Adipose Tissue- and Bone Marrow-Derived Mesenchymal Stem Cells from Sheep: Culture Characteristics
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals
2.3. Bone Marrow and Adipose Tssue Collection
2.4. Stem Cell Isolation and Culture Expansion
2.4.1. Bone Marrow
2.4.2. Adipose Tissue
2.5. Percent Adherent Cells (PAC)
2.6. Characterization of MSCs
Morphology
2.7. Colony Forming Unit-f (CFU-f)
2.8. Growth Kinetics and Population Doubling Time (PDT)
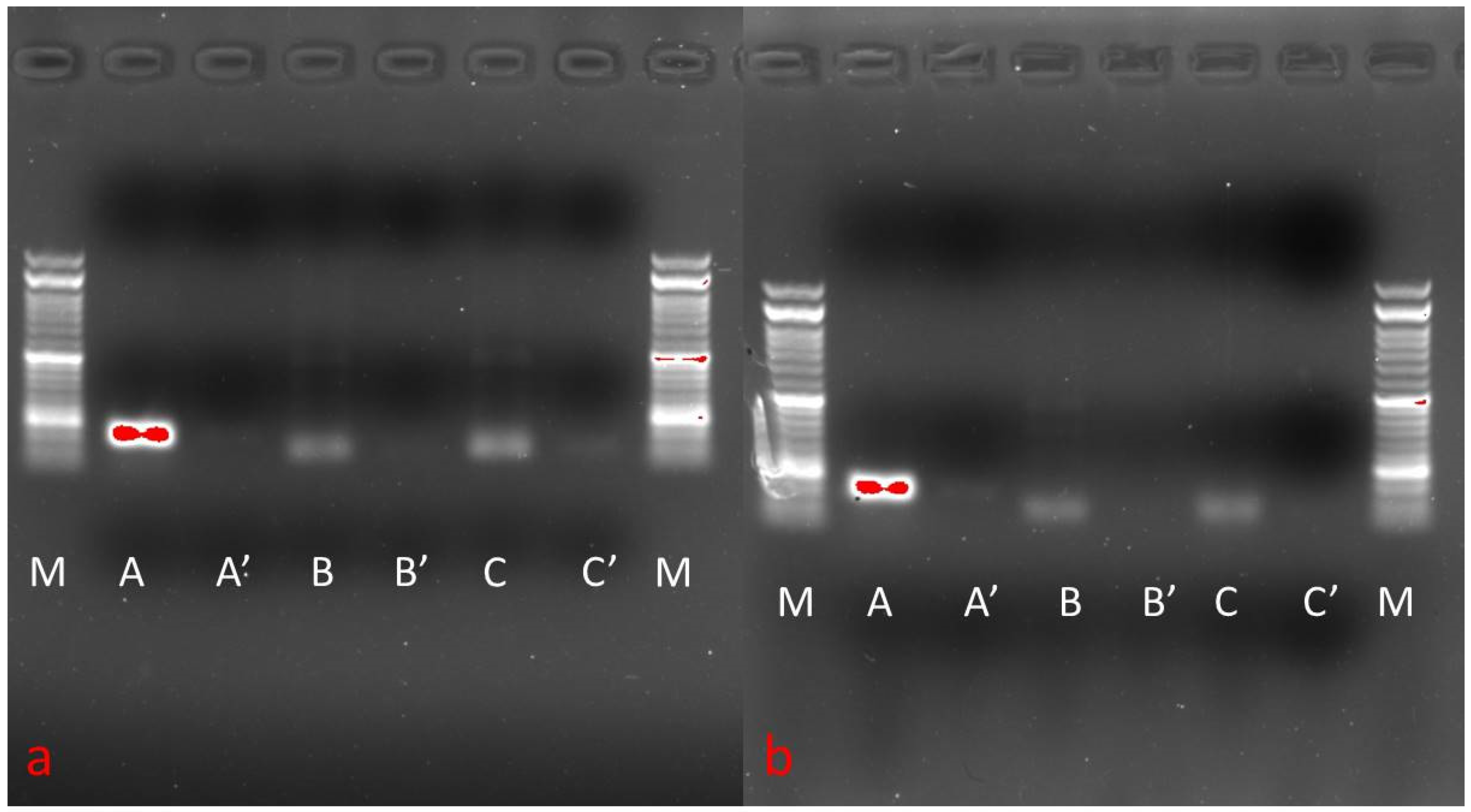
2.9. Surface Marker Expression Analysis
Semi-Quantitative RT-PCR
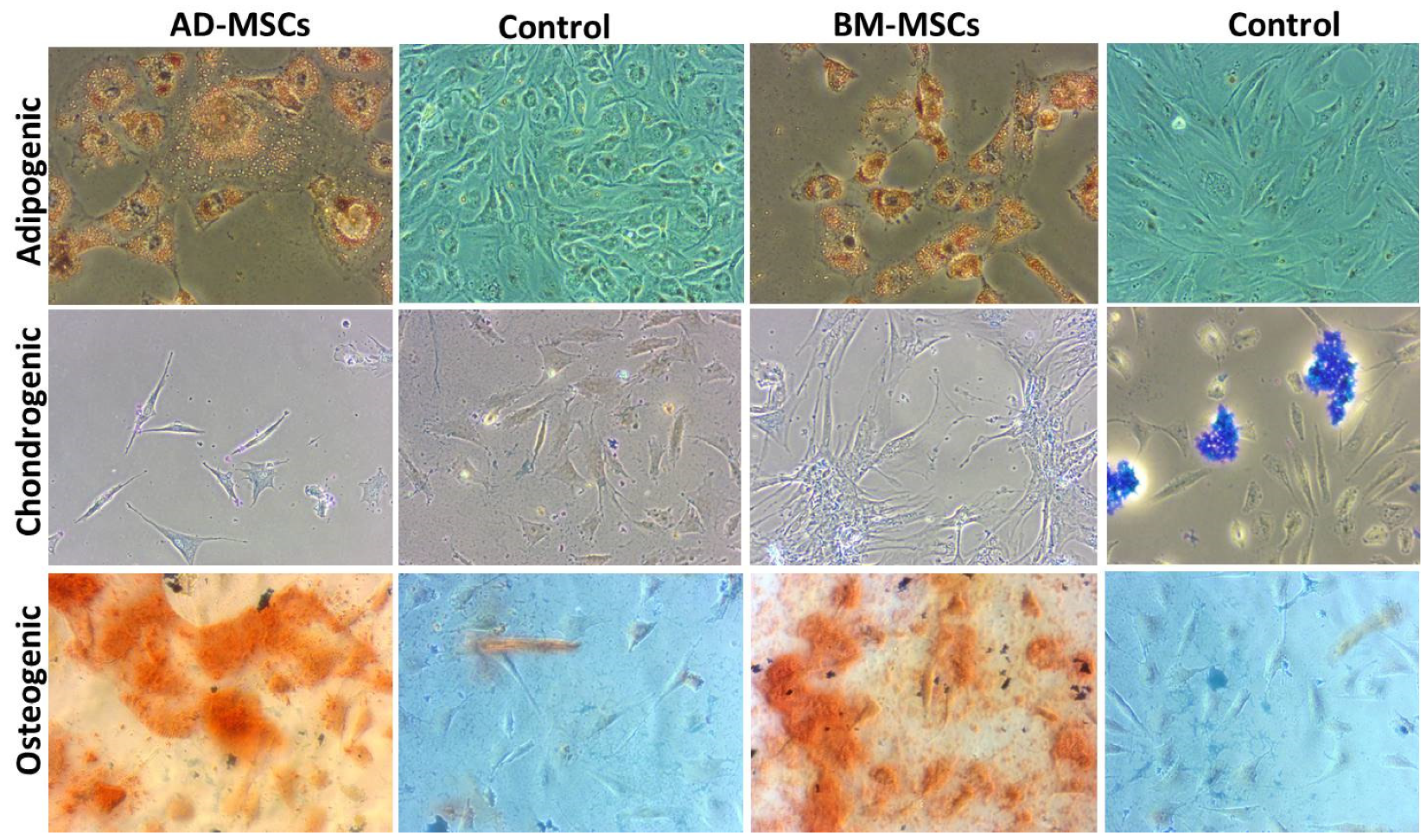
2.10. Tri-Lineage Differentiation
2.10.1. Adipogenic Differentiation
2.10.2. Chondrogenic Differentiation
2.10.3. Osteogenic Differentiation
2.11. Cryopreservation and Post-Thaw Viability
2.11.1. Cell Viability
2.11.2. Cellular Expansion and CFU (f)
2.11.3. Differentiation of Cells
2.12. Statistical Analysis
3. Results
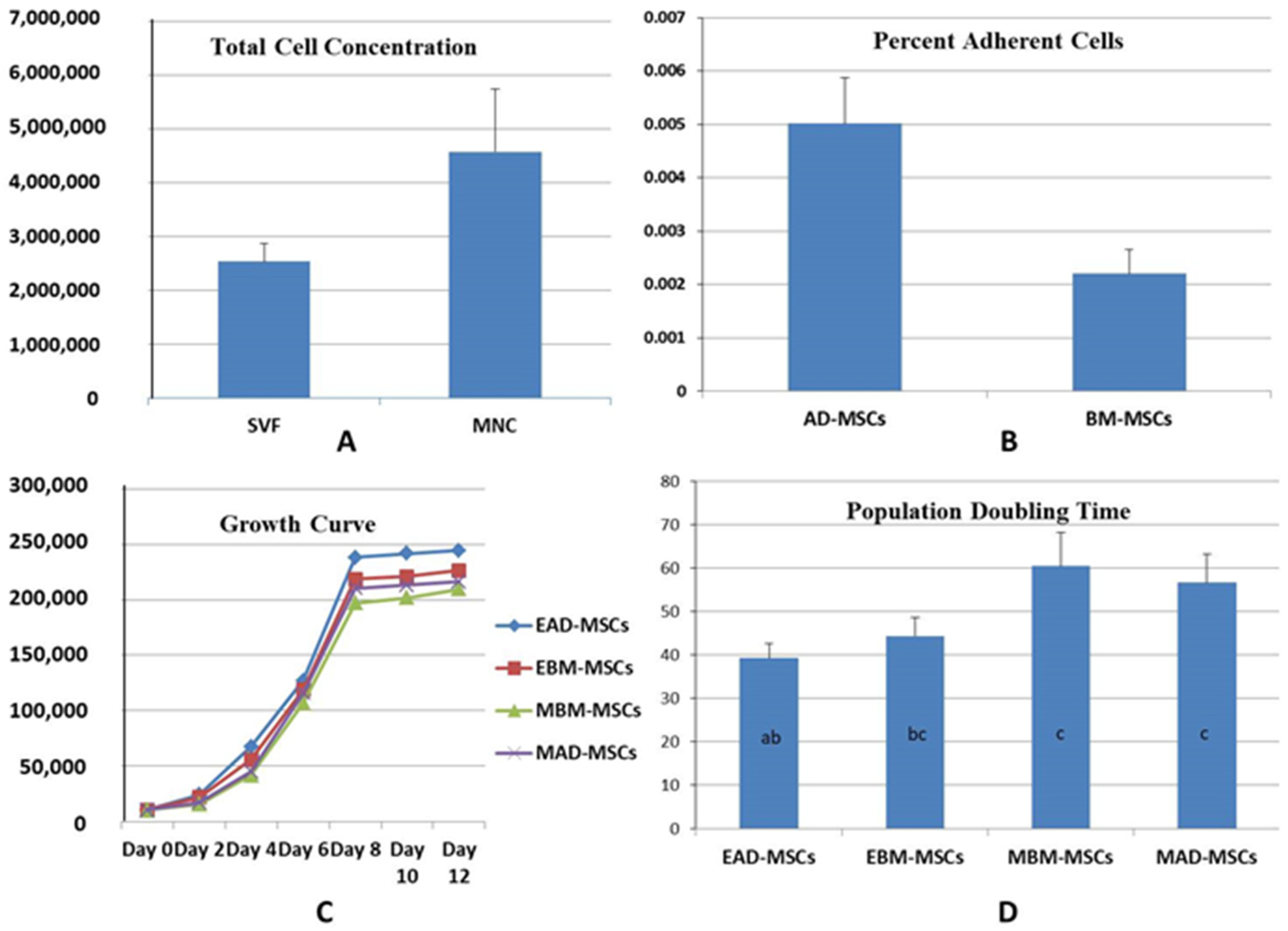
3.1. Bone Marrow and Adipose Tissue Collection
3.2. Cell Population and Percent Cell Adherence (PAC)
3.3. Growth Kinetics and PDT
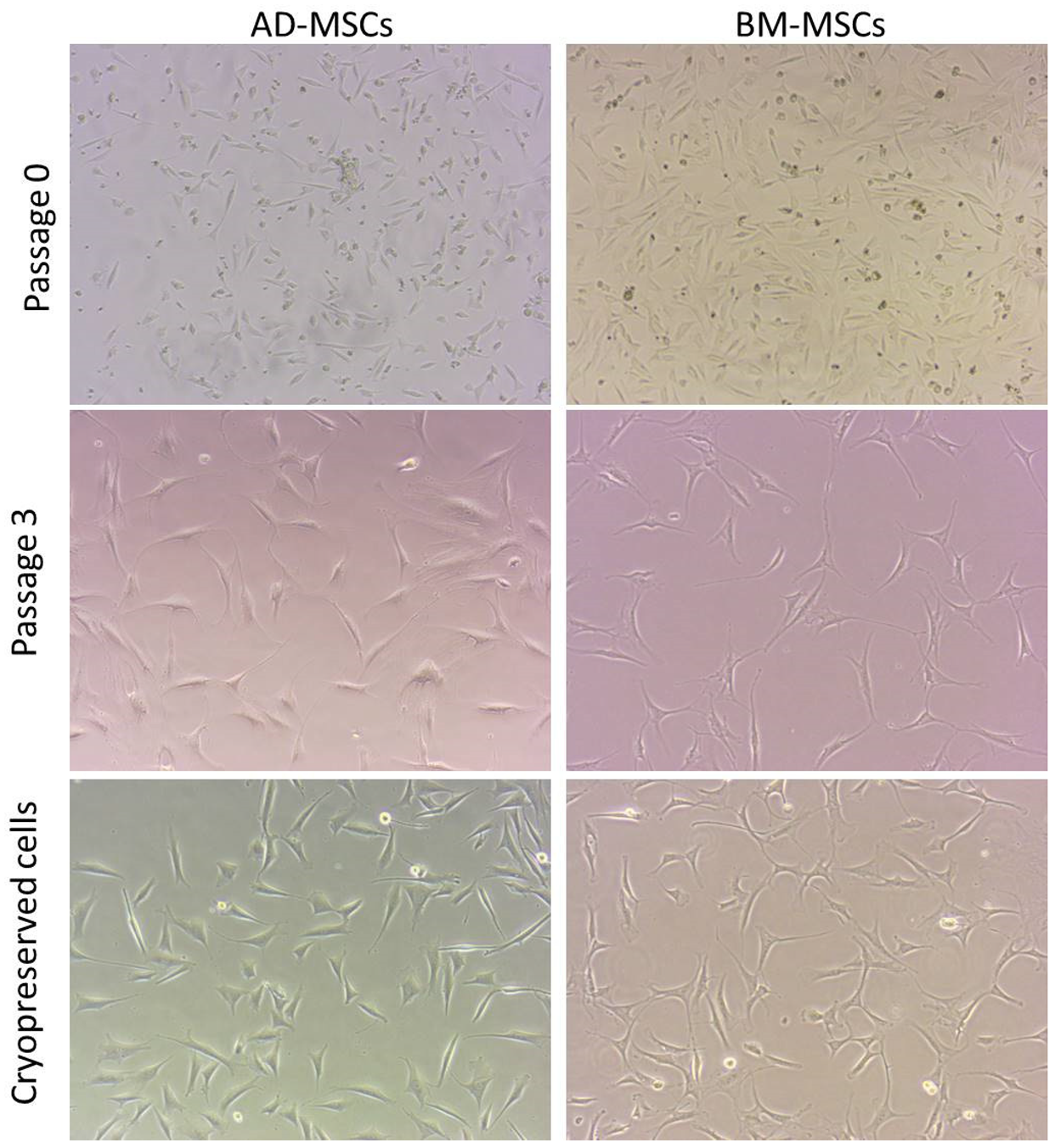
3.4. Cell Morphology
3.5. Colony Forming Unit (Fibroblasts) Assay
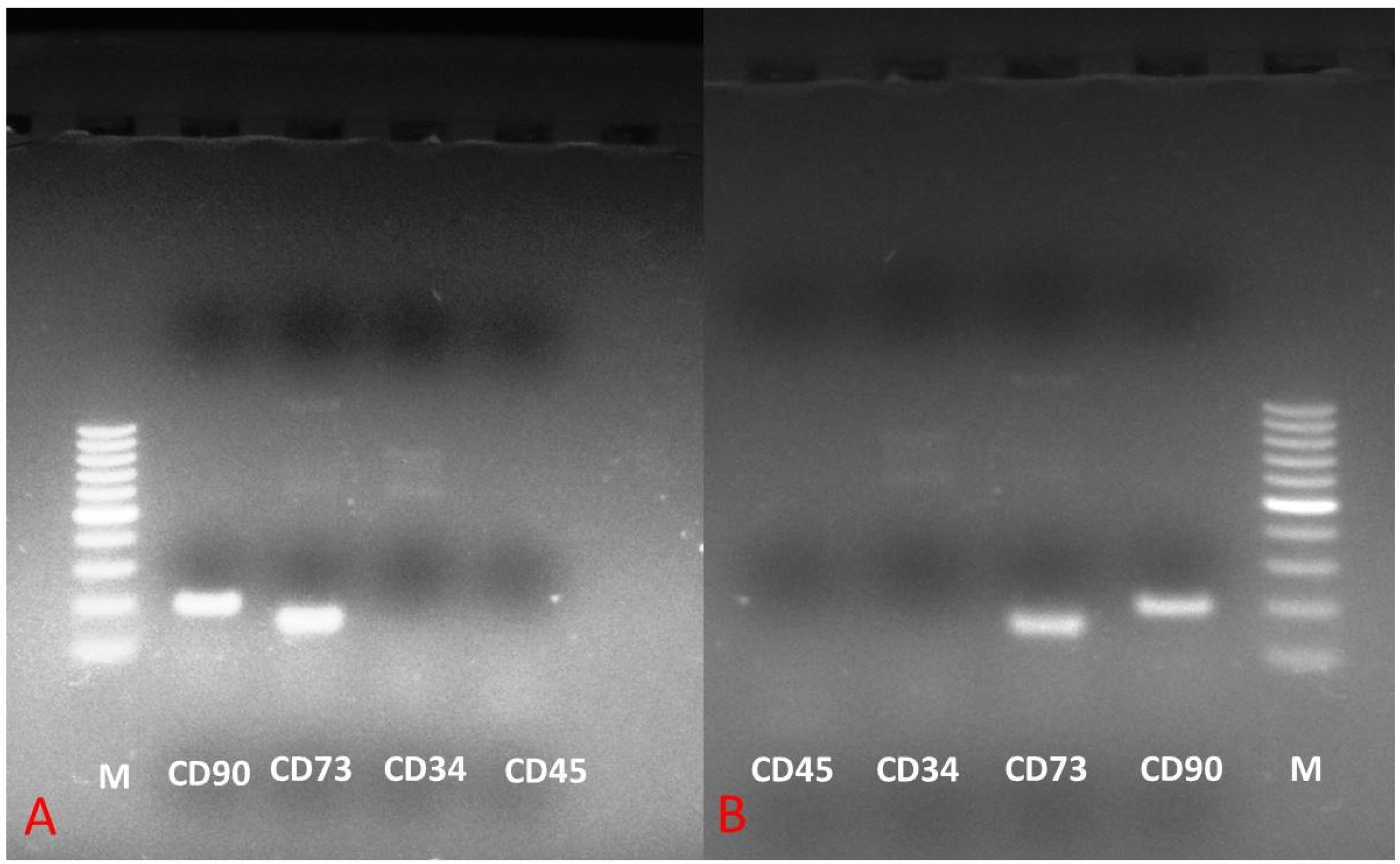
3.6. Phenotypic Gene Expression
3.7. Tri-Lineage Differentiation
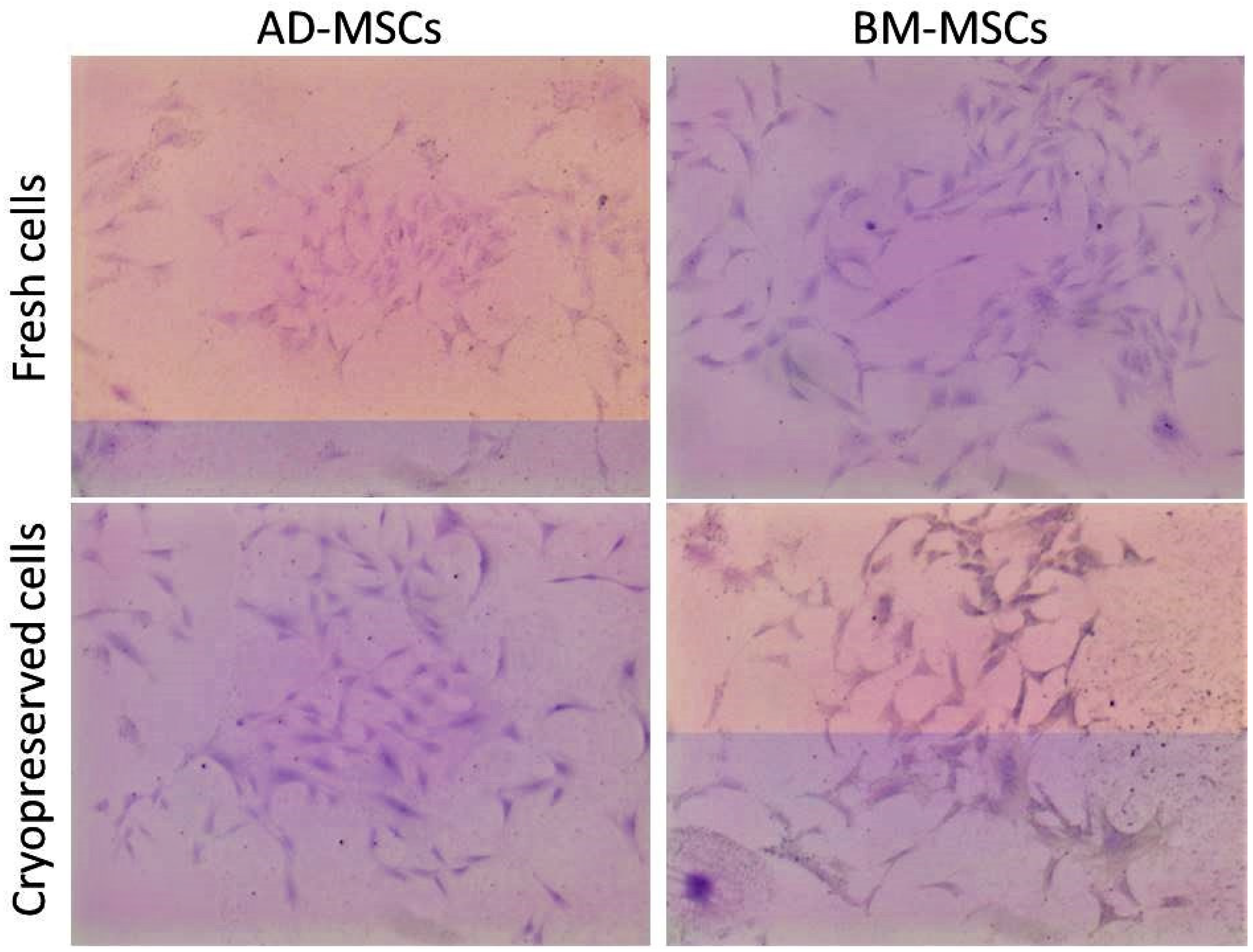
3.8. MSCs Cryopreservation and Post-Thaw Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Gugjoo, M.B. Amarpal Mesenchymal stem cell research in sheep: Current status and future prospects. Small Rumin. Res. 2018, 169, 46–56. [Google Scholar] [CrossRef]
