The Chemical Ecology of Elephants: 21st Century Additions to Our Understanding and Future Outlooks
Abstract
Simple Summary
Abstract
1. Introduction
2. Background up to Turn of 21st Century
3. 21st Century Gains in Understanding
3.1. Introduction
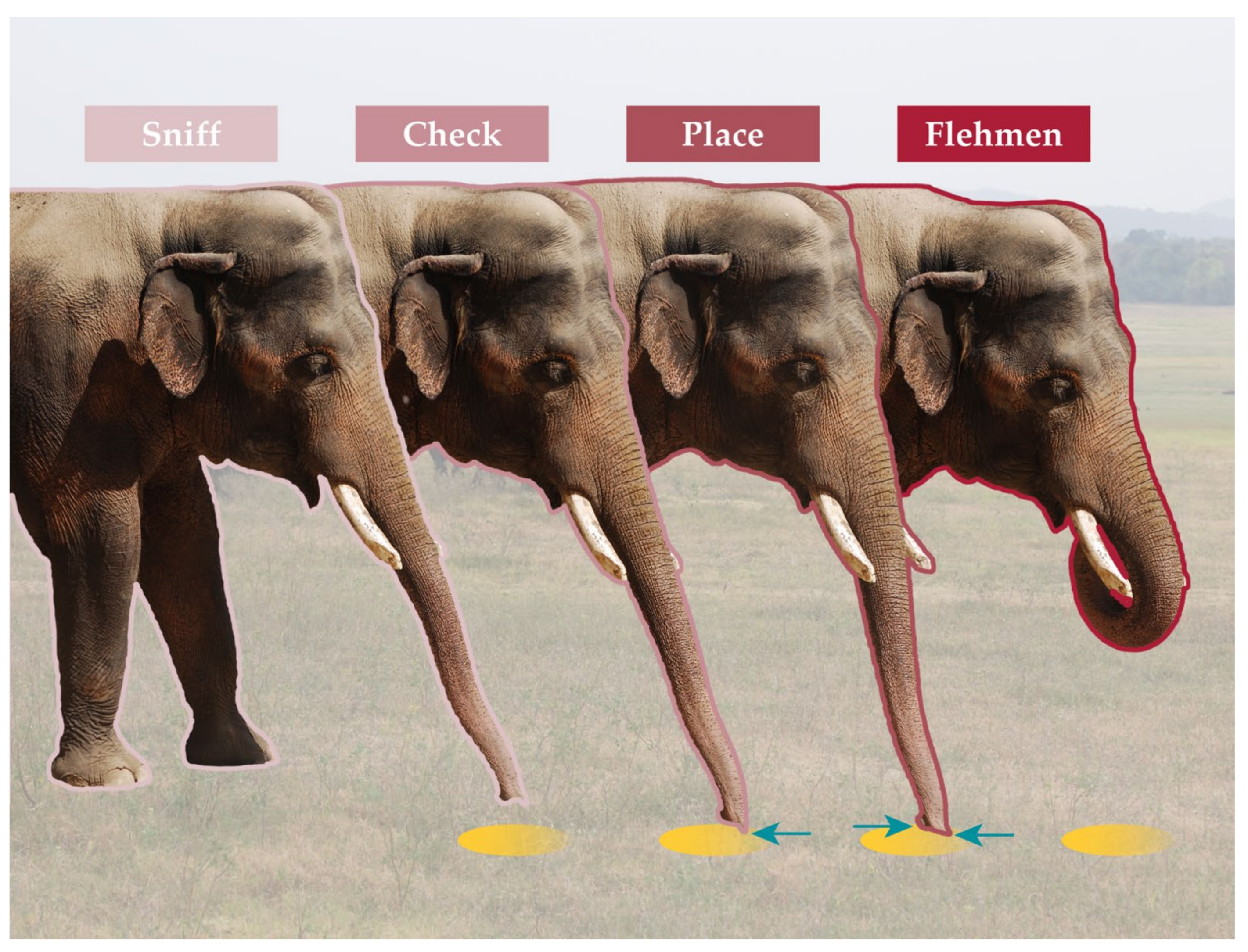
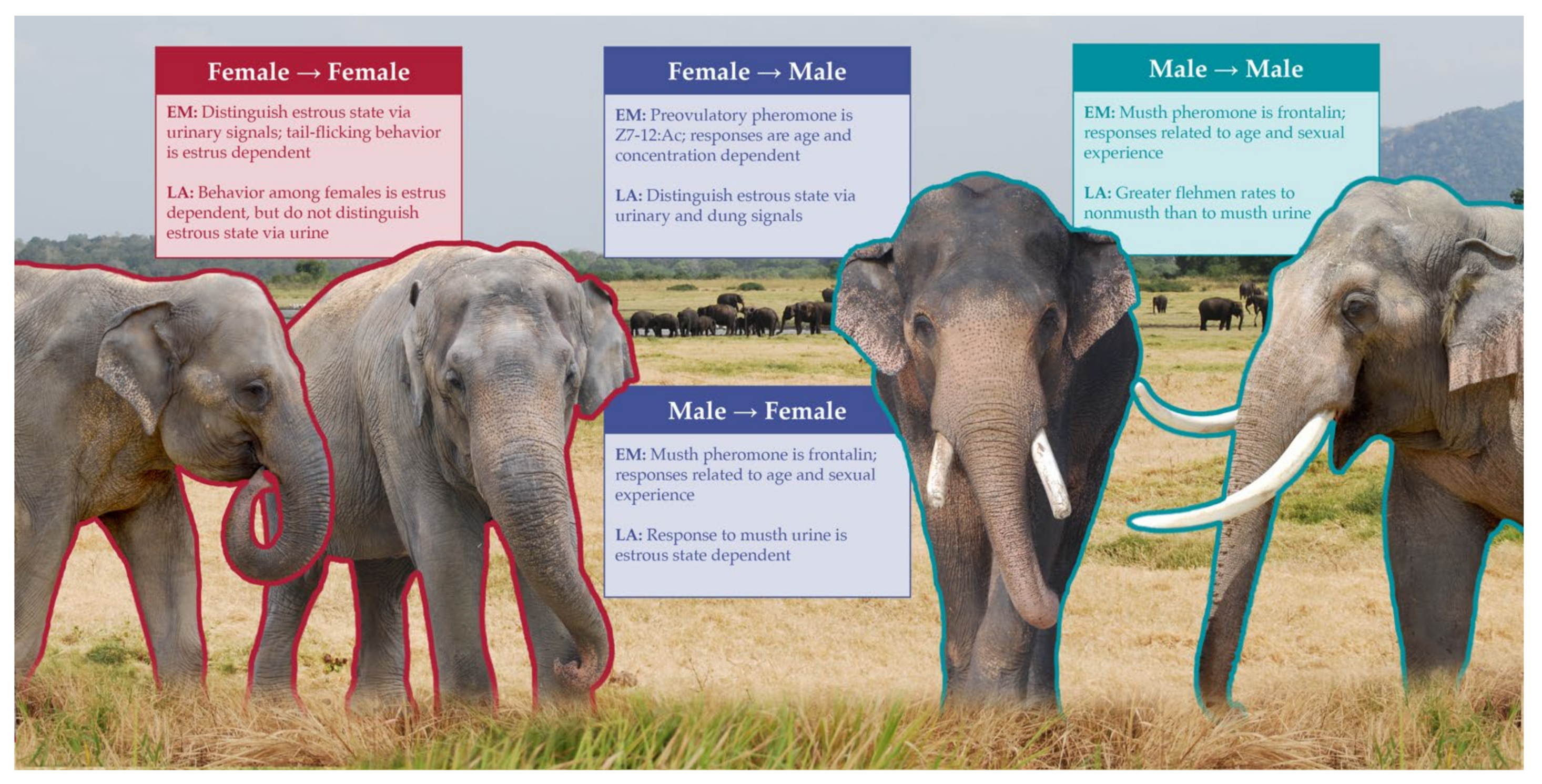
3.2. Intraspecific Chemical Signaling and Capabilities
3.3. Interspecific Chemical Ecology
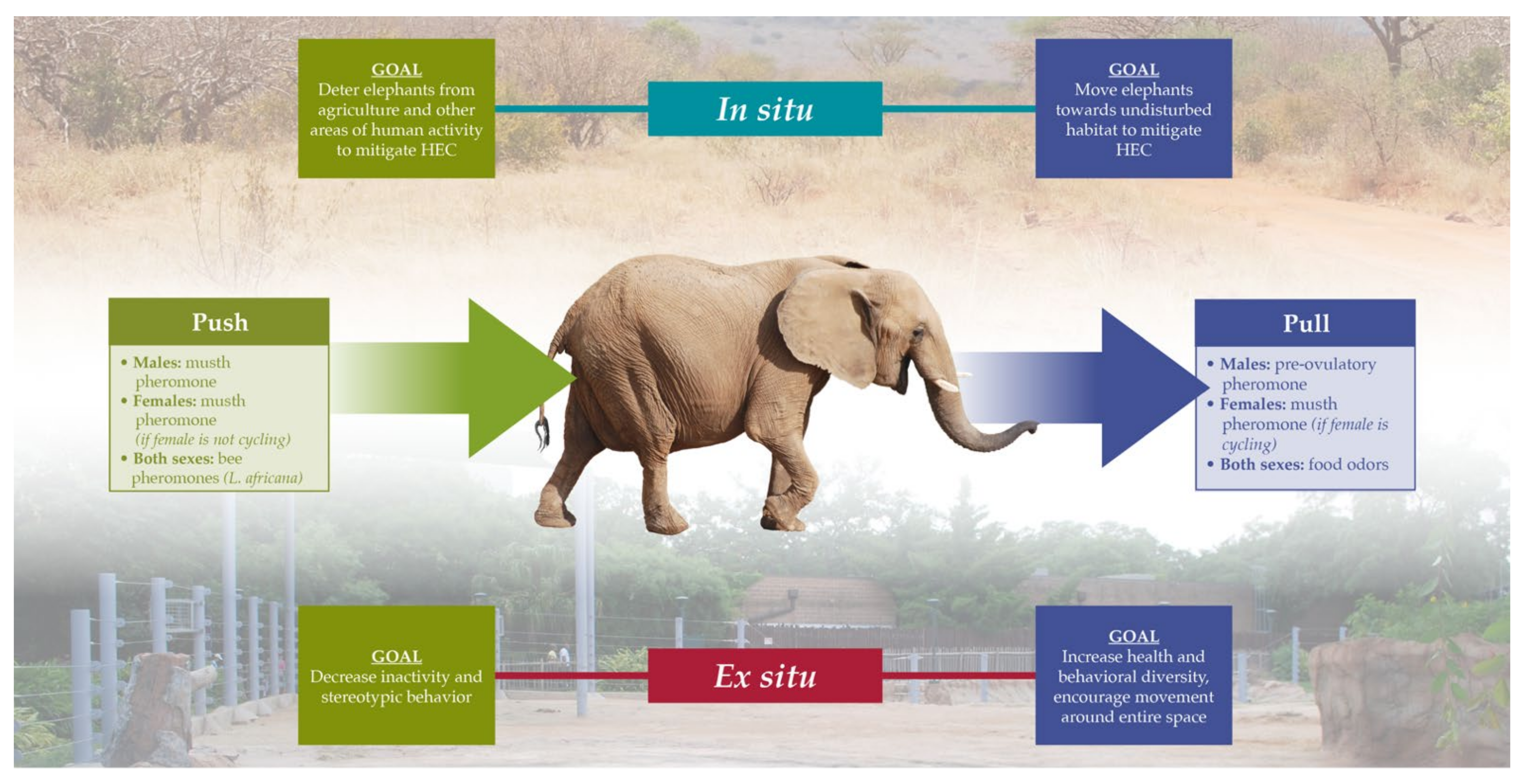
3.4. Conservation and Management Applications of Chemical Ecology of Elephants
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Sukumar, R. The Living Elephants: Evolutionary Ecology, Behavior, and Conservation; Oxford University Press: Oxford, UK, 2003; p. 478. [Google Scholar]
- De Silva, S.; Wittemyer, G. A Comparison of Social Organization in Asian Elephants and African Savannah Elephants. Int. J. Primatol. 2012, 33, 1125–1141. [Google Scholar] [CrossRef]
- Moss, C.J.; Poole, J.H. Relationships and social structure of African elephants. In Primate Social Relationships: An Integrated Approach; Hinde, R.A., Ed.; Blackwell Scientific: Oxford, UK, 1983; pp. 315–325. [Google Scholar]
- Wittemyer, G.; Getz, W. Hierarchical dominance structure and social organization in African elephants, Loxodonta africana. Anim. Behav. 2007, 73, 671–681. [Google Scholar] [CrossRef]
- Fishlock, V.; Lee, P. Forest elephants: Fission–fusion and social arenas. Anim. Behav. 2013, 85, 357–363. [Google Scholar] [CrossRef]
- Schuttler, S.G.; Philbrick, J.A.; Jeffery, K.J.; Eggert, L.S. Fine-Scale Genetic Structure and Cryptic Associations Reveal Evidence of Kin-Based Sociality in the African Forest Elephant. PLoS ONE 2014, 9, e88074. [Google Scholar] [CrossRef]
- Wittemyer, G.; Douglas-Hamilton, I.; Getz, W. The socioecology of elephants: Analysis of the processes creating multitiered social structures. Anim. Behav. 2005, 69, 1357–1371. [Google Scholar] [CrossRef]
- Archie, E.A.; Moss, C.J.; Alberts, S.C. The ties that bind: Genetic relatedness predicts the fission and fusion of social groups in wild African elephants. Proc. R. Soc. Biol. Sci. 2006, 273, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Evans, K.E.; Harris, S. Adolescence in male African elephants, Loxodonta africana, and the importance of sociality. Anim. Behav. 2008, 76, 779–787. [Google Scholar] [CrossRef]
- Chiyo, P.I.; Archie, E.A.; Hollister-Smith, J.A.; Lee, P.; Poole, J.H.; Moss, C.J.; Alberts, S.C. Association patterns of African elephants in all-male groups: The role of age and genetic relatedness. Anim. Behav. 2011, 81, 1093–1099. [Google Scholar] [CrossRef]
- Chiyo, P.I.; Wilson, J.W.; Archie, E.A.; Lee, P.C.; Moss, C.J.; Alberts, S.C. The influence of forage, protected areas, and mating prospects on grouping patterns of male elephants. Behav. Ecol. 2014, 25, 1494–1504. [Google Scholar] [CrossRef]
- Keerthipriya, P.; Nandini, S.; Vidya, T.N.C. Effects of Male Age and Female Presence on Male Associations in a Large, Polygynous Mammal in Southern India: The Asian Elephant. Front. Ecol. Evol. 2021, 9, 616666. [Google Scholar] [CrossRef]
- Jainudeen, M.R.; McKay, G.M.; Eisenberg, J.F. Observations on musth in the domesticated Asiatic elephant (Elephas maximus). Mammalia 1972, 36, 247–261. [Google Scholar] [CrossRef]
- Poole, J.H. Rutting behavior in African elephants: The phenomenon of musth. Behaviour 1987, 102, 283–316. [Google Scholar] [CrossRef]
- Poole, J.H.; Kasman, L.H.; Ramsay, E.C.; Lasley, B.L. Musth and urinary testosterone concentrations in the African elephant (Loxodonta africana). J. Reprod. Fertil. 1984, 70, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Sukumar, R. The Asian Elephant: Ecology and Management; Cambridge University Press: Cambridge, UK, 1989; p. 255. [Google Scholar]
- Freeman, E.W.; Whyte, I.; Brown, J.L. Reproductive evaluation of elephants culled in Kruger National Park, South Africa between 1975 and 1995. Afr. J. Ecol. 2009, 47, 192–201. [Google Scholar] [CrossRef]
- Moss, C.J.; Lee, P.C. Female reproductive strategies: Individual life histories. In The Amboseli Elephants: A Long-Term Perspective on a Long-Lived Mammal; Moss, C.J., Croze, H., Lee, P.C., Eds.; The University of Chicago Press: Chicago, IL, USA, 2011; pp. 187–204. [Google Scholar]
- Brown, J.L. Comparative reproductive biology of elephants. In Reproductive Sciences in Animal Conservation: Progress and Prospects; Holt, W.V., Brown, J.L., Comizzoli, P., Eds.; Springer: New York, NY, USA, 2014; pp. 135–169. [Google Scholar]
- Owen-Smith, R.N. Megaherbivores: The Influence of Very Large Body Size on Ecology; Cambridge University Press: Cambridge, UK, 1988. [Google Scholar]
- Sukumar, R. Ecology of the Asian elephant in southern India. II. Feeding habits and crop raiding patterns. J. Trop. Ecol. 1990, 6, 33–53. [Google Scholar] [CrossRef]
- Agam, A.; Barkai, R. Elephant and mammoth hunting during the Paleolithic: A review of the relevant archaeological, ethnographic and ethno-historical records. Quaternary 2018, 1, 3. [Google Scholar] [CrossRef]
- LaDue, C.A.; Schulte, B.A.; Kiso, W.K.; Freeman, E.W. Musth and sexual selection in elephants: A review of signaling properties and potential fitness consequences. Behaviour 2021. [Google Scholar] [CrossRef]
