Patterns of Feeding by Householders Affect Activity of Hedgehogs (Erinaceus europaeus) during the Hibernation Period
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
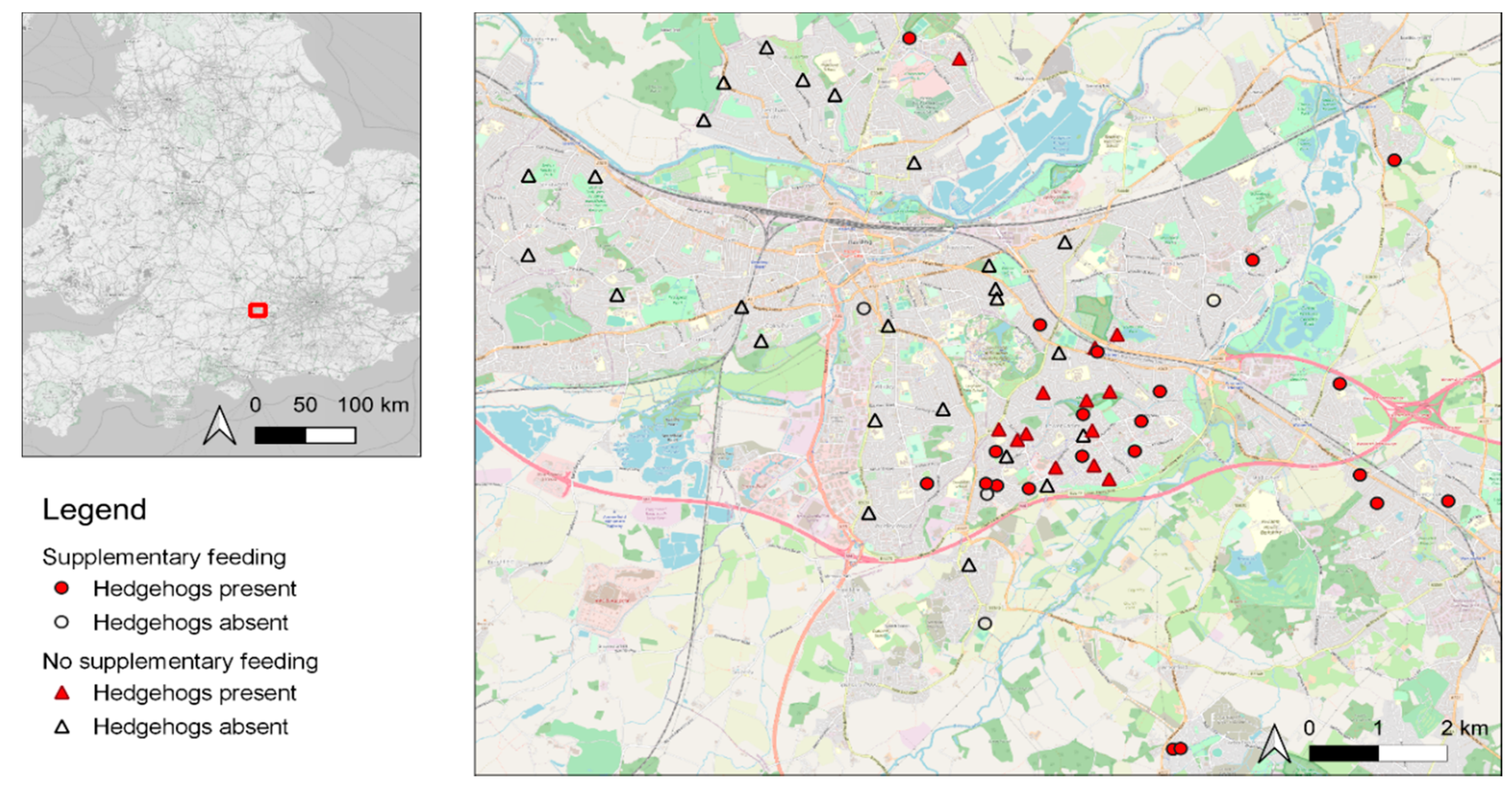
2.1. Footprint Tunnel Survey
2.2. Dividing the Data into Seasons
2.3. Data Analyses
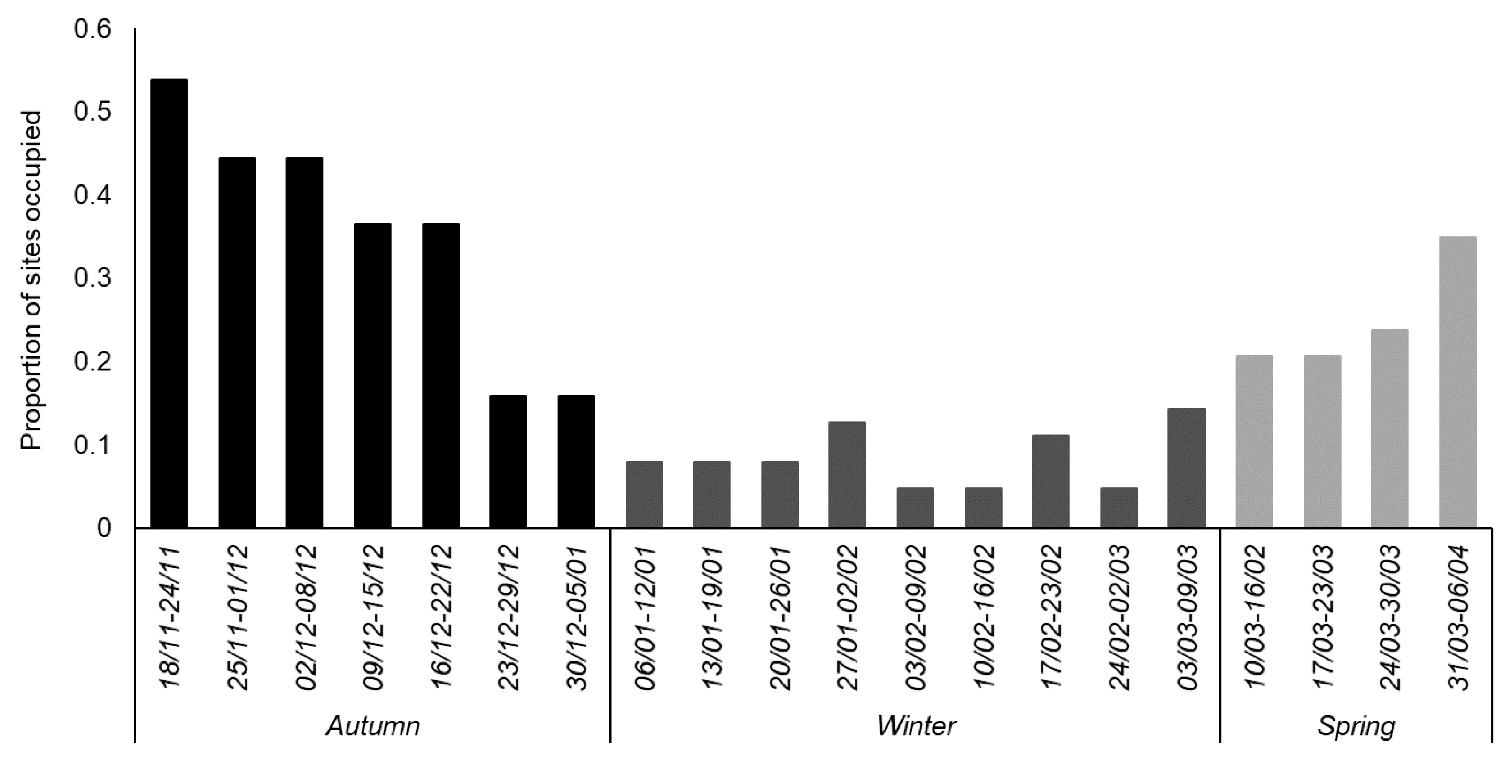
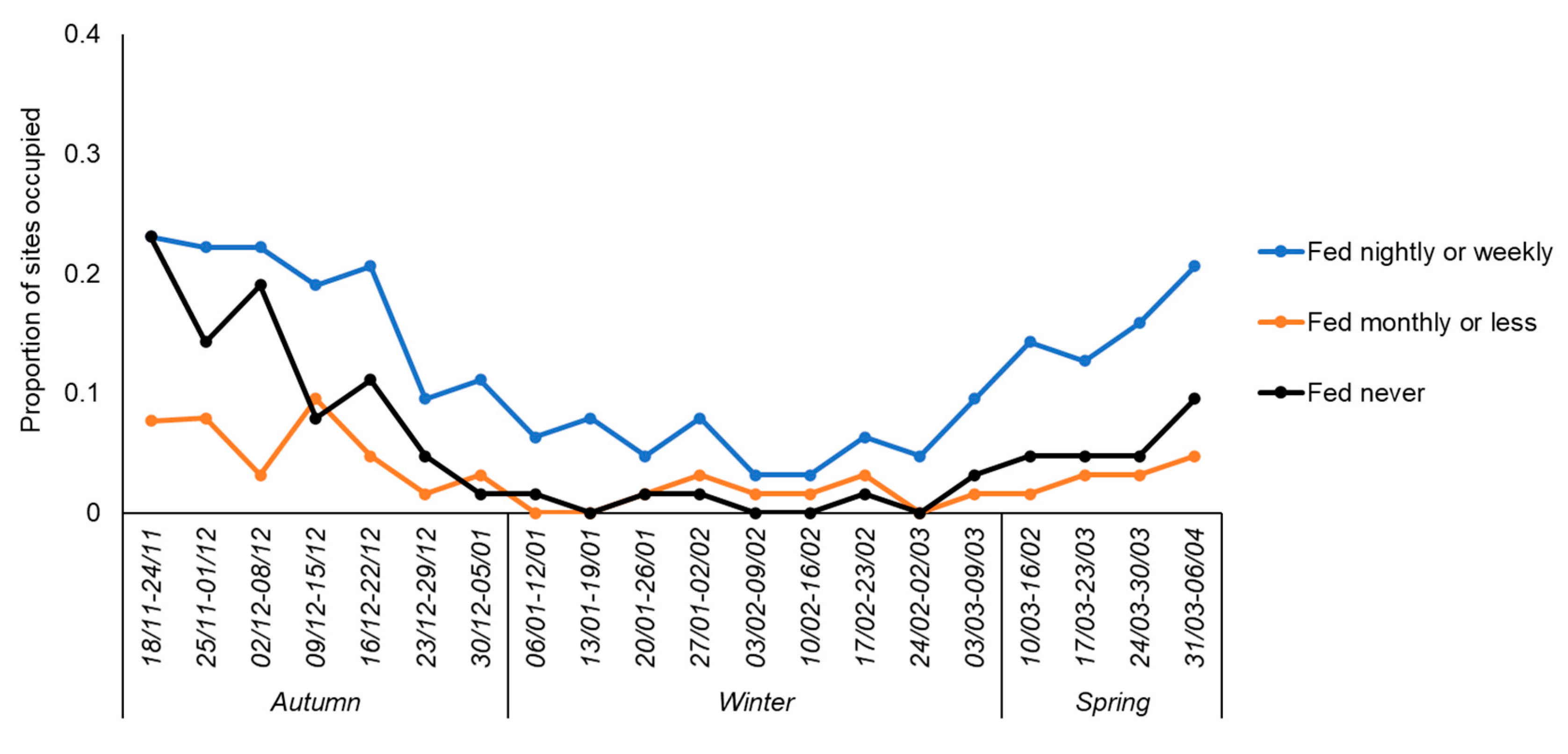
3. Results
3.1. General Trends
3.2. Factors Affecting Hedgehog Occupancy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Leather, S.R.; Walters, K.F.A.; Bale, J.S. The Ecology of Insect Overwintering; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar]
- Cáceres, C.E. Dormancy in invertebrates. Invert. Biol. 1997, 116, 371–383. [Google Scholar] [CrossRef]
- Wells, K.D. The Ecology and Behavior of Amphibians; University of Chicago Press: Chicago, IL, USA, 2007. [Google Scholar]
- Ruf, T.; Geiser, F. Daily torpor and hibernation in birds and mammals. Biol. Rev. 2015, 90, 891–926. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.L.; Yoder, A.D. Theme and variations: Heterothermy in mammals. Integ. Comp. Biol. 2014, 54, 439–442. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Reeve, N. Hedgehogs; Academic Press: London, UK, 1994. [Google Scholar]
- Morris, P. Hedgehog; William Collins: London, UK, 2018. [Google Scholar]
- Kristiansson, H. Population variables and causes of mortality in a hedgehog (Erinaceus europaeus) population in southern Sweden. J. Zool. 1990, 220, 391–404. [Google Scholar] [CrossRef]
- Jensen, A.B. Overwintering of European hedgehogs Erinaceus europaeus in a Danish rural area. Acta Theriol. 2004, 49, 145–155. [Google Scholar] [CrossRef]
- Morris, P. Winter nests of the hedgehog (Erinaceus europaeus L.). Oecologia 1973, 11, 299–313. [Google Scholar] [CrossRef]
