Effects of Exercise Combined with Undenatured Type II Collagen on Endurance Capacity, Antioxidant Status, Muscle Lipogenic Genes and E3 Ubiquitin Ligases in Rats
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design
2.3. Exercise Procedure
2.4. Sample Collection
2.5. Biochemical Analysis
2.6. Western Blot Method
2.7. Statistical Analysis
3. Results
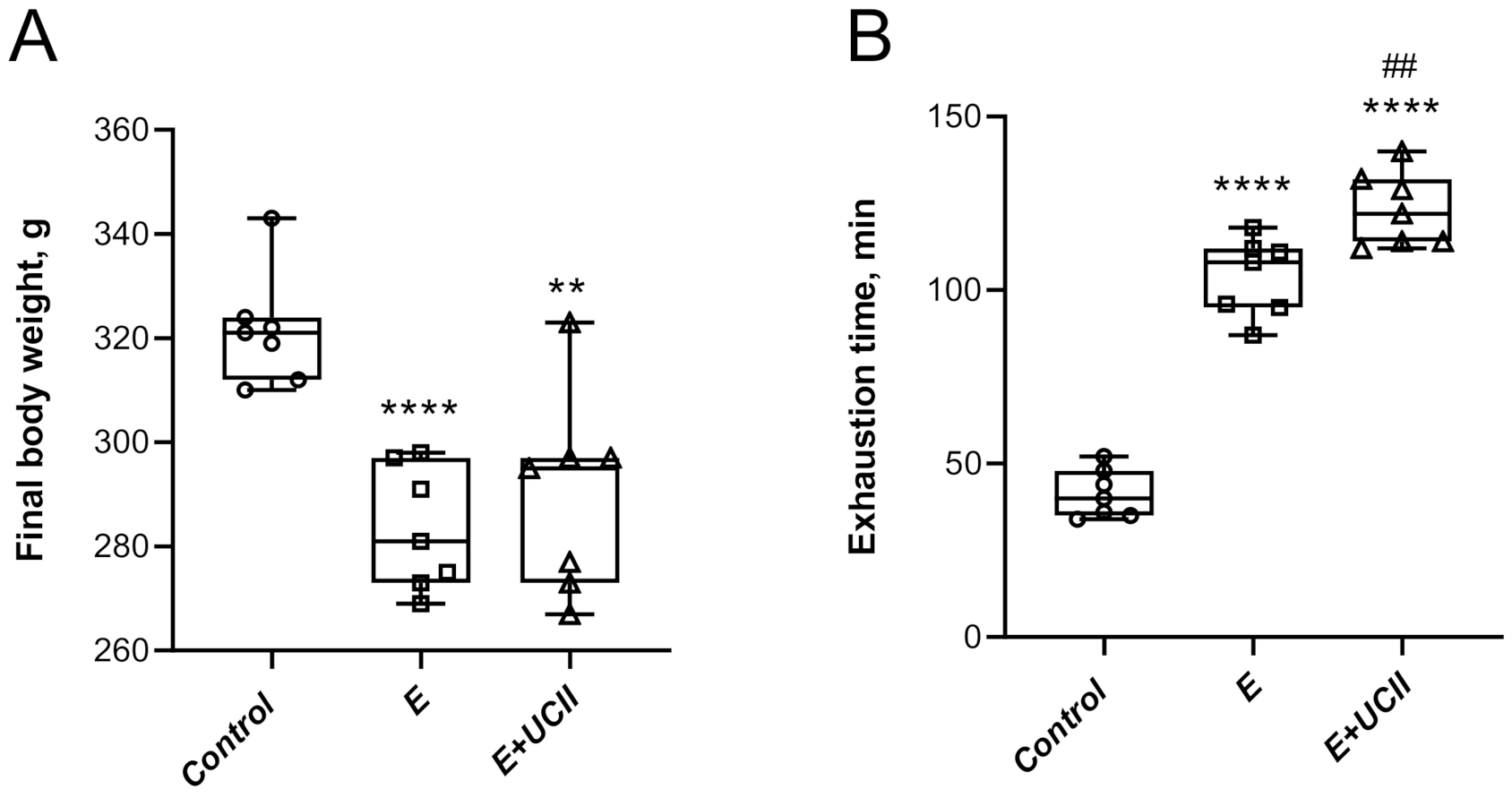
3.1. Performance and Serum Analyses
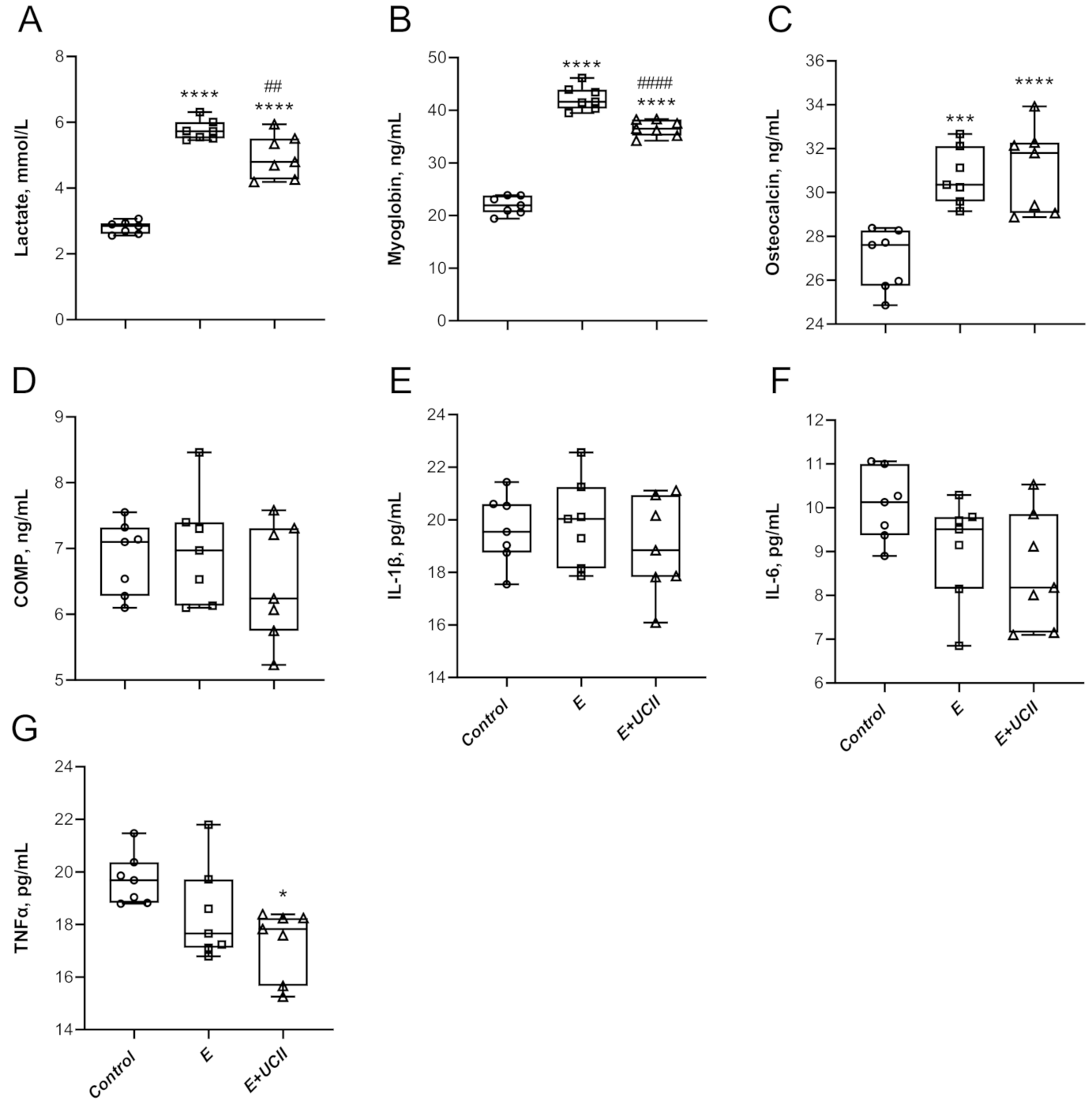
3.2. Inflammatory and Cartilage Markers
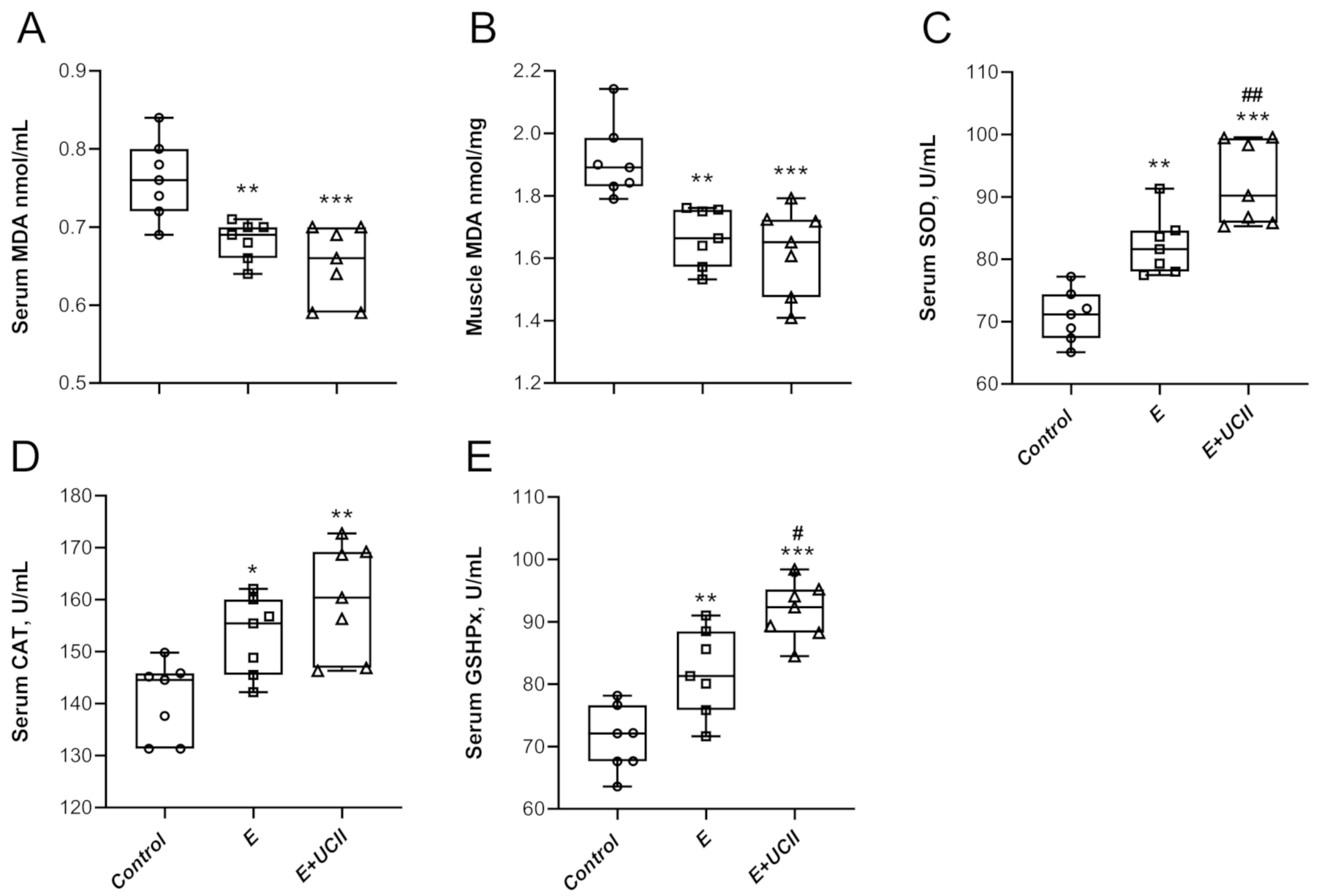
3.3. Oxidative Stress and Antioxidant Properties
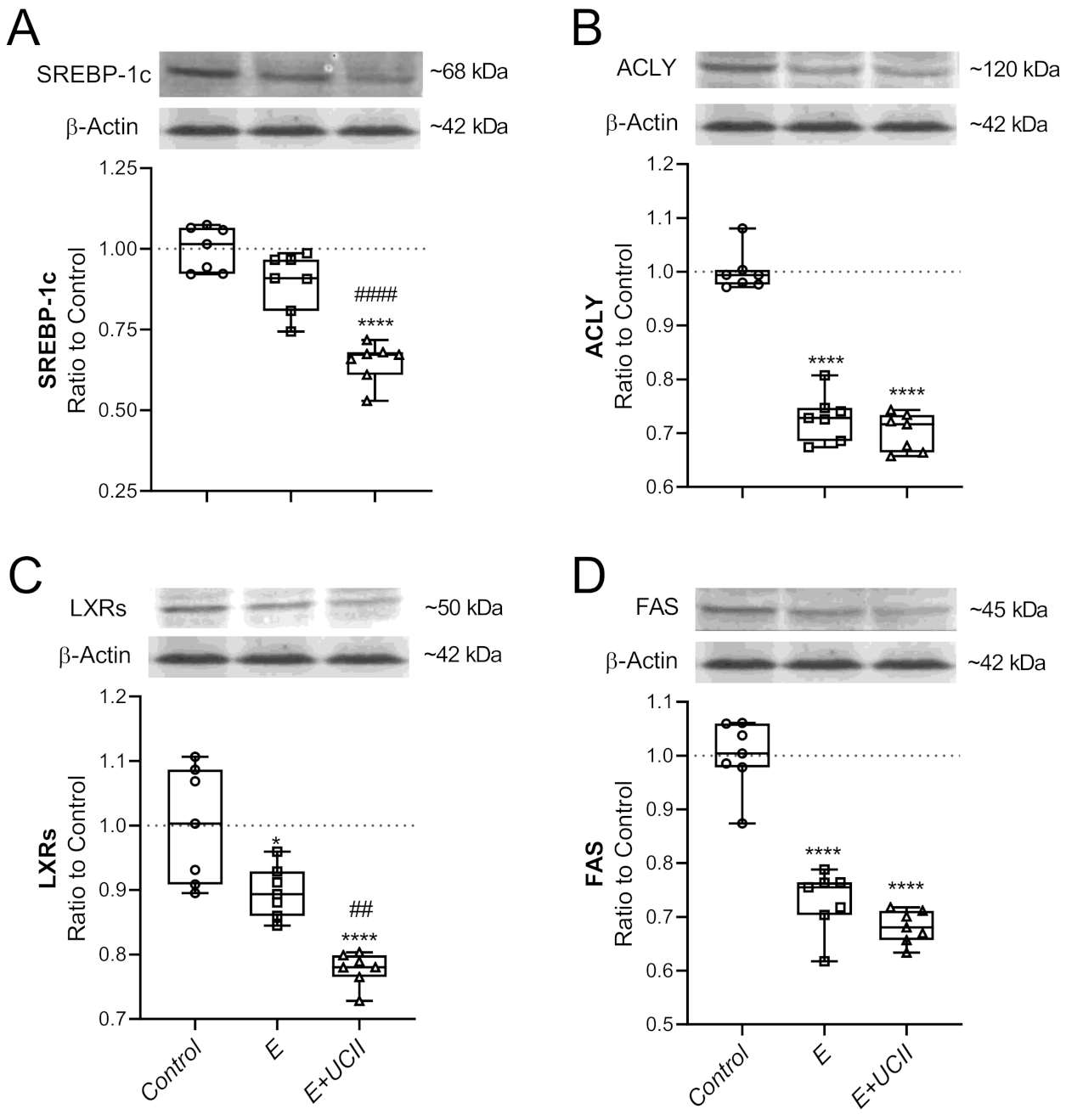
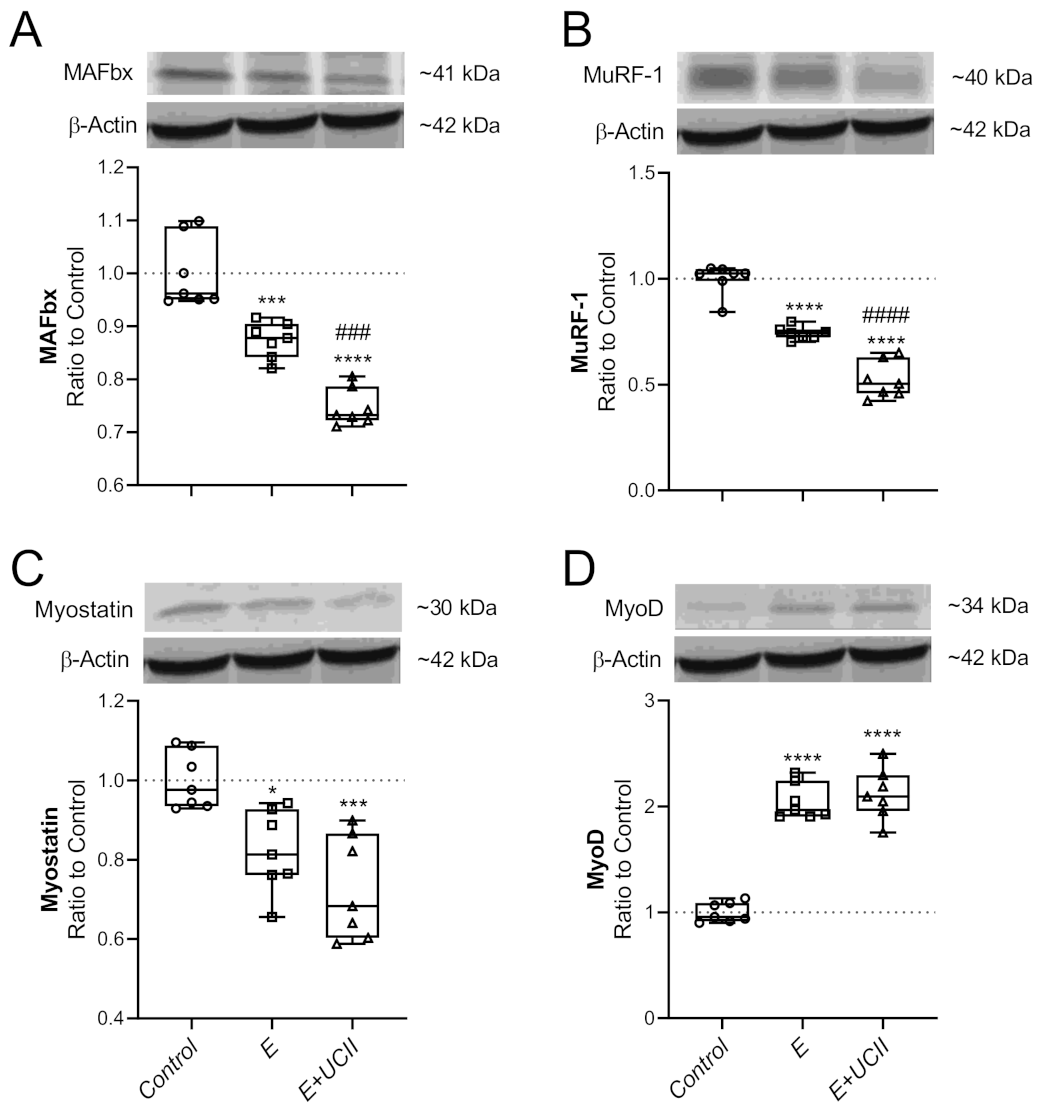
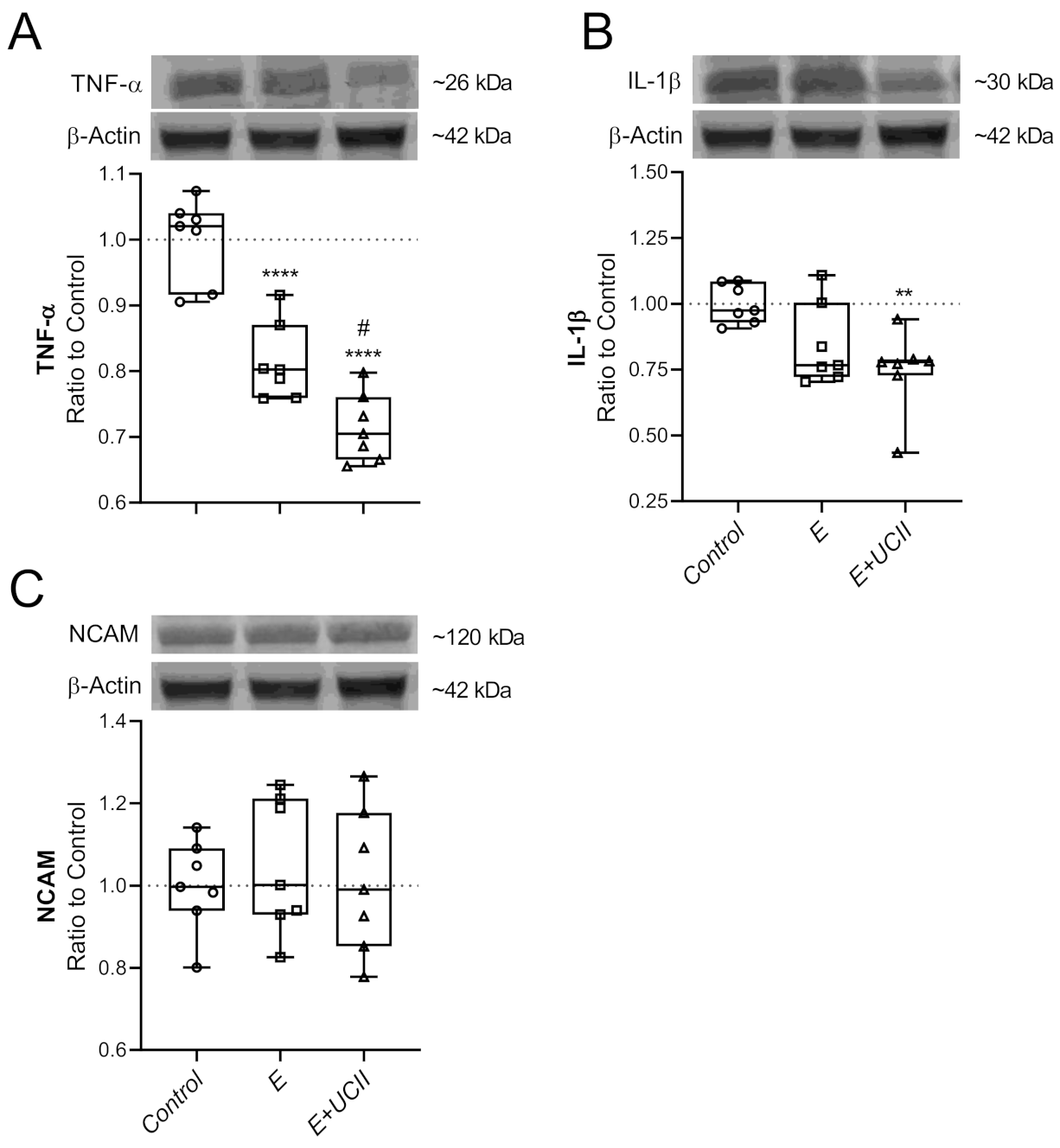
3.4. Muscle Proteins and Inflammatory Cytokines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steele, J.; Fisher, J.; Skivington, M.; Dunn, C.; Arnold, J.; Tew, G.; Batterham, A.M.; Nunan, D.; O’Driscoll, J.M.; Mann, S.; et al. A higher effort-based paradigm in physical activity and exercise for public health: Making the case for a greater emphasis on resistance training. BMC Public Health 2017, 17, 300. [Google Scholar] [CrossRef] [PubMed]
- Elokda, A.S.; Nielsen, D.H. Effects of exercise training on the glutathione antioxidant system. Eur. J. Cardiovasc. Prev. Rehabil. 2007, 14, 630–637. [Google Scholar] [CrossRef]
- Kemmler, W.; Von Stengel, S.; Engelke, K.; Kalender, W.A. Exercise decreases the risk of metabolic syndrome in elderly females. Med. Sci. Sports Exerc. 2009, 41, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Rosety-Rodriguez, M.; Rosety, I.; Fornieles-Gonzalez, G.; Diaz-Ordonez, A.J.; Camacho, A.; Rosety, M.A.; Pardo, A.; Rosety, M.; Alvero, R.; Ordonez, F.J. A 6-week training program increased muscle antioxidant system in elderly diabetic fatty rats. Med. Sci. Monit. 2012, 18, BR346–BR350. [Google Scholar] [CrossRef]
- Silva, E.P., Jr.; Borges, L.S.; Mendes-da-Silva, C.; Hirabara, S.M.; Lambertucci, R.H. l-Arginine supplementation improves rats’ antioxidant system and exercise performance. Free Radic. Res. 2017, 51, 281–293. [Google Scholar] [CrossRef]