- Gugjoo, M.B.; Pal, A.; Fazili, M.R.; Shah, R.A.; Mir, M.S.; Sharma, G.T. Goat Mesenchymal Stem Cell Basic Research and Potential Applications. Mesenchymal Stem Cell Vet. Sci. 2020, 153–179. [Google Scholar] [CrossRef]
- Toupadakis, C.A.; Wong, A.; Genetos, D.C.; Cheung, W.K.; Borjesson, D.L.; Ferraro, G.L.; Galuppo, L.D.; Leach, J.K.; Owens, S.D.; Yellowley, C.E. Comparison of the osteogenic potential of equine mesenchymal stem cells from bone marrow, adipose tissue, umbilical cord blood, and umbilical cord tissue. Am. J. Vet. Res. 2010, 71, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Cortes, Y.; Ojeda, M.; Araya, D.; Dueñas, F.; Fernández, M.S.; Peralta, O.A. Isolation and multilineage differentiation of bone marrow mesenchymal stem cells from abattoir-derived bovine fetuses. BMC Vet. Res. 2013, 9. [Google Scholar] [CrossRef] [PubMed]
- Heidari, B.; Shirazi, A.; Akhondi, M.M.; Hassanpour, H.; Behzadi, B.; Naderi, M.M.; Sarvari, A.; Borjian, S. Comparison of proliferative and multilineage differentiation potential of sheep mesenchymal stem cells derived from bone marrow, liver, and adipose tissue. Avicenna J. Med. Biotechnol. 2013, 5, 104–117. [Google Scholar]
- Xiong, H.; Bai, C.; Wu, S.; Gao, Y.; Lu, T.; Hu, Q.; Guan, W.; Ma, Y. Biological characterization of mesenchymal stem cells from bovine umbilical cord. Animal Cells Syst. 2014, 18, 59–67. [Google Scholar] [CrossRef][Green Version]