- Rasmussen, L.E.L.; Schulte, B. Chemical signals in the reproduction of Asian (Elephas maximus) and African (Loxodonta africana) elephants. Anim. Reprod. Sci. 1998, 53, 19–34. [Google Scholar] [CrossRef]
- Rasmussen, L.E.L.; Schulte, B.A. Ecological and biochemical constraints on pheromonal signaling systems in Asian elephants and their evolutionary implications. In Advances in Chemical Signals in Vertebrates; Johnston, R.E., Muller-Schwarze, D., Sorenson, P.W., Eds.; Springer: Boston, MA, USA, 1999. [Google Scholar]
- Rasmussen, L.E.L. Evolution of chemical signals in the Asian elephant, Elephas maximus: Behavioural and ecological influences. J. Biosci. 1999, 24, 241–251. [Google Scholar] [CrossRef]
- Rasmussen, L.E.L. Elephant olfaction: Smell detectors extraordinaire. ChemoSense 1999, 2, 4–5. [Google Scholar]
- Langbauer, W.R. Elephant communication. Zoo Biol. 2000, 19, 425–445. [Google Scholar] [CrossRef]
- Poole, J.H.; Moss, C.J. Musth in the African elephant, Loxodonta africana. Nature 1981, 292, 830–831. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.E.L.; Krishnamurthy, V. How chemical signals integrate Asian elephant society: The known and the unknown. Zoo Biol. 2000, 19, 405–423. [Google Scholar] [CrossRef]
- Schulte, B.A.; Rasmussen, L.E.L. Musth, sexual selection, testosterone, and metabolites. In Advances in Chemical Signals in Vertebrates; Johnston, R.E., Müller-Schwarze, D., Sorensen, P.W., Eds.; Springer: New York, NY, USA, 1999; pp. 383–397. [Google Scholar]
- Ananth, D. Musth in elephants. Zoos Print J. 2000, 15, 259–262. [Google Scholar] [CrossRef]
- Rasmussen, L.E.L.; Lee, T.D.; Roelofs, W.L.; Zhang, A.; Daves, G.D., Jr. Insect pheromone in elephants. Nature 1996, 379, 684. [Google Scholar] [CrossRef]
- Rasmussen, L.E.L.; Lee, T.D.; Zhang, A.; Roelofs, W.L.; Daves, G.D., Jr. Purification, identification, concentration and bioactivity of (Z)-7-dodecen-1-yl acetate: Sex pheromone of the female Asian elephant, Elephas maximus. Chem. Senses 1997, 22, 417–437. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rasmussen, L.E.L.; Perrin, T.E. Physiological correlates of musth: Lipid metabolites and chemical composition of exudates. Physiol. Behav. 1999, 67, 539–549. [Google Scholar] [CrossRef]
- Scott, N.L.; Rasmussen, L.E.L. Chemical communication of musth in captive male Asian elephants, Elephas maximus. In Chemical Signals in Vertebrates 10; Mason, R.T., LeMaster, M.P., Müller-Schwarze, D., Eds.; Springer: Corvallis, OR, USA, 2005; pp. 118–127. [Google Scholar]
- Rasmussen, L.E.L.; Hall-Martin, A.J.; Hess, D.L. Chemical profiles of male African elephants, Loxodonta africana: Physiological and ecological implications. J. Mammal. 1996, 77, 422–439. [Google Scholar] [CrossRef][Green Version]
- Poole, J.H. Announcing intent: The aggressive state of musth in African elephants. Anim. Behav. 1989, 37, 140–152. [Google Scholar] [CrossRef]
- Rasmussen, L.E.L. Chemosensory responses in two species of elephants to constituents of temporal gland secretion and musth urine. J. Chem. Ecol. 1988, 14, 1687–1711. [Google Scholar] [CrossRef]
- Goodwin, T.E.; Rasmussen, E.L.; Guinn, A.C.; McKelvey, S.S.; Gunawardena, R.; Riddle, S.W.; Riddle, H.S. African Elephant Sesquiterpenes. J. Nat. Prod. 1999, 62, 1570–1572. [Google Scholar] [CrossRef]
- Rasmussen, L.E.L. Chemical communication: An integral part of functional Asian elephant (Elephas maximus) society. Écoscience 1998, 5, 410–426. [Google Scholar] [CrossRef]
- Rasmussen, L.E.L. Chemical, tactile, and taste sensory systems. In Biology, Medicine, and Surgery of Elephants; Fowler, M.E., Mikota, S.K., Eds.; Blackwell Publishing: Ames, IA, USA, 2006; pp. 409–414. [Google Scholar]
- Rasmussen, L.E.L.; Munger, B.L. The sensorineural specializations of the trunk tip (finger) of the Asian elephant, Elephas maximus. Anat. Rec. 1996, 246, 127–134. [Google Scholar] [CrossRef]
- Rasmussen, L.E.; Schmidt, M.J.; Henneous, R.; Groves, D.; Daves, G.D., Jr. Asian bull elephants: Flehmen-like responses to extractable components in female elephant estrous urine. Science 1982, 217, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Schulte, B.A.; Rasmussen, L.E.L. Signal-receiver interplay in the communication of male condition by Asian elephants. Anim. Behav. 1999, 57, 1265–1274. [Google Scholar] [CrossRef] [PubMed]
- LaDue, C.A.; Goodwin, T.E.; Schulte, B.A. Concentration-dependent chemosensory responses towards pheromones are influenced by receiver attributes in Asian elephants. Ethology 2018, 124, 387–399. [Google Scholar] [CrossRef]
- Bax, P.N.; Sheldrick, D.L.W. Some preliminary observations on the food of elephant in the Tsavo Royal National Park (East) of Kenya. East Afr. Wildl. J. 1963, 1, 40–51. [Google Scholar] [CrossRef]
- Field, C.R.; Ross, I.C. The savanna ecology of Kidepo Valley National Park, II. Feeding ecology of elephant and giraffe. East Afr. Wildl. J. 1976, 14, 1–15. [Google Scholar] [CrossRef]
- Short, J. Diet and feeding behaviour of the forest elephant. Mammalia 1981, 45, 177–185. [Google Scholar] [CrossRef]
- Tchamba, M.N.; Seme, P.M. Diet and feeding behaviour of the forest elephant in the Santchou Reserve, Cameroon. Afr. J. Ecol. 1993, 31, 165–171. [Google Scholar] [CrossRef]