- Sahin, K.; Orhan, C.; Tuzcu, M.; Sahin, N.; Erten, F.; Juturu, V. Capsaicinoids improve consequences of physical activity. Toxicol. Rep. 2018, 5, 598–607. [Google Scholar] [CrossRef]
- Musumeci, G. Effects of exercise on physical limitations and fatigue in rheumatic diseases. World J. Orthop. 2015, 6, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Hurley, M.; Dickson, K.; Hallett, R.; Grant, R.; Hauari, H.; Walsh, N.; Stansfield, C.; Oliver, S. Exercise interventions and patient beliefs for people with hip, knee or hip and knee osteoarthritis: A mixed methods review. Cochrane Database Syst. Rev. 2018, 4, CD010842. [Google Scholar] [CrossRef] [PubMed]
- Iijima, H.; Aoyama, T.; Ito, A.; Tajino, J.; Yamaguchi, S.; Nagai, M.; Kiyan, W.; Zhang, X.; Kuroki, H. Exercise intervention increases expression of bone morphogenetic proteins and prevents the progression of cartilage-subchondral bone lesions in a post-traumatic rat knee model. Osteoarthr. Cartil. 2016, 24, 1092–1102. [Google Scholar] [CrossRef]
- Smith, J.K. Exercise as an adjuvant to cartilage regeneration therapy. Int. J. Mol. Sci. 2020, 21, 9471. [Google Scholar] [CrossRef] [PubMed]
- Thyfault, J.P.; Bergouignan, A. Exercise and metabolic health: Beyond skeletal muscle. Diabetologia 2020, 63, 1464–1474. [Google Scholar] [CrossRef] [PubMed]
- Hemmingsen, B.; Gimenez-Perez, G.; Mauricio, D.; Roque, I.F.M.; Metzendorf, M.I.; Richter, B. Diet, physical activity or both for prevention or delay of type 2 diabetes mellitus and its associated complications in people at increased risk of developing type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2017, 12, CD003054. [Google Scholar] [CrossRef] [PubMed]
- Solinas, G.; Boren, J.; Dulloo, A.G. De novo lipogenesis in metabolic homeostasis: More friend than foe? Mol. Metab. 2015, 4, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Booth, F.W.; Roberts, C.K.; Laye, M.J. Lack of exercise is a major cause of chronic diseases. Compr. Physiol. 2012, 2, 1143–1211. [Google Scholar] [PubMed]