- Rasmussen, S.L.; Berg, T.B.; Dabelsteen, T.; Jones, O.R. The ecology of suburban juvenile European hedgehogs (Erinaceus europaeus) in Denmark. Ecol. Evol. 2019, 9, 13174–13187. [Google Scholar] [CrossRef]
- Roos, S.; Johnston, A.; Noble, D. UK Hedgehog Datasets and Their Potential for Long-Term Monitoring. BTO Research Report, 598; The British Trust for Ornithology: Thetford, UK, 2012. [Google Scholar]
- Mathews, F.; Kubasiewicz, L.; Gurnell, J.; Harrower, C.; McDonald, R.; Shore, R. A Review of the Population and Conservation Status of British Mammals. A Report by the Mammal Society under Contract to Natural England, Natural Resources Wales and Scottish Natural Heritage; Natural England: Peterborough, UK, 2018. [Google Scholar]
- Becher, S.A.; Griffiths, R. Genetic differentiation among local populations of the European hedgehog (Erinaceus europaeus) in mosaic habitats. Mol. Ecol. 1998, 7, 1599–1604. [Google Scholar] [CrossRef]
- Rondinini, C.; Doncaster, C. Roads as barriers to movement for hedgehogs. Funct. Ecol. 2002, 16, 504–509. [Google Scholar] [CrossRef]
- Hof, A.R.; Bright, P.W. The value of green-spaces in built-up areas for western hedgehogs. Lutra 2009, 52, 69–92. [Google Scholar]
- Hof, A.R.; Bright, P.W. The value of agri-environment schemes for macro-invertebrate feeders: Hedgehogs on arable farms in Britain. Anim. Conserv. 2010, 13, 467–473. [Google Scholar] [CrossRef]
- Moorhouse, T.P.; Palmer, S.C.F.; Travis, J.M.J.; Macdonald, D.W. Hugging the hedges: Might agri-environment manipulations affect landscape permeability for hedgehogs? Biol. Conserv. 2014, 176, 109–116. [Google Scholar] [CrossRef]
- Huijser, M.P.; Bergers, P.J.M. The effect of roads and traffic on hedgehog (Erinaceus europaeus) populations. Biol. Cons. 2000, 95, 111–116. [Google Scholar] [CrossRef]