- White, L.J.T.; Tutin, C.E.G.; Fernandez, M. Group composition and diet of forest elephants, Loxodonta africana cyclotis Matschie 1900, in the Lopé Reserve, Gabon. Afr. J. Ecol. 1993, 31, 181–199. [Google Scholar] [CrossRef]
- Stokke, S. Sex differences in feeding-patch choice in a megaherbivore: Elephants in Chobe National Park, Botswana. Can. J. Zool. 1999, 77, 1723–1732. [Google Scholar] [CrossRef]
- Loveridge, A.J.; Hunt, J.E.; Murindagomo, F.; Macdonald, D.W. Influence of drought on predation of elephant (Loxodonta africana) calves by lions (Panthera leo) in an African wooded savannah. J. Zool. 2006, 270, 523–530. [Google Scholar] [CrossRef]
- Naughton, L.; Rose, R.; Treves, A. The Social Dimensions of Human-Elephant Conflict in AFRICA: A Literature Review and Case Studies from Uganda and Cameroon; IUCN African Elephant Specialist Group, Human-Elephant Task Conflict Task Force: Glands, Switzerland, 1999. [Google Scholar]
- Niimura, Y.; Matsui, A.; Touhara, K. Extreme expansion of the olfactory receptor gene repertoire in African elephants and evolutionary dynamics of orthologous gene groups in 13 placental mammals. Genome Res. 2014, 24, 1485–1496. [Google Scholar] [CrossRef] [PubMed]
- Arvidsson, J.; Amundin, M.; Laska, M. Successful acquisition of an olfactory discrimination test by Asian elephants, Elephas maximus. Physiol. Behav. 2011, 105, 809–814. [Google Scholar] [CrossRef]
- Rizvanovic, A.; Amundin, M.; Laska, M. Olfactory discrimination ability of Asian elephants (Elephas maximus) for structurally related odorants. Chem. Senses 2012, 38, 107–118. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miller, A.K.; Hensman, M.C.; Hensman, S.; Schultz, K.; Reid, P.; Shore, M.; Brown, J.; Furton, K.G.; Lee, S. African elephants (Loxodonta africana) can detect TNT using olfaction: Implications for biosensor application. Appl. Anim. Behav. Sci. 2015, 171, 177–183. [Google Scholar] [CrossRef]
- Ngwenya, A.; Patzke, N.; Ihunwo, A.O.; Manger, P.R. Organisation and chemical neuroanatomy of the African elephant (Loxodonta africana) olfactory bulb. Brain Struct. Funct. 2011, 216, 403–416. [Google Scholar] [CrossRef]
- Halpern, M.; Martínez-Marcos, A. Structure and function of the vomeronasal system: An update. Prog. Neurobiol. 2003, 70, 245–318. [Google Scholar] [CrossRef]
- Mohrhardt, J.; Nagel, M.; Fleck, D.; Ben-Shaul, Y.; Spehr, M. Signal detection and coding in the accessory olfactory system. Chem. Senses 2018, 43, 667–695. [Google Scholar] [CrossRef]
- Johnson, E.W.; Rasmussen, L.E.L. Morphological characteristics of the vomeronasal organ of the newborn Asian elephant (Elephas maximus). Anat. Rec. 2002, 267, 252–259. [Google Scholar] [CrossRef]
- Goöbbel, L.; Fischer, M.S.; Smith, T.D.; Wible, J.R.; Bhatnagar, K.P. The vomeronasal organ and associated structures of the fetal African elephant, Loxodonta africana (Proboscidea, Elephantidae). Acta Zool. 2004, 85, 41–52. [Google Scholar] [CrossRef]
- Lamps, L.W.; Smoller, B.R.; Goodwin, T.E.; Rasmussen, L.E.L. Hormone receptor expression in interdigital glands of the Asian elephant (Elephas maximus). Zoo Biol. 2004, 23, 463–469. [Google Scholar] [CrossRef]
- Lamps, L.W.; Smoller, B.R.; Rasmussen, L.E.L.; Slade, B.E.; Fritsch, G.; Goodwin, T.E. Characterization of interdigital glands in the Asian elephant (Elephas maximus). Res. Vet. Sci. 2001, 71, 197–200. [Google Scholar] [CrossRef]
- Meyer, W. Demonstration of lysozyme and antimicrobial peptides in the temporal gland of the African elephant (Loxodonta africana). Mamm. Biol. 2007, 72, 251–255. [Google Scholar] [CrossRef]
- Rasmussen, L.E.L.; Greenwood, D.R. Frontalin: A chemical message of musth in Asian elephants (Elephas maximus). Chem. Senses 2003, 28, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.E.L.; Riddle, H.S.; Krishnamurthy, V. Mellifluous matures to malodorous in musth. Nature 2002, 415, 975–976. [Google Scholar] [CrossRef]
- Greenwood, D.R.; Comeskey, D.; Hunt, M.B.; Rasmussen, L.E.L. Chirality in elephant pheromones. Nature 2005, 438, 1097–1098. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.E.L.B.; Greenwood, D.R.; Goodwin, T.E.; Schulte, B.A. Asian elephant reflections: Chirality counts. In Chemical Signals in Vertebrates 13; Schulte, B.A., Goodwin, T.E., Ferkin, M.H., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 229–244. [Google Scholar] [CrossRef]
- Rasmussen, L.E.L.; Riddle, H.S. Elephant breath: Clues about health, disease, metabolism and social signals. J. Elephant Man. Assoc. 2004, 15, 24–33. [Google Scholar]
- Rasmussen, L.E.L.; Wittemyer, G. Chemosignalling of musth by individual wild African elephants (Loxodonta africana): Implications for conservation and management. Proc. R. Soc. Lond. B Biol. Sci. 2002, 269, 853–860. [Google Scholar] [CrossRef]
- Goodwin, T.E.; Eggert, M.S.; House, S.J.; Weddell, M.E.; Schulte, B.A.; Rasmussen, L.E.L. Insect pheromones and precursors in female African elephant urine. J. Chem. Ecol. 2006, 32, 1849–1853. [Google Scholar] [CrossRef]