- Irving, B.A.; Davis, C.K.; Brock, D.W.; Weltman, J.Y.; Swift, D.; Barrett, E.J.; Gaesser, G.A.; Weltman, A. Effect of exercise training intensity on abdominal visceral fat and body composition. Med. Sci. Sports Exerc. 2008, 40, 1863–1872. [Google Scholar] [CrossRef]
- Yoon, M.S. mTOR as a Key Regulator in Maintaining Skeletal Muscle Mass. Front. Physiol. 2017, 8, 788. [Google Scholar] [CrossRef]
- Glass, D.J. Signaling pathways perturbing muscle mass. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Lokireddy, S.; Wijesoma, I.W.; Sze, S.K.; McFarlane, C.; Kambadur, R.; Sharma, M. Identification of atrogin-1-targeted proteins during the myostatin-induced skeletal muscle wasting. Am. J. Physiol. Cell Physiol. 2012, 303, C512–C529. [Google Scholar] [CrossRef]
- Cohen, S.; Brault, J.J.; Gygi, S.P.; Glass, D.J.; Valenzuela, D.M.; Gartner, C.; Latres, E.; Goldberg, A.L. During muscle atrophy, thick, but not thin, filament components are degraded by MuRF1-dependent ubiquitylation. J. Cell Biol. 2009, 185, 1083–1095. [Google Scholar] [CrossRef]
- Cunha, J.E.; Barbosa, G.M.; Castro, P.; Luiz, B.L.F.; Silva, A.C.A.; Russo, T.L.; Vasilceac, F.A.; Cunha, T.M.; Cunha, F.Q.; Salvini, T.F. Knee osteoarthritis induces atrophy and neuromuscular junction remodeling in the quadriceps and tibialis anterior muscles of rats. Sci. Rep. 2019, 9, 6366. [Google Scholar] [CrossRef] [PubMed]
- Heiden, T.L.; Lloyd, D.G.; Ackland, T.R. Knee joint kinematics, kinetics and muscle co-contraction in knee osteoarthritis patient gait. Clin. Biomech. 2009, 24, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Van der Esch, M.; Steultjens, M.; Knol, D.L.; Dinant, H.; Dekker, J. Joint laxity and the relationship between muscle strength and functional ability in patients with osteoarthritis of the knee. Arthritis Rheum. 2006, 55, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Gencoglu, H.; Orhan, C.; Sahin, E.; Sahin, K. Undenatured Type II Collagen (UC-II) in Joint Health and Disease: A Review on the Current Knowledge of Companion Animals. Animals 2020, 10, 697. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, D.; Misner, B.; Bagchi, M.; Kothari, S.C.; Downs, B.W.; Fafard, R.D.; Preuss, H.G. Effects of orally administered undenatured type II collagen against arthritic inflammatory diseases: A mechanistic exploration. Int. J. Clin. Pharmacol. Res. 2002, 22, 101–110. [Google Scholar]