- Wembridge, D.E.; Newman, M.R.; Bright, P.W.; Morris, P.A. An estimate of the annual number of hedgehog (Erinaceus europaeus) road casualties in Great Britain. Mamm. Comm. 2016, 2, 8–14. [Google Scholar]
- Wright, P.G.R.; Coomber, F.G.; Bellamy, C.C.; Perkins, S.E.; Mathews, F. Predicting hedgehog mortality risks on British roads using habitat suitability modelling. PeerJ 2020, 7, e8154. [Google Scholar] [CrossRef]
- Dowding, C.V.; Shore, R.F.; Worgan, A.; Baker, P.J.; Harris, S. Accumulation of anticoagulant rodenticides in a non-target insectivore, the European hedgehog (Erinaceus europaeus). Environ. Pollut. 2010, 158, 161–166. [Google Scholar] [CrossRef]
- Young, R.P.; Davison, J.; Trewby, I.D.; Wilson, G.J.; Delahay, R.J.; Doncaster, C.P. Abundance of hedgehogs (Erinaceus europaeus) in relation to the density and distribution of badgers (Meles meles). J. Zool. 2006, 269, 349–356. [Google Scholar] [CrossRef]
- Trewby, I.D.; Young, R.; McDonald, R.A.; Wilson, G.J.; Davison, J.; Walker, N.; Robertson, A.; Doncaster, C.P.; Delahay, R.J. Impacts of removing badgers on localised counts of hedgehogs. PLoS ONE 2014, 9, e95477. [Google Scholar] [CrossRef]
- Pettett, C.E.; Moorhouse, T.P.; Johnson, P.J.; Macdonald, D.W. Factors affecting hedgehog (Erinaceus europaeus) attraction to rural villages in arable landscapes. Eur. J. Wildl. Res. 2018, 63, 54. [Google Scholar] [CrossRef]
- Williams, B.; Baker, P.J.; Thomas, E.; Wilson, G.; Judge, J.; Yarnell, R.W. Reduced occupancy of hedgehogs (Erinaceus europaeus) in rural England and Wales: The influence of habitat and an asymmetric intraguild predator. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Yarnell, R.W.; Surgery, J.; Grogan, A.; Thompson, R.; Davies, K.; Kimbrough, C.; Scott, D.M. Should rehabilitated hedgehogs be released in winter? A comparison of survival, nest use and weight change in wild and rescued animals. Eur. J. Wildl. Res. 2019, 65, 6. [Google Scholar] [CrossRef]