- Akram, T.; Shah, R.A.; Fazili, M.R.; Mudasir, H.; Mir, B.A.; Hussain, S.S.; Ahmad, S.M.; Dar, P.A.; Ganai, N.A.; Shabir, N. Comparative efficiency of goat mesenchymal stem cell isolation from bone marrow and bone chip. Small Rumin. Res. 2017, 153, 87–94. [Google Scholar] [CrossRef]
- Bearden, R.N.; Huggins, S.S.; Cummings, K.J.; Smith, R.; Gregory, C.A.; Saunders, W.B. In-vitro characterization of canine multipotent stromal cells isolated from synovium, bone marrow, and adipose tissue: A donor-matched comparative study. Stem Cell Res. Ther. 2017, 8. [Google Scholar] [CrossRef]
- Ribitsch, I.; Chang-Rodriguez, S.; Egerbacher, M.; Gabner, S.; Gueltekin, S.; Huber, J.; Schuster, T.; Jenner, F. Sheep Placenta Cotyledons: A Noninvasive Source of Ovine Mesenchymal Stem Cells. Tissue Eng. Part C Methods 2017, 23, 298–310. [Google Scholar] [CrossRef]
- Sasaki, A.; Mizuno, M.; Ozeki, N.; Katano, H.; Otabe, K.; Tsuji, K.; Koga, H.; Mochizuki, M.; Sekiya, I. Canine mesenchymal stem cells from synovium have a higher chondrogenic potential than those from infrapatellar fat pad, adipose tissue, and bone marrow. PLoS ONE 2018, 13. [Google Scholar] [CrossRef]
- Cao, L.; Liu, G.; Gan, Y.; Fan, Q.; Yang, F.; Zhang, X.; Tang, T.; Dai, K. The use of autologous enriched bone marrow MSCs to enhance osteoporotic bone defect repair in long-term estrogen deficient goats. Biomaterials 2012, 33, 5076–5084. [Google Scholar] [CrossRef]