- Park, K.S.; Park, M.J.; Cho, M.L.; Kwok, S.K.; Ju, J.H.; Ko, H.J.; Park, S.H.; Kim, H.Y. Type II collagen oral tolerance; mechanism and role in collagen-induced arthritis and rheumatoid arthritis. Mod. Rheumatol. 2009, 19, 581–589. [Google Scholar] [CrossRef]
- Lugo, J.P.; Saiyed, Z.M.; Lau, F.C.; Molina, J.P.; Pakdaman, M.N.; Shamie, A.N.; Udani, J.K. Undenatured type II collagen (UC-II(R)) for joint support: A randomized, double-blind, placebo-controlled study in healthy volunteers. J. Int. Soc. Sports Nutr. 2013, 10, 48. [Google Scholar] [CrossRef] [PubMed]
- Crowley, D.C.; Lau, F.C.; Sharma, P.; Evans, M.; Guthrie, N.; Bagchi, M.; Bagchi, D.; Dey, D.K.; Raychaudhuri, S.P. Safety and efficacy of undenatured type II collagen in the treatment of osteoarthritis of the knee: A clinical trial. Int. J. Med. Sci. 2009, 6, 312–321. [Google Scholar] [CrossRef]
- Lugo, J.P.; Saiyed, Z.M.; Lane, N.E. Efficacy and tolerability of an undenatured type II collagen supplement in modulating knee osteoarthritis symptoms: A multicenter randomized, double-blind, placebo-controlled study. Nutr. J. 2016, 15, 14. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.; Zhao, W.; Wu, Y.Q.; Chang, Y.; Wang, Q.T.; Zhang, L.L.; Wei, W. Chicken type II collagen induced immune balance of main subtype of helper T cells in mesenteric lymph node lymphocytes in rats with collagen-induced arthritis. Inflamm. Res. 2010, 59, 369–377. [Google Scholar] [CrossRef]
- Radak, Z.; Chung, H.Y.; Koltai, E.; Taylor, A.W.; Goto, S. Exercise, oxidative stress and hormesis. Ageing Res. Rev. 2008, 7, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Bo, H.; Kang, W.; Jiang, N.; Wang, X.; Zhang, Y.; Ji, L.L. Exercise-induced neuroprotection of hippocampus in APP/PS1 transgenic mice via upregulation of mitochondrial 8-oxoguanine DNA glycosylase. Oxid. Med. Cell. Longev. 2014, 2014, 834502. [Google Scholar] [CrossRef]