- Walhovd, H. Partial arousals from hibernation in hedgehogs in outdoor hibernacula. Oecologia 1979, 40, 141–153. [Google Scholar] [CrossRef]
- Webb, P.I.; Ellison, J. Normothermy, torpor, and arousal in hedgehogs (Erinaceus europaeus) from Dunedin, New Zealand. J. Zool. 1998, 25, 85–90. [Google Scholar] [CrossRef]
- Doncaster, C.P. Factors regulating local variations in abundance: Field tests on hedgehogs, Erinaceus europaeus. Oikos 1994, 69, 182–192. [Google Scholar] [CrossRef]
- Hubert, P.; Julliard, R.; Biagianti, S.; Poulle, M.L. Ecological factors driving the higher hedgehog (Erinaceus europeaus) density in an urban area compared to the adjacent rural area. Landsc. Urban Plan. 2011, 103, 34–43. [Google Scholar] [CrossRef]
- Van de Poel, J.L.; Dekker, J.; van Langevelde, F. Dutch hedgehogs Erinaceus europaeus are nowadays mainly found in urban areas, possibly due to the negative effects of badgers Meles meles. Wild. Biol. 2015, 21, 51–55. [Google Scholar] [CrossRef]
- Schaus, J.; Uzal, A.; Gentle, L.K.; Baker, P.J.; Bearman-Brown, L.; Bullion, S.; Gazzard, A.; Lockwood, H.; North, A.; Reader, T.; et al. Application of the Random Encounter Model in citizen science projects to monitor animal densities. Remote Sens. Ecol. Cons. 2020. [Google Scholar] [CrossRef]
- Soivio, A.; Tähti, H.; Kristoffersson, R. Studies on the periodicity of hibernation in the hedgehog (Erinaceus europaeus L.): III. Hibernation in a constant ambient temperature of −5° C. Ann. Zoo. Fenn. 1968, 5, 224–226. [Google Scholar]
- Parkes, J. Some aspects of the biology of the hedgehog (Erinaceus europaeus L.) in the Manawatu, New Zealand. New Zeal. J. Zool. 1975, 2, 463–472. [Google Scholar] [CrossRef]
