis isolate (Czechia) Production of β-hemolysin Atypical strain Phosphatase and clumping factor negative | |
| CCM 6188 | MSSA | Bovine mammary gland isolate Loss of hemolysins production | |
| EMRSA-15 | MRSA | Human origin Epidemic strain Oxacillin-resistant Penicillin-resistant | |
| SA 2 | MRSA | Human origin Clinical drug-resistant isolate Oxacillin-resistant Penicillin-resistant Gentamicin-resistant Tetracycline-resistant | |
| SA 3 | MRSA | Human origin Clinical drug-resistant isolate Gentamicin-resistant Penicillin-resistant |
| Bioactive Compound | Average Content ± STD (ng/mg) | Precision (RSD) | LOD (ng/mg) | LOQ (ng/mg) |
|---|---|---|---|---|
| Soyasaponin I | 515.40 ± 0.46 | 0.089% | 0.19 | 2.91 |
| 7,4′-dihydroxyisoflavone (daidzein) | 236.30 ± 4.85 | 2.05% | 0.22 | 0.73 |
| 5,7,4′-trihydroxyisoflavone (genistein) | 131.23 ± 2.32 | 1.77% | 0.33 | 1.11 |
| 7,4′-dihydroxy-6-methoxyisoflavone (glycitein) | 29.00 ± 0.26 | 0.91% | 0.14 | 0.47 |
| 7-hydroxy-6-methoxyisoflavone | tr. | - | 0.08 | 0.25 |
| 7-methoxyisoflavone (methoxyisoflavone) | tr. | - | 0.09 | 0.30 |
| 7-hydroxy-4′-methoxyisoflavone (formonetin) | tr. | - | 0.10 | 0.34 |
| 6,7,4′-trihydroxyisoflavone (demethyltexasin) | tr. | - | 0.22 | 0.75 |
| 7,3′4′-trihydroxyisoflavone | tr. | - | 0.24 | 0.80 |
| 7,4′-dimethoxy-5-hydroxyisoflavone (dimethylgenistein) | tr. | - | 0.16 | 0.52 |
| 7-hydroxyisoflavone | ND | - | 0.10 | 0.34 |
| 5,7,4′-trimethoxyisoflavone | ND | - | 0.06 | 0.21 |
| 6,7,4′-trimethoxyisoflavone | ND | - | 0.08 | 0.27 |
| 4,7,8’-trimethoxyisoflavone | ND | - | 0.08 | 0.27 |
| 5,7-dihydroxy-4′-methoxyisoflavone (biochanin A) | ND | - | 0.23 | 0.75 |
| 6,4′-dimethoxy-7-hydroxyisoflavone (afrormosin) | ND | - | 0.08 | 0.26 |
| 6,7-dimethoxyisoflavone | ND | - | 0.09 | 0.29 |
| 7,4′-dimethoxyisoflavone | ND | - | 0.08 | 0.28 |
| 7,12-dihydroxycoumestan (coumestrol) | ND | - | 0.93 | 3.12 |
| Staphylococcus aureus Strain | MIC (μg/mL) 1 | ||
|---|---|---|---|
| MED | Penicillin G | DMSO | |
| ATCC 29213 * | 2048 | 0.125 | >10,000 |
| ATCC 33591 † | 4096 | 512 | >10,000 |
| ATCC 43300 † | 4096 | 64 | >10,000 |
| ATCC BAA 976 † | 4096 | 32 | >10,000 |
| CCM 4442 * | 2048 | 0.01563 | >10,000 |
| CCM 6188 * | 4096 | 0.01563 | >10,000 |
| EMRSA-15 † | 4096 | 32 | >10,000 |
| SA 2 † | 2048 | 1 | >10,000 |
| SA 3 † | 4096 | 2 | >10,000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lalouckova, K.; Mala, L.; Marsik, P.; Skrivanova, E. In Vitro Antibacterial Effect of the Methanolic Extract of the Korean Soybean Fermented Product Doenjang against Staphylococcus aureus. Animals 2021, 11, 2319. https://doi.org/10.3390/ani11082319
Lalouckova K, Mala L, Marsik P, Skrivanova E. In Vitro Antibacterial Effect of the Methanolic Extract of the Korean Soybean Fermented Product Doenjang against Staphylococcus aureus. Animals. 2021; 11(8):2319. https://doi.org/10.3390/ani11082319
Chicago/Turabian StyleLalouckova, Klara, Lucie Mala, Petr Marsik, and Eva Skrivanova. 2021. "In Vitro Antibacterial Effect of the Methanolic Extract of the Korean Soybean Fermented Product Doenjang against Staphylococcus aureus" Animals 11, no. 8: 2319. https://doi.org/10.3390/ani11082319
APA StyleLalouckova, K., Mala, L., Marsik, P., & Skrivanova, E. (2021). In Vitro Antibacterial Effect of the Methanolic Extract of the Korean Soybean Fermented Product Doenjang against Staphylococcus aureus. Animals, 11(8), 2319. https://doi.org/10.3390/ani11082319