- Castelda, S.M.; Goodwin, T.E.; Schulte, B.A. Investigating chemical signals in African elephants for convergence with insects and similarities with Asian elephants. In Proceedings of the 2007 International Elephant Conservation & Research Symposium, International Elephant Foundation, Orlando, FL, USA, 2–4 November 2007; pp. 81–91. [Google Scholar]
- Archie, E.A.; Theis, K.R. Animal behaviour meets microbial ecology. Anim. Behav. 2011, 82, 425–436. [Google Scholar] [CrossRef]
- Goodwin, T.E.; Broederdorf, L.J.; Burkert, B.A.; Hirwa, I.H.; Mark, D.B.; Waldrip, Z.J.; Kopper, R.A.; Sutherland, M.V.; Freeman, E.W.; Hollister-Smith, J.A.; et al. Chemical signals of elephant musth: Temporal aspects of microbially-mediated modifications. J. Chem. Ecol. 2012, 38, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, T.E.; Harelimana, I.H.; MacDonald, L.J.; Mark, D.B.; Juru, A.U.; Yin, Q.; Engman, J.A.; Kopper, R.A.; Lichti, C.F.; Mackintosh, S.G.; et al. The role of bacteria in chemical signals of elephant musth: Proximate causes and biochemical pathways. In Chemical Signals in Vertebrates 13; Schulte, B.A., Goodwin, T.E., Ferkin, M.H., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 63–85. [Google Scholar] [CrossRef]
- Ganswindt, A.; Heistermann, M.; Hodges, K. Physical, physiological, and behavioral correlates of musth in captive African elephants (Loxodonta africana). Physiol. Biochem. Zool. 2005, 78, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Ganswindt, A.; Rasmussen, H.B.; Heistermann, M.; Hodges, J.K. The sexually active states of free-ranging male African elephants (Loxodonta africana): Defining musth and non-musth using endocrinology, physical signals, and behavior. Horm. Behav. 2005, 47, 83–91. [Google Scholar] [CrossRef]
- Schulte, B.A.; Bagley, K.R.; Groover, M.; Loizi, H.; Merte, C.; Meyer, J.M.; Napora, E.; Stanley, L.; Vyas, D.K.; Wollett, K.; et al. Comparisons of state and likelihood of performing chemosensory event behaviors in two populations of African elephants (Loxodonta africana). In Chemical Signals in Vertebrates 11; Hurst, J., Beynon, R.J., Roberts, S.C., Wyatt, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 81–92. [Google Scholar]
- Schulte, B.A.; Loizi, H.; Bagley, K.; Gray, A.; Stanley, L.; Correll, M.; Goodwin, T.E.; Brown, P.A.; Davis, B.L.; Dill, W.M.; et al. Elephant chemotactile communication and conservation. J. Elephant Man. Assoc. 2004, 15, 16–23. [Google Scholar]
- Schulte, B.A.; Bagley, K.; Correll, M.; Gray, A.; Heineman, S.M.; Loizi, H.; Malament, M.; Scott, N.L.; Slade, B.E.; Stanley, L.; et al. Assessing chemical communication in elephants. In Chemical Signals in Vertebrates 10; Mason, R.T., LeMaster, M.P., Müller-Schwarze, D., Eds.; Springer: Boston, MA, USA, 2005; pp. 140–151. [Google Scholar]
- Schulte, B.A.; Bagley, K.R.; Castelda, S.; Loizi, H.; Nasseri, N.; Vyas, D.K.; Goodwin, T.E. From exploration to selective information gathering: The development of chemosensory investigation in male African elephants (Loxodonta africana). In Chemical Signals in Vertebrates 12; East, M.L., Dehnhard, M., Eds.; Springer Science Business Media: New York, NY, USA, 2013; pp. 135–143. [Google Scholar]
- Bagley, K.R.; Goodwin, T.E.; Rasmussen, L.E.L.; Schulte, B.A. Male African elephants, Loxodonta africana, can distinguish oestrous status via urinary signals. Anim. Behav. 2006, 71, 1439–1445. [Google Scholar] [CrossRef]
- Rasmussen, L.E.L.; Schulte, B.; Goodwin, T.; Whitehouse, A.; Loizi, H. Sexual dimorphism in the performance of chemosensory investigatory behaviours by African elephants (Loxodonta africana). Behaviour 2009, 146, 373–392. [Google Scholar] [CrossRef]
- Merte, C.E.; Goodwin, T.E.; Schulte, B.A. Male and female developmental differences in chemosensory investigations by African elephants (Loxodonta africana) approaching waterholes. Behav. Ecol. Sociobiol. 2010, 64, 401–408. [Google Scholar] [CrossRef]
- Dürckheim, K.E.M.v. Olfaction and Scent Discrimination in African Elephants (Loxodonta africana); Stellenbosch University: Stellenbosch, South Africa, 2021. [Google Scholar]
- Weissenböck, N.M.; Schwammer, H.M.; Ruf, T. Estrous synchrony in a group of African elephants (Loxodonta africana) under human care. Anim. Reprod. Sci. 2009, 113, 322–327. [Google Scholar] [CrossRef]
- Goodwin, T.E.; Rasmussen, L.E.L.; Schulte, B.A.; Brown, P.A.; Davis, B.L.; Dill, W.M.; Dowdy, N.C.; Hicks, A.R.; Morshedi, R.G.; Mwanza, D.; et al. Chemical analysis of preovulatory female African elephant urine: A search for putative pheromones. In Chemical Signals in Vertebrates 10; Mason, R.T., LeMaster, M.P., Muller-Schwarze, D., Eds.; Springer: Boston, MA, USA, 2005; pp. 128–139. [Google Scholar]
- Goodwin, T.E.; Schulte, B.A. Prospecting for mammalian chemical signals via solventless extraction techniques: An elephantine task. ChemoSense 2009, 11, 9–15. [Google Scholar]
- Goodwin, T.E.; Brown, P.A.; Eggert, M.S.; Evola, M.G.; House, S.J.; Morshedi, R.G.; Weddell, M.E.; Chen, C.J.; Jackson, S.R.; Aubut, Y.; et al. Use of automated solid phase dynamic extraction (SPDE)/GC-MS and novel macros in the search for African elephant pheromones. In Chemical Signals in Vertebrates 11; Hurst, J., Beynon, R.J., Roberts, S.C., Wyatt, T., Eds.; Springer: Boston, MA, USA, 2007; pp. 25–35. [Google Scholar]