- Tähti, H.; Soivio, A. Respiratory and circulatory differences between induced and spontaneous arousals in hibernating hedgehogs (Erinaceus europaeus L.). Ann. Zool. Fenn. 1977, 14, 198–203. [Google Scholar]
- Dmi’el, R.; Schwarz, M. Hibernation patterns and energy expenditure in hedgehogs from semi-arid and temperate habitats. J. Comp. Physiol. B 1984, 117–123. [Google Scholar] [CrossRef]
- Fowler, P.A.; Racey, P.A. Daily and seasonal cycles of body temperature and aspects of heterothermy in the hedgehog Erinaceus europaeus. J. Comp. Physiol. B 1984, 160, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Stocker, L. The Complete Hedgehog; Chatto and Windus: London, UK, 1987. [Google Scholar]
- Rast, W.; Barthel, L.M.F.; Berger, A. Music festival makes hedgehogs move: How individuals cope behaviorally in response to human-induced stressors. Animals 2019, 9, 455. [Google Scholar] [CrossRef] [PubMed]
- Finch, D.; Smith, B.R.; Marshall, C.; Coomber, F.G.; Kubasiewicz, L.M.; Anderson, M.; Wright, P.G.R.; Mathews, F. Effects of Artificial Light at Night (ALAN) on European hedgehog activity at supplementary feeding stations. Animals 2020, 10, 768. [Google Scholar] [CrossRef] [PubMed]
- Perini, K.; Magliocco, A. Effects of vegetation, urban density, building height, and atmospheric conditions on local temperatures and thermal comfort. Urban For. Urban Green. 2014, 13, 495–506. [Google Scholar] [CrossRef]
- Chapman, S.; Watson, J.E.; Salazar, A.; Thatcher, M.; McAlpine, C.A. The impact of urbanization and climate change on urban temperatures: A systematic review. Landsc. Ecol. 2017, 32, 1921–1935. [Google Scholar] [CrossRef]
- Keep Feeding Hedgehogs in the Autumn—University of Brighton. Available online: https://www.brighton.ac.uk/about-us/news-and-events/news/2017/09-13-keep-feeding-hedgehogs-in-the-autumn.aspx (accessed on 6 June 2020).