- Sallam, N.; Laher, I. Exercise Modulates Oxidative Stress and Inflammation in Aging and Cardiovascular Diseases. Oxid. Med. Cell. Longev. 2016, 2016, 7239639. [Google Scholar] [CrossRef]
- Sahin, K.; Pala, R.; Tuzcu, M.; Ozdemir, O.; Orhan, C.; Sahin, N.; Juturu, V. Curcumin prevents muscle damage by regulating NF-kappaB and Nrf2 pathways and improves performance: An in vivo model. J. Inflamm. Res. 2016, 9, 147–154. [Google Scholar]
- Yan, Z.; Zhao, H.; Liu, A.; Liu, S.; Zou, G.; Wang, H. The Effects of Undenatured Type II Collagen on Inflammatory Mediators and Oxidative Stress in an Osteoarthritis Rat Model. IOP Conf. Ser. Earth Environ. Sci. 2020, 598, 012067. [Google Scholar] [CrossRef]
- Shin, J.W.; Seol, I.C.; Son, C.G. Interpretation of animal dose and human equivalent dose for drug development. Korean J. Intern. Med. 2010, 31, 1–7. [Google Scholar]
- Feng, R.; Wang, L.; Li, Z.; Yang, R.; Liang, Y.; Sun, Y.; Yu, Q.; Ghartey-Kwansah, G.; Sun, Y.; Wu, Y.; et al. A systematic comparison of exercise training protocols on animal models of cardiovascular capacity. Life Sci. 2019, 217, 128–140. [Google Scholar] [CrossRef]
- Rivas-Estany, E.; Sixto-Fernandez, S.; Barrera-Sarduy, J.; Hernandez-Garcia, S.; Gonzalez-Guerra, R.; Stusser-Beltranena, R. Effects of long-term exercise training on left ventricular function and remodeling in patients with anterior wall myocardial infarction. Arch. Cardiol. Mex. 2013, 83, 167–173. [Google Scholar]
- Wojdasiewicz, P.; Poniatowski, L.A.; Szukiewicz, D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediat. Inflamm. 2014, 2014, 561459. [Google Scholar] [CrossRef] [PubMed]
- Haseeb, A.; Haqqi, T.M. Immunopathogenesis of osteoarthritis. Clin. Immunol. 2013, 146, 185–196. [Google Scholar] [CrossRef]
- Mueller, M.B.; Tuan, R.S. Anabolic/Catabolic balance in pathogenesis of osteoarthritis: Identifying molecular targets. PM&R 2011, 3, S3–S11. [Google Scholar]
- Varney, J.L.; Fowler, J.W.; Coon, C.N. PSVI-33 Undenatured type II collagen mitigates inflammation and cartilage degeneration in healthy untrained Labrador retrievers after exercise. J. Anim. Sci. 2020, 98, 313–314. [Google Scholar] [CrossRef]