- Marycz, K.; Kornicka, K.; Basinska, K.; Czyrek, A. Equine Metabolic Syndrome Affects Viability, Senescence, and Stress Factors of Equine Adipose-Derived Mesenchymal Stromal Stem Cells: New Insight into EqASCs Isolated from EMS Horses in the Context of Their Aging. Oxidative Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Lara, E.; Velásquez, A.; Cabezas, J.; Rivera, N.; Pacha, P.; Rodríguez-Alvarez, L.; Saravia, F.; Castro, F.O. Endometritis and in vitro PGE2 challenge modify properties of cattle endometrial mesenchymal stem cells and their transcriptomic profile. Stem Cells Int. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- de Moraes, C.N.; Maia, L.; Dias, M.C.; Dell’Aqua, C.P.F.; da Mota, L.S.L.S.; Chapwanya, A.; Landim-Alvarenga, F.D.C.; Oba, E. Bovine endometrial cells: A source of mesenchymal stem/progenitor cells. Cell Biol. Int. 2016, 40, 1332–1339. [Google Scholar] [CrossRef] [PubMed]
- Tamadon, A.; Mehrabani, D.; Zarezadeh, Y.; Rahmanifar, F.; Dianatpour, M.; Zare, S. Caprine Endometrial Mesenchymal Stromal Stem Cell: Multilineage Potential, Characterization, and Growth Kinetics in Breeding and Anestrous Stages. Vet. Med. Int. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, N.P.; Srivastava, J.K.; Smith, R.F.; Longinotti, C. Heterogeneity in proliferative potential of ovine mesenchymal stem cell colonies. J. Mater. Sci. Mater. Med. 2004, 15, 397–402. [Google Scholar] [CrossRef]
- Gugjoo, M.B.; Kinjavdekar, A.P.; Aithal, H.P.; Ansari, M.M.; Pawde, A.M.R.; Sharma, G.T. Isolation, culture and characterization of New Zealand white rabbit mesenchymal stem cells derived from bone marrow. Asian J. Anim. Vet. Adv. 2015, 10, 537–548. [Google Scholar] [CrossRef]