- Meyer, J.M.; Goodwin, T.E.; Schulte, B.A. Intrasexual chemical communication and social responses of captive female African elephants, Loxodonta africana. Anim. Behav. 2008, 76, 163–174. [Google Scholar] [CrossRef]
- Hollister-Smith, J.A.; Alberts, S.C.; Rasmussen, L.E.L. Do male African elephants, Loxodonta africana, signal musth via urine dribbling? Anim. Behav. 2008, 76, 1829–1841. [Google Scholar] [CrossRef]
- Apps, P.J. Are mammal olfactory signals hiding right under our noses? Naturwissenschaften 2013, 100, 487–506. [Google Scholar] [CrossRef] [PubMed]
- Ghosal, R.; Seshagiri, P.B.; Sukumar, R. Dung as a potential medium for inter-sexual chemical signaling in Asian elephants (Elephas maximus). Behav. Process. 2012, 91, 15–21. [Google Scholar] [CrossRef]
- Bates, L.A.; Sayialel, K.N.; Njiraini, N.W.; Poole, J.H.; Moss, C.J.; Byrne, R.W. African elephants have expectations about the locations of out-of-sight family members. Biol. Lett. 2008, 4, 34–36. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.R.B.; Brent, L.J.N.; Motsentwa, T.; Croft, D.P. Field evidence supporting monitoring of chemical information on pathways by male African elephants. Anim. Behav. 2021, 176, 193–206. [Google Scholar] [CrossRef]
- Esposito, R.M. Effect of Matriarchs on Group Interactions, Kinship Fitness, and Differences in Chemosensory Behavior of African Elephants (Loxodonta africana); Georgia Southern University: Statesboro, GA, USA, 2008. [Google Scholar]
- Riddle, H.S.; Riddle, S.W.; Rasmussen, L.E.L.; Goodwin, T.E. First disclosure and preliminary investigation of a liquid released from the ears of African elephants. Zoo Biol. 2000, 19, 475–480. [Google Scholar] [CrossRef]
- Gröschl, M. The physiological role of hormones in saliva. Bioessays 2009, 31, 843–852. [Google Scholar] [CrossRef]
- Booth, W.D. Factors affecting the pheromone composition of voided boar saliva. J. Reprod. Fertil. 1987, 81, 427–431. [Google Scholar] [CrossRef]
- Stopka, P.; Kuntová, B.; Klempt, P.; Havrdová, L.; Černá, M.; Stopková, R. On the saliva proteome of the Eastern European house mouse (Mus musculus musculus) focusing on sexual signalling and immunity. Sci. Rep. 2016, 6, 32481. [Google Scholar] [CrossRef]
- Allen, C.R.B.; Croft, D.P.; Testard, C.; Brent, L.J.N. Function of trunk-mediated “greeting” behaviours between male African elephants: Insights from choice of partners. Animals 2021, 11, 2718. [Google Scholar] [CrossRef]
- Poole, J.H.; Granli, P. Signals, gestures, and behavior of African elephants. In The Amboseli Elephants: A Long-Term Perspective on a Long-Lived Mammal; Moss, C.J., Croze, H., Lee, P.C., Eds.; The University of Chicago Press: Chicago, IL, USA, 2011; pp. 109–124. [Google Scholar]
- Sharma, N.; Pokharel, S.S.; Kohshima, S.; Sukumar, R. Behavioural responses of free-ranging Asian elephants (Elephas maximus) towards dying and dead conspecifics. Primates 2019, 61, 128–129. [Google Scholar] [CrossRef]
- Goldenberg, S.Z.; Wittemyer, G. Elephant behavior toward the dead: A review and insights from field observations. Primates 2020, 61, 119–128. [Google Scholar] [CrossRef]
- Stephan, C.; Bahamboula, J.J.D.; Brncic, T.M. Responses to a poached conspecific in wild forest elephants (Loxodonta africana cyclotis). Behaviour 2020, 157, 823–833. [Google Scholar] [CrossRef]
- Budd, K.; Gunn, J.C.; Finch, T.; Klymus, K.; Sitati, N.; Eggert, L.S. Effects of diet, habitat, and phylogeny on the fecal microbiome of wild African savanna (Loxodonta africana) and forest elephants (L. cyclotis). Ecol. Evol. 2020, 10, 5637–5650. [Google Scholar] [CrossRef] [PubMed]
- Parsons, M.H.; Apfelbach, R.; Banks, P.B.; Cameron, E.Z.; Dickman, C.R.; Frank, A.S.K.; Jones, M.E.; McGregor, I.S.; McLean, S.; Müller-Schwarze, D.; et al. Biologically meaningful scents: A framework for understanding predator–prey research across disciplines. Biol. Rev. 2018, 93, 98–114. [Google Scholar] [CrossRef] [PubMed]
- Bates, L.A.; Sayialel, K.N.; Njiraini, N.W.; Moss, C.J.; Poole, J.H.; Byrne, R.W. Elephants classify human ethnic groups by odor and garment color. Curr. Biol. 2007, 17, 1938–1942. [Google Scholar] [CrossRef] [PubMed]
- Power, R.J.; Compion, R.X.S. Lion predation on elephants in the Savuti, Chobe National Park, Botswana. Afr. Zool. 2009, 44, 36–44. [Google Scholar] [CrossRef]
- Kumaraguru, A.; Saravanamuthu, R.; Brinda, K.; Asokan, S. Prey preference of large carnivores in Anamalai Tiger Reserve, India. Eur. J. Wildl. Res. 2011, 57, 627–637. [Google Scholar] [CrossRef]
- McComb, K.; Shannon, G.; Durant, S.M.; Sayialel, K.; Slotow, R.; Poole, J.; Moss, C. Leadership in elephants: The adaptive value of age. Proc. R. Soc. Lond. B Biol. Sci. 2011, 278, 3270–3276. [Google Scholar] [CrossRef]
- Thuppil, V.; Coss, R.G. Wild Asian elephants distinguish aggressive tiger and leopard growls according to perceived danger. Biol. Lett. 2013, 9, 20130518. [Google Scholar] [CrossRef]
- Valenta, K.; Schmitt, M.H.; Ayasse, M.; Nevo, O. The sensory ecology of fear: African elephants show aversion to olfactory predator signals. Conserv. Sci. Pract. 2021, 3, e333. [Google Scholar] [CrossRef]