- Bagi, C.M.; Berryman, E.R.; Teo, S.; Lane, N.E. Oral administration of undenatured native chicken type II collagen (UC-II) diminished deterioration of articular cartilage in a rat model of osteoarthritis (OA). Osteoarthr. Cartil. 2017, 25, 2080–2090. [Google Scholar] [CrossRef] [PubMed]
- Giles, L.V.; Koehle, M.S. The health effects of exercising in air pollution. Sports Med. 2014, 44, 223–249. [Google Scholar] [CrossRef]
- Powers, S.K.; Ji, L.L.; Leeuwenburgh, C. Exercise training-induced alterations in skeletal muscle antioxidant capacity: A brief review. Med. Sci. Sports Exerc. 1999, 31, 987–997. [Google Scholar] [CrossRef] [PubMed]
- Porstmann, T.; Santos, C.R.; Griffiths, B.; Cully, M.; Wu, M.; Leevers, S.; Griffiths, J.R.; Chung, Y.L.; Schulze, A. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab. 2008, 8, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, S.; Miyazaki, H.; Nakatani, T.; Kai, Y.; Kamei, Y.; Miura, S.; Tsuboyama-Kasaoka, N.; Ezaki, O. Up-regulation of SREBP-1c and lipogenic genes in skeletal muscles after exercise training. Biochem. Biophys. Res. Commun. 2002, 296, 395–400. [Google Scholar] [CrossRef]
- Jeong, J.H.; Park, H.G.; Lee, Y.R.; Lee, W.L. Moderate exercise training is more effective than resveratrol supplementation for ameliorating lipid metabolic complication in skeletal muscle of high fat diet-induced obese mice. J. Exerc. Nutr. Biochem. 2015, 19, 131–137. [Google Scholar] [CrossRef]
- Yu, Q.; Xia, Z.; Liong, E.C.; Tipoe, G.L. Chronic aerobic exercise improves insulin sensitivity and modulates Nrf2 and NFkappaB/IkappaBalpha pathways in the skeletal muscle of rats fed with a high fat diet. Mol. Med. Rep. 2019, 20, 4963–4972. [Google Scholar]
- Smith, I.J.; Huffman, K.M.; Durheim, M.T.; Duscha, B.D.; Kraus, W.E. Sex-specific alterations in mRNA level of key lipid metabolism enzymes in skeletal muscle of overweight and obese subjects following endurance exercise. Physiol. Genom. 2009, 36, 149–157. [Google Scholar] [CrossRef][Green Version]