- Vahedi, P.; Soleimanirad, J.; Roshangar, L.; Shafaei, H.; Jarolmasjed, S.; Charoudeh, H.N. Advantages of sheep infrapatellar fat pad adipose tissue derived stem cells in tissue engineering. Adv. Pharm. Bull. 2016, 6, 105–110. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Somal, A.; Bhat, I.A.; Indu, B.; Singh, A.P.; Panda, B.S.K.; Desingu, P.A.; Pandey, S.; Bharti, M.K.; Pal, A.; Saikumar, G.; et al. Impact of Cryopreservation on Caprine Fetal Adnexa Derived Stem Cells and Its Evaluation for Growth Kinetics, Phenotypic Characterization, and Wound Healing Potential in Xenogenic Rat Model. J. Cell. Physiol. 2017, 232, 2186–2200. [Google Scholar] [CrossRef]
- Lyahyai, J.; Mediano, D.R.; Ranera, B.; Sanz, A.; Remacha, A.R.; Bolea, R.; Zaragoza, P.; Rodellar, C.; Martín-Burriel, I. Isolation and characterization of ovine mesenchymal stem cells derived from peripheral blood. BMC Vet. Res. 2012, 8. [Google Scholar] [CrossRef]
- Tian, Y.; Tao, L.; Zhao, S.; Tai, D.; Liu, D.; Liu, P. Isolation and morphological characterization of sheep amniotic fluid mesenchymal stem cells. Exp. Anim. 2016, 65, 125–134. [Google Scholar] [CrossRef][Green Version]
- Fan, X.L.; Zhang, Y.; Li, X.; Fu, Q.L. Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cell. Mol. Life Sci. 2020, 77, 2771–2794. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.W.; Yang, M.S.; Park, J.S.; Kim, H.C.; Kim, Y.J.; Choi, J. Isolation of mesenchymal stem cells from cryopreserved human umbilical cord blood. Int. J. Hematol. 