- Wittemyer, G.; Polansky, L.; Douglas-Hamilton, I.; Getz, W.M. Disentangling the effects of forage, social rank, and risk on movement autocorrelation of elephants using Fourier and wavelet analyses. Proc. Natl. Acad. Sci. USA 2008, 105, 19108–19113. [Google Scholar] [CrossRef]
- Iason, G. The role of plant secondary metabolites in mammalian herbivory: Ecological perspectives. Proc. Nutr. Soc. 2005, 64, 123–131. [Google Scholar] [CrossRef]
- Hansen, S.C.; Stolter, C.; Imholt, C.; Jacob, J. Plant secondary metabolites as rodent repellents: A systematic review. J. Chem. Ecol. 2016, 42, 970–983. [Google Scholar] [CrossRef] [PubMed]
- Scogings, P.F.; Hjältén, J.; Skarpe, C. Secondary metabolites and nutrients of woody plants in relation to browsing intensity in African savannas. Oecologia 2011, 167, 1063–1073. [Google Scholar] [CrossRef] [PubMed]
- Scogings, P.F.; Hjältén, J.; Skarpe, C.; Hattas, D.; Zobolo, A.; Dziba, L.; Rooke, T. Nutrient and secondary metabolite concentrations in a savanna are independently affected by large herbivores and shoot growth rate. Plant Ecol. 2014, 215, 73–82. [Google Scholar] [CrossRef]
- Stokke, S.; du Toit, J.T. Sexual segregation in habitat use by elephants in Chobe National Park, Botswana. Afr. J. Ecol. 2002, 40, 360–371. [Google Scholar] [CrossRef]
- Makhabu, S.W. Interactions between Woody Plants, Elephants and Other Browsers in the Chobe Riverfront Botswana. Ph.D. Thesis, Norwegian University of Science and Technology, Trondheim, Norway, 2005. [Google Scholar]
- Makhabu, S.W.; Skarpe, C. Rebrowsing by elephants three years after simulated browsing on five woody plant species in northern Botswana. S. Afr. J. Wildl. Res. 2006, 36, 99–102. [Google Scholar]
- Hemborg, Å.M.; Bond, W.J. Do browsing elephants damage female trees more? Afr. J. Ecol. 2007, 45, 41–48. [Google Scholar] [CrossRef]
- Joshi, R.; Singh, R. Feeding behaviour of wild Asian elephants (Elephas maximus) in the Rajaji National Park. J. Am. Sci. 2008, 4, 34–48. [Google Scholar]
- Bal, P.; Nath, C.D.; Nanaya, K.M.; Kushalappa, C.G.; Garcia, C. Elephants also like coffee: Trends and drivers of human–elephant conflicts in coffee agroforestry landscapes of Kodagu, Western Ghats, India. Environ. Manag. 2011, 47, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Shannon, G.; Mackey, R.L.; Slotow, R. Diet selection and seasonal dietary switch of a large sexually dimorphic herbivore. Acta Oecologica 2013, 46, 48–55. [Google Scholar] [CrossRef]
- English, M.; Gillespie, G.; Ancrenaz, M.; Ismail, S.; Goossens, B.; Nathan, S.; Linklater, W. Plant selection and avoidance by the Bornean elephant (Elephas maximus borneensis) in tropical forest: Does plant recovery rate after herbivory influence food choices? J. Trop. Ecol. 2014, 30, 371–379. [Google Scholar] [CrossRef]
- English, M.; Gillespie, G.; Goosens, B.; Ismail, S.; Ancrenaz, M.; Linklater, W. Recursion to food plants by free-ranging Bornean elephant. PeerJ 2015, 3, e1030. [Google Scholar] [CrossRef] [PubMed]
- Branco, P.S.; Merkle, J.A.; Pringle, R.M.; Pansu, J.; Potter, A.B.; Reynolds, A.; Stalmans, M.; Long, R.A. Determinants of elephant foraging behaviour in a coupled human-natural system: Is brown the new green? J. Anim. Ecol. 2019, 88, 780–792. [Google Scholar] [CrossRef] [PubMed]
- Codron, J.; Lee-Thorp, J.A.; Sponheimer, M.; Codron, D.; Grant, R.C.; de Ruiter, D.J. Elephant (Loxodonta africana) diets in Kruger National Park, South Africa: Spatial and landscape differences. J. Mammal. 2006, 87, 27–34. [Google Scholar] [CrossRef]
- Owen-Smith, N.; Chafota, J. Selective feeding by a megaherbivore, the African elephant (Loxodonta africana). J. Mammal. 2012, 93, 698–705. [Google Scholar] [CrossRef]
- Shrader, A.M.; Bell, C.; Bertolli, L.; Ward, D. Forest or the trees: At what scale do elephants make foraging decisions? Acta Oecologica 2012, 42, 3–10. [Google Scholar] [CrossRef]
- Schmitt, M.H.; Shuttleworth, A.; Shrader, A.M.; Ward, D. The role of volatile plant secondary metabolites as pre-ingestive cues and potential toxins dictating diet selection by African elephants. Oikos 2019, 129, 24–34. [Google Scholar] [CrossRef]
- Schmitt, M.H.; Shuttleworth, A.; Ward, D.; Shrader, A.M. African elephants use plant odours to make foraging decisions across multiple spatial scales. Anim. Behav. 2018, 141, 17–27. [Google Scholar] [CrossRef]
- Plotnik, J.M.; Shaw, R.C.; Brubaker, D.L.; Tiller, L.N.; Clayton, N.S. Thinking with their trunks: Elephants use smell but not sound to locate food and exclude nonrewarding alternatives. Anim. Behav. 2014, 88, 91–98. [Google Scholar] [CrossRef]
- Plotnik, J.M.; Brubaker, D.L.; Dale, R.; Tiller, L.N.; Mumby, H.S.; Clayton, N.S. Elephants have a nose for quantity. Proc. Natl. Acad. Sci. USA 2019, 116, 12566–12571. [Google Scholar] [CrossRef]
- Wood, M.; Chamaillé-Jammes, S.; Hammerbacher, A.; Shrader, A.M. African elephants can detect water from natural and artificial sources via olfactory cues. Anim. Cogn. 2021. [Google Scholar] [CrossRef] [PubMed]
- Gobush, K.S.; Edwards, C.T.T.; Balfour, D.; Wittemyer, G.; Maisels, F.; Taylor, R.D. Loxodonta africana. The IUCN Red List of Threatened Species. 2021. Available online: https://dx.doi.org/10.2305/IUCN.UK.2021-2.RLTS.T181008073A204401095.en (accessed on 12 August 2021).