- De Souza Cordeiro, L.M.; Mario, E.G.; Moreira, C.C.L.; Rodrigues, A.H.; Wanner, S.P.; Soares, D.D.; Botion, L.M.; Ferreira, A.V.M. Aerobic training induces differential expression of genes involved in lipid metabolism in skeletal muscle and white adipose tissues. J. Cell Biochem. 2019, 120, 18883–18893. [Google Scholar] [CrossRef]
- Nadeau, K.J.; Ehlers, L.B.; Aguirre, L.E.; Moore, R.L.; Jew, K.N.; Ortmeyer, H.K.; Hansen, B.C.; Reusch, J.E.; Draznin, B. Exercise training and calorie restriction increase SREBP-1 expression and intramuscular triglyceride in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E90–E98. [Google Scholar] [CrossRef] [PubMed]
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.I.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-Related Loss of Muscle Mass and Function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.M.S.; Alabarse, P.V.G.; Teixeira, V.O.N.; Freitas, E.C.; de Oliveira, F.H.; Chakr, R.; Xavier, R.M. Muscle wasting in osteoarthritis model induced by anterior cruciate ligament transection. PLoS ONE 2018, 13, e0196682. [Google Scholar] [CrossRef] [PubMed]
- Okada, A.; Ono, Y.; Nagatomi, R.; Kishimoto, K.N.; Itoi, E. Decreased muscle atrophy F-box (MAFbx) expression in regenerating muscle after muscle-damaging exercise. Muscle Nerve 2008, 38, 1246–1253. [Google Scholar] [CrossRef]
- Koyama, S.; Hata, S.; Witt, C.C.; Ono, Y.; Lerche, S.; Ojima, K.; Chiba, T.; Doi, N.; Kitamura, F.; Tanaka, K.; et al. Muscle RING-finger protein-1 (MuRF1) as a connector of muscle energy metabolism and protein synthesis. J. Mol. Biol. 2008, 376, 1224–1236. [Google Scholar] [CrossRef] [PubMed]
- Mascher, H.; Tannerstedt, J.; Brink-Elfegoun, T.; Ekblom, B.; Gustafsson, T.; Blomstrand, E. Repeated resistance exercise training induces different changes in mRNA expression of MAFbx and MuRF-1 in human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E43–E51. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Liang, J.; Wu, L.; Zhang, H.; Lv, J.; Chen, N. Exercise-Induced Autophagy Suppresses Sarcopenia Through Akt/mTOR and Akt/FoxO3a Signal Pathways and AMPK-Mediated Mitochondrial Quality Control. Front. Physiol. 2020, 11, 583478. [Google Scholar] [CrossRef] [PubMed]
| Items | Control | Exercise (E) | E + UCII |