2005, 81, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Fujii, S.; Miura, Y.; Iwasa, M.; Yoshioka, S.; Fujishiro, A.; Sugino, N.; Kaneko, H.; Nakagawa, Y.; Hirai, H.; Takaori-Kondo, A.; et al. Isolation of mesenchymal stromal/stem cells from cryopreserved umbilical cord blood cells. J. Clin. Exp. Hematop. 2017, 57, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Skiles, M.L.; Brown, K.S.; Tatz, W.; Swingle, K.; Brown, H.L. Quantitative analysis of composite umbilical cord tissue health using a standardized explant approach and an assay of metabolic activity. Cytotherapy 2018, 20, 564–575. [Google Scholar] [CrossRef] [PubMed]
- Nixon, A.J.; Dahlgren, L.A.; Haupt, J.L.; Yeager, A.E.; Ward, D.L. Effect of adipose-derived nucleated cell fractions on tendon repair in horses with collagenase-induced tendinitis. Am. J. Vet. Res. 2008, 69, 928–937. [Google Scholar] [CrossRef]
- Baghaban Eslaminejad, M.R.; Taghiyar, L.; Dehghan, M.M.; Falahi, F.; Kazemi Mehrjerdi, H. Equine marrow-derived mesenchymal stem cells: Isolation, differentiation and culture optimization. Iran. J. Vet. Res. 2009, 10, 1–11. [Google Scholar]
- Hong, L.; Zhang, G.; Sultana, H.; Yu, Y.; Wei, Z. The effects of 17-β estradiol on enhancing proliferation of human bone marrow mesenchymal stromal cells in vitro. Stem Cells Dev. 2011, 20, 925–931. [Google Scholar] [CrossRef]
- Marycz, K.; Śmieszek, A.; Grzesiak, J.; Nicpoń, J. Effects of steroids on the morphology and proliferation of canine and equine mesenchymal stem cells of adipose origin-In vitro research. Acta Vet. Hung. 2014, 62, 317–333. [Google Scholar] [CrossRef]