- Gobush, K.S.; Edwards, C.T.T.; Balfour, D.; Wittemyer, G.; Maisels, F.; Taylor, R.D. Loxodonta cyclotis. The IUCN Red List of Threatened Species. 2021. Available online: https://dx.doi.org/10.2305/IUCN.UK.2021-1.RLTS.T181007989A204404464.en (accessed on 12 August 2021).
- Williams, C.; Tiwari, S.K.; Goswami, V.R.; de Silva, S.; Kumar, A.; Baskaran, N.; Yoganand, K.; Menon, V. Elephas maximus. The IUCN Red List of Threatened Species. 2020. Available online: https://dx.doi.org/10.2305/IUCN.UK.2020-3.RLTS.T7140A45818198.en (accessed on 12 August 2021).
- Cook, S.M.; Khan, Z.R.; Pickett, J.A. The use of push-pull strategies in integrated pest management. Annu. Rev. Entomol. 2007, 52, 375–400. [Google Scholar] [CrossRef]
- Schulte, B.A. Learning and applications of chemical signals in vertebrates for human–wildlife conflict mitigation. In Chemical Signals in Vertebrates 13; Schulte, B.A., Goodwin, T.E., Ferkin, M.H., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 499–510. [Google Scholar] [CrossRef]
- Schulte, B.A.; Freeman, E.W.; Goodwin, T.E.; Hollister-Smith, J.; Rasmussen, L.E.L. Honest signalling through chemicals by elephants with applications for care and conservation. Appl. Anim. Behav. Sci. 2007, 102, 344–363. [Google Scholar] [CrossRef]
- Jackson, J.; Childs, D.Z.; Mar, K.U.; Htut, W.; Lummaa, V. Long-term trends in wild-capture and population dynamics point to an uncertain future for captive elephants. Proc. R. Soc. B 2019, 286, 20182810. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.L. Update on comparative biology of elephants: Factors affecting reproduction, health and welfare. In Reproductive Sciences in Animal Conservation, 2nd ed.; Comizzoli, P., Brown, J.L., Holt, W.V., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 243–273. [Google Scholar] [CrossRef]
- Schulte, B.A. Social structure and helping behavior in captive elephants. Zoo Biol. 2000, 19, 447–459. [Google Scholar] [CrossRef]
- Brown, J.L.; Paris, S.; Prado-Oviedo, N.A.; Meehan, C.L.; Hogan, J.N.; Morfeld, K.A.; Carlstead, K. Reproductive health assessment of female elephants in North American zoos and association of husbandry practices with reproductive dysfunction in African elephants (Loxodonta africana). PLoS ONE 2016, 11, e0145673. [Google Scholar] [CrossRef]
- Meehan, C.L.; Hogan, J.N.; Bonaparte-Saller, M.K.; Mench, J.A. Housing and social environments of African (Loxodonta africana) and Asian (Elephas maximus) elephants in North American zoos. PLoS ONE 2016, 11, e0146703. [Google Scholar] [CrossRef]
- Bechert, U.S.; Brown, J.L.; Dierenfeld, E.S.; Ling, P.D.; Molter, C.M.; Schulte, B.A. Zoo elephant research: Contributions to conservation of captive and free-ranging species. Int. Zoo Yearb. 2019, 53, 89–115. [Google Scholar] [CrossRef]
- Meehan, C.; Greco, B.; Lynn, B.; Morfeld, K.; Vicino, G.; Orban, D.; Gorsuch, C.; Quick, M.; Ripple, L.; Fournier, K.; et al. The Elephant Welfare Initiative: A model for advancing evidence-based zoo animal welfare monitoring, assessment and enhancement. Int. Zoo Yearb. 2019, 53, 45–61. [Google Scholar] [CrossRef]
- Wyatt, T.D. Pheromones and Animal Behavior: Chemical Signals and Signatures, 2nd ed.; Cambridge University Press: Cambridge, UK, 2014; p. 405. [Google Scholar]
- Swaisgood, R.R.; Schulte, B.A. Applying knowledge of mammalian social organization, mating systems, and communication to management. In Wild Mammals in Captivity: Principles and Techniques for Zoo Management, Second Edition; Kleiman, D.G., Thompson, K.V., Eds.; University of Chicago Press: Chicago, IL, USA, 2010; pp. 329–343. [Google Scholar]
- Clark, F.; King, A.J. A.J. A critical review of zoo-based olfactory enrichment. In Chemical Signals in Vertebrates 11; Hurst, J., Beynon, R.J., Roberts, S.C., Wyatt, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 391–398. [Google Scholar] [CrossRef]
- Mellen, J.; MacPhee, M.S. Philosophy of environmental enrichment: Past, present, and future. Zoo Biol. 2001, 20, 211–226. [Google Scholar] [CrossRef]
- Alligood, C.; Leighty, K. Putting the “E” in SPIDER: Evolving trends in the evaluation of environmental enrichment efficacy in zoological settings. Anim. Behav. Cogn. 2015, 2, 200–217. [Google Scholar] [CrossRef]
- Greco, B.J.; Meehan, C.L.; Hogan, J.N.; Leighty, K.A.; Mellen, J.; Mason, G.J.; Mench, J.A. The days and nights of zoo elephants: Using epidemiology to better understand stereotypic behavior of African elephants (Loxodonta africana) and Asian elephants (Elephas maximus) in North American Zoos. PLoS ONE 2016, 11, e0144276. [Google Scholar] [CrossRef] [PubMed]
- LaDue, C.A.; Schulte, B.A. Pheromonal enrichment in the zoo: An empirical approach with Asian elephants (Elephas maximus). Appl. Anim. Behav. Sci. 2021, 235, 105228. [Google Scholar] [CrossRef]
- Mason, G.J. Species differences in responses to captivity: Stress, welfare and the comparative method. Trends Ecol. Evol. 2010, 25, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.S.; Owen, M.; Wintle, N.J.P.; Zhang, G.; Zhang, H.; Swaisgood, R.R. Stereotypic behaviour predicts reproductive performance and litter sex ratio in giant pandas. Sci. Rep. 2020, 10, 7263. [Google Scholar] [CrossRef] [PubMed]
- Swaisgood, R.R.; Lindburg, D.; White, A.M.; Zhang, H.; Zhou, X. Chemical communication in giant pandas. In Giant Pandas: Biology and Conservation; Lindburg, D., Baragona, K., Eds.; University of California Press: Berkeley, CA, USA, 2004; pp. 106–124. [Google Scholar]
- Thouless, C.R.; Dublin, H.T.; Blanc, J.J.; Skinner, D.P.; Daniel, T.E.; Taylor, R.D.; Maisels, F.; Frederick, H.L.; Bouché, P. African Elephant Status Report 2016: An Update from the African Elephant Database; International Union for the Conservation of Nature and Natural Resources: Gland, Switzerland, 2016. [Google Scholar]
- Calabrese, A.; Calabrese, J.M.; Songer, M.; Wegmann, M.; Hedges, S.; Rose, R.; Leimgruber, P. Conservation status of Asian elephants: The influence of habitat and governance. Biodivers. Conserv. 2017, 26, 2067–2081. [Google Scholar] [CrossRef]
- Shaffer, L.J.; Khadka, K.K.; Hoek, J.V.D.; Naithani, K.J. Human-elephant conflict: A review of current management strategies and future directions. Front. Ecol. Evol. 2019, 6, 235. [Google Scholar] [CrossRef]