|---|---|---|---|
| Glucose, mg/dL | 108.29 ± 3.19 | 102.14 ± 1.77 | 102.43 ± 1.65 |
| TC, mg/dL | 98.51 ± 1.66 | 92.06 ± 1.57 * | 95.70 ± 1.48 |
| Triglyceride, mg/dL | 103.51 ± 1.17 | 96.58 ± 1.74 * | 102.40 ± 1.82 # |
| TP, g/dL | 6.56 ± 0.19 | 6.59 ± 0.11 | 6.52 ± 0.17 |
| Albumin, g/dL | 3.43 ± 0.10 | 3.51 ± 0.09 | 3.53 ± 0.12 |
| Globulin, g/dL | 3.09 ± 0.12 | 3.29 ± 0.10 | 3.19 ± 0.10 |
| ALT, U/L | 97.29 ± 4.65 | 98.43 ± 5.42 | 95.43 ± 3.00 |
| AST, U/L | 118.43 ± 6.21 | 116.86 ± 6.28 | 114.43 ± 7.75 |
| TBil, mg/dL | 0.24 ± 0.01 | 0.24 ± 0.01 | 0.24 ± 0.01 |
| CK, IU/L | 125.80 ± 1.75 | 193.60 ± 2.86 **** | 179.98 ± 2.81 ****, ## |
| Creatine, mg/dL | 0.48 ± 0.03 | 0.47 ± 0.02 | 0.48 ± 0.04 |
| BUN, mg/dL | 20.47 ± 0.77 | 20.86 ± 0.23 | 19.44 ± 0.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orhan, C.; Sahin, E.; Er, B.; Tuzcu, M.; Lopes, A.P.; Sahin, N.; Juturu, V.; Sahin, K. Effects of Exercise Combined with Undenatured Type II Collagen on Endurance Capacity, Antioxidant Status, Muscle Lipogenic Genes and E3 Ubiquitin Ligases in Rats. Animals 2021, 11, 851. https://doi.org/10.3390/ani11030851
Orhan C, Sahin E, Er B, Tuzcu M, Lopes AP, Sahin N, Juturu V, Sahin K. Effects of Exercise Combined with Undenatured Type II Collagen on Endurance Capacity, Antioxidant Status, Muscle Lipogenic Genes and E3 Ubiquitin Ligases in Rats. Animals. 2021; 11(3):851. https://doi.org/10.3390/ani11030851
Chicago/Turabian StyleOrhan, Cemal, Emre Sahin, Besir Er, Mehmet Tuzcu, Andrey P. Lopes, Nurhan Sahin, Vijaya Juturu, and Kazim Sahin. 2021. "Effects of Exercise Combined with Undenatured Type II Collagen on Endurance Capacity, Antioxidant Status, Muscle Lipogenic Genes and E3 Ubiquitin Ligases in Rats" Animals 11, no. 3: 851. https://doi.org/10.3390/ani11030851
APA StyleOrhan, C., Sahin, E., Er, B., Tuzcu, M., Lopes, A. P., Sahin, N., Juturu, V., & Sahin, K. (2021). Effects of Exercise Combined with Undenatured Type II Collagen on Endurance Capacity, Antioxidant Status, Muscle Lipogenic Genes and E3 Ubiquitin Ligases in Rats. Animals, 11(3), 851. https://doi.org/10.3390/ani11030851