- Mauro, A.; Sanyal, H.; Canciello, A.; Berardinelli, P.; Russo, V.; Bernabò, N.; Valbonetti, L.; Barboni, B. In Vitro Effect of Estradiol and Progesterone on Ovine Amniotic Epithelial Cells. Stem Cells Int. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Desantis, S.; Accogli, G.; Zizza, S.; Mastrodonato, M.; Blasi, A.; Francioso, E.; Rossi, R.; Crovace, A.; Resta, L. Ultrastructural study of cultured ovine bone marrow-derived mesenchymal stromal cells. Ann. Anat. 2015, 201, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Somal, A.; Bhat, I.A.; Indu, B.; Pandey, S.; Panda, B.S.K.; Thakur, N.; Sarkar, M.; Chandra, V.; Saikumar, G.; Sharma, G.T. A comparative study of growth kinetics, in vitro differentiation potential and molecular characterization of fetal adnexa derived caprine mesenchymal stem cells. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Cui, P.; He, X.; Pu, Y.; Zhang, W.; Zhang, P.; Li, C.; Guan, W.; Li, X.; Ma, Y. Biological characterization and pluripotent identification of sheep dermis-derived mesenchymal stem/progenitor cells. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
| Sl. No | Gene | Primer Sequence | Annealing Temperature | Amplicon Size (bp) | References |
|---|---|---|---|---|---|
| 1. | CD73 | F-CTGAGACACCCGGATGAGAT R-ACTGGACCAGGTCAAAGGTG | 55 °C | 160 | [20] |
| 2. | CD90 | F-GTGAACCAGAGCCTTCGTCT R-GGTGGTGAAGTTGGACAGGT | 55 °C | 201 | |
| 3. | CD34 | F-TGGGCATCGAGGACATCTCT R-GATCAAGATGGCCAGCAGGAT | 60 °C | 107 | [21] |
| 4. | CD45 | F-CCTGGACACCACCTCAAAGCT R-TCCGTCCTGGGTTTTATCCTG | 60 °C | 101 | |