- De Sales, A.R.; Anastácio, R.S.S.; Pereira, M.J. The African elephant (Loxodonta africana): Mini-review of an endangered species. Nat. Resour. 2020, 11, 317–350. [Google Scholar] [CrossRef]
- Gross, E.M.; Lahkar, B.P.; Subedi, N.; Nyirenda, V.R.; Klebelsberg, E.; Jakoby, O. Elephants in the village: Causes and consequences of property damage in Asia and Africa. Conserv. Sci. Pract. 2021, 3, e343. [Google Scholar] [CrossRef]
- Brunnermeier, M.J.; Schmied, S.A.K.; Schupfner, R. Distribution of 14C, 90Sr, and 228Th in an elephant tusk. J. Radioanal. Nucl. Chem. 2012, 292, 1285–1290. [Google Scholar] [CrossRef]
- Wasser, S.K.; Mailand, C.; Booth, R.; Mutayoba, B.; Kisamo, E.; Clark, B.; Stephens, M. Using DNA to track the origin of the largest ivory seizure since the 1989 trade ban. Proc. Natl. Acad. Sci. USA 2007, 104, 4228–4233. [Google Scholar] [CrossRef] [PubMed]
- Guldemond, R.A.R.; Purdon, A.; van Aarde, R.J. A systematic review of elephant impact across Africa. PLoS ONE 2017, 12, e0178935. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, M.H.; Ward, D.; Shrader, A.M. Incorporating secondary metabolites, tannin-binding proteins, and diet breadth into carrying-capacity models for African elephants. Ecol. Model. 2016, 332, 8–18. [Google Scholar] [CrossRef]
- Wright, M.G.; Spencer, C.; Cook, R.M.; Henley, M.D.; North, W.; Mafra-Neto, A. African bush elephants respond to a honeybee alarm pheromone blend. Curr. Biol. 2018, 28, R778–R780. [Google Scholar] [CrossRef]
- Mafra-Neto, A.; Lame, F.M.d.; Fettig, C.J.; Munson, A.S.; Perring, T.M.; Stelinski, L.L.; Stoltman, L.L.; Mafra, L.E.J.; Borges, R.; Vargas, R.I. Manipulation of insect behavior with specialized pheromone and lure application technology (SPLAT®). In Pest Management with Natural Products; Beck, J.J., Coats, J.R., Duke, S.O., Koivunen, M.E., Eds.; American Chemical Society: Washington, DC, USA, 2013; pp. 31–58. [Google Scholar]
- Gross, E.M.; Drouet-Hoguet, N.; Subedi, N.; Gross, J. The potential of medicinal and aromatic plants (MAPs) to reduce crop damages by Asian elephants (Elephas maximus). Crop. Prot. 2017, 100, 29–37. [Google Scholar] [CrossRef]
- Gross, E.M.; McRobb, R.; Gross, J. Cultivating alternative crops reduces crop losses due to African elephants. J. Pest. Sci. 2015, 89, 497–506. [Google Scholar] [CrossRef]
- Hedges, S.; Gunaryadi, D. Reducing human-elephant conflict: Do chillies help deter elephants from entering crop fields? Oryx 2009, 44, 139–146. [Google Scholar] [CrossRef]
- Le Bel, S. Repelling elephants with a chilli pepper gas dispenser: Field tests and practical use in Mozambique, Zambia and Zimbabwe. Pachyderm 2015, 56, 87–96. [Google Scholar]
- Le Bel, S.; Taylor, R.; Lagrange, M.; Ndoro, O.; Barra, M.; Madzikanda, H. An easy-to-use capsicum delivery system for crop-raiding elephants in Zimbabwe: Preliminary results of a field test in Hwange National Park. Pachyderm 2010, 47, 80–89. [Google Scholar]
- Chang’a, A.; de Souza, N.; Muya, J.; Keyyu, J.; Mwakatobe, A.; Malugu, L.; Ndossi, H.P.; Konuche, J.; Omondi, R.; Mpinge, A.; et al. Scaling-up the use of chili fences for reducing human-elephant conflict across landscapes in Tanzania. Trop. Conserv. Sci. 2016, 9, 921–930. [Google Scholar] [CrossRef]
- Von Hagen, R.L.; Kasaine, S.; Githiru, M.; Amakobe, B.; Mutwiwa, U.N.; Schulte, B.A. Metal strip fences for preventing African elephant (Loxodonta africana) crop foraging in the Kasigau Wildlife Corridor, Kenya. Afr. J. Ecol. 2020, 59, 293–298. [Google Scholar] [CrossRef]
- Kiffner, C.; Schaal, I.; Cass, L.; Peirce, K.; Sussman, O.; Grueser, A.; Wachtel, E.; Adams, H.; Clark, K.; König, H.J.; et al. Perceptions and realities of elephant crop raiding and mitigation methods. Conserv. Sci. Pract. 2021, 3, e372. [Google Scholar] [CrossRef]
- Von Hagen, R.L.; Norris, P.; Schulte, B.A. Quantifying capsaicinoids from chili pepper and motor oil mixtures used in elephant deterrent fences. Chromatographia 2020, 83, 1153–1157. [Google Scholar] [CrossRef]
- Santiapillai, C.; Read, B. Would masking the smell of ripening paddy-fields help mitigate human-elephant conflict in Sri Lanka? Oryx 2010, 44, 509–511. [Google Scholar] [CrossRef]
- Oniba, E.; Robertson, M.R. Trialling a new scent-based repellent to mitigate elephant crop-raiding around Murchison Falls National Park, Uganda. Pachyderm 2019, 60, 123–125. [Google Scholar]
- Rasmussen, L.E.L.; Riddle, S.W. Development and initial testing of pheromone-enhanced mechanical devices for deterring crop raiding elephants: A positive conservation step. J. Elephant Man. Assoc. 2004, 15, 30–37. [Google Scholar]
- Greenwood, D.R.; Rasmussen, L.E.L. The elephant as an ideal olfactory model mammal. ChemoSense 2009, 11, 1–8. [Google Scholar]
- Polla, E.J.; Grueter, C.C.; Smith, C.L. Asian elephants (Elephas maximus) discriminate between familiar and unfamiliar human visual and olfactory cues. Anim. Behav. Cogn. 2018, 5, 279–291. [Google Scholar] [CrossRef]
- Dürckheim, K.E.M.v.; Hoffman, L.C.; Leslie, A.; Hensman, M.C.; Hensman, S.; Schultz, K.; Lee, S. African elephants (Loxodonta africana) display remarkable olfactory acuity in human scent matching to sample performance. Appl. Anim. Behav. Sci. 2018, 200, 123–129. [Google Scholar] [CrossRef]
- Partan, S.; Marler, P. Communication goes multimodal. Science 1999, 283, 1272–1273. [Google Scholar] [CrossRef] [PubMed]
- Apps, P.J.; Weldon, P.J.; Kramer, M. Chemical signals in terrestrial vertebrates: Search for design features. Nat. Prod. Rep. 2015, 32, 1131–1153. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schulte, B.A.; LaDue, C.A. The Chemical Ecology of Elephants: 21st Century Additions to Our Understanding and Future Outlooks. Animals 2021, 11, 2860. https://doi.org/10.3390/ani11102860
Schulte BA, LaDue CA. The Chemical Ecology of Elephants: 21st Century Additions to Our Understanding and Future Outlooks. Animals. 2021; 11(10):2860. https://doi.org/10.3390/ani11102860
Chicago/Turabian StyleSchulte, Bruce A., and Chase A. LaDue. 2021. "The Chemical Ecology of Elephants: 21st Century Additions to Our Understanding and Future Outlooks" Animals 11, no. 10: 2860. https://doi.org/10.3390/ani11102860
APA StyleSchulte, B. A., & LaDue, C. A. (2021). The Chemical Ecology of Elephants: 21st Century Additions to Our Understanding and Future Outlooks. Animals, 11(10), 2860. https://doi.org/10.3390/ani11102860