| 5. | BGLAP | F-CCCAGGAGGGAGGTGTGTG R-CTAGACCGGGCCGTAGAAGC | 58 °C | 99 | |
| 6. | BGN | F-AACATGAACTGCATTGAGATGGG R-GCGAAGGTAGTTGAGCTTCAGG | 58 °C | 93 | |
| 7. | PPARG | F-ACGGGAAAGACGACAGACA R-AAACTGACACCCCTGGAAGATG | 56 °C | 150 | [22] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dar, E.R.; Gugjoo, M.B.; Javaid, M.; Hussain, S.; Fazili, M.R.; Dhama, K.; Alqahtani, T.; Alqahtani, A.M.; Shah, R.A.; Emran, T.B. Adipose Tissue- and Bone Marrow-Derived Mesenchymal Stem Cells from Sheep: Culture Characteristics. Animals 2021, 11, 2153. https://doi.org/10.3390/ani11082153
Dar ER, Gugjoo MB, Javaid M, Hussain S, Fazili MR, Dhama K, Alqahtani T, Alqahtani AM, Shah RA, Emran TB. Adipose Tissue- and Bone Marrow-Derived Mesenchymal Stem Cells from Sheep: Culture Characteristics. Animals. 2021; 11(8):2153. https://doi.org/10.3390/ani11082153
Chicago/Turabian StyleDar, Ejaz R., Mudasir B. Gugjoo, Moien Javaid, Shahid Hussain, Mujeeb R. Fazili, Kuldeep Dhama, Taha Alqahtani, Ali M. Alqahtani, Riaz A. Shah, and Talha Bin Emran. 2021. "Adipose Tissue- and Bone Marrow-Derived Mesenchymal Stem Cells from Sheep: Culture Characteristics" Animals 11, no. 8: 2153. https://doi.org/10.3390/ani11082153
APA StyleDar, E. R., Gugjoo, M. B., Javaid, M., Hussain, S., Fazili, M. R., Dhama, K., Alqahtani, T., Alqahtani, A. M., Shah, R. A., & Emran, T. B. (2021). Adipose Tissue- and Bone Marrow-Derived Mesenchymal Stem Cells from Sheep: Culture Characteristics. Animals, 11(8), 2153. https://doi.org/10.3390/ani11082153