- Hedgehog Street: Should I Keep Feeding Hedgehogs Overwinter? Available online: https://www.hedgehogstreet.org/should-i-keep-feeding-hedgehogs-over-winter/ (accessed on 16 May 2020).
- British Hedgehog Preservation Society: Feeding. Available online: https://www.britishhedgehogs.org.uk/feeding/ (accessed on 16 May 2020).
- Tiggywinkles Wildlife Hospital Hedgehog Fact Sheet. Available online: https://www.sttiggywinkles.org.uk/hedgehog-fact-sheet/ (accessed on 16 May 2020).
- Robb, G.N.; McDonald, R.A.; Chamberlain, D.E.; Bearhop, S. Food for thought: Supplementary feeding as a driver of ecological change in avian populations. Front. Ecol. Environ. 2008, 6, 476–484. [Google Scholar] [CrossRef]
- Ewen, J.G.; Walker, L.; Canessa, S.; Groombridge, J.J. Improving supplementary feeding in species conservation. Conserv. Biol. 2014, 29, 341–349. [Google Scholar] [CrossRef]
- Murray, M.H.; Becker, D.J.; Hall, R.J.; Hernandez, S.M. Wildlife health and supplemental feeding: A review and management recommendations. Biol. Conserv. 2016, 205, 163–174. [Google Scholar] [CrossRef]
- Bojarskaa, K.; Drobniak, S.; Jakubiec, Z.; Zyśk-Gorczyńsk, E. Winter insomnia: How weather conditions and supplementary feeding affect the brown bear activity in a long-term study. Glob. Ecol. Conserv. 2019, 17, e00523. [Google Scholar] [CrossRef]
- Krofel, M.; Spacapan, M.; Jerina, K. Winter sleep with room service: Denning behaviour of brown bears with access to anthropogenic food. J. Zool. 2017, 302, 8–14. [Google Scholar] [CrossRef]
- Kirby, R.; Johnson, H.E.; Alldredge, M.W.; Pauli, J.N. The cascading effects of human food on hibernation and cellular aging in free-ranging black bears. Sci. Rep. 2019, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mann, N.; Neumann, J.L.; Yarnell, R.W.; Baker, P.J. A prickly problem: Developing a volunteer-friendly tool for monitoring populations of a terrestrial urban mammal, the West European hedgehog (Erinaceus europaeus). Urban Ecosyst. 2018, 21, 1075–1086. [Google Scholar] [CrossRef]
- Yarnell, R.W.; Pacheco, M.; Williams, B.; Neumann, J.L.; Rymer, D.J.; Baker, P.J. Using occupancy analysis to validate the use of footprint tunnels as a method for monitoring the hedgehog Erinaceus europaeus. Mamm. Rev. 2014, 44, 234–238. [Google Scholar] [CrossRef]
- Williams, R.L.; Stafford, R.; Goodenough, A. Biodiversity in urban gardens: Assessing the accuracy of citizen science data on garden hedgehogs. Urban Ecosyst. 2014, 18, 1–15. [Google Scholar] [CrossRef]
- MacKenzie, D.I.; Royle, J.A. Designing occupancy studies: General advice and allocating survey effort. J. Appl. Ecol. 2005, 42, 1105–1114. [Google Scholar] [CrossRef]
- Land Cover Map 2015 (Vector, GB). Available online: https://catalogue.ceh.ac.uk/documents/6c6c9203-7333-4d96-88ab-78925e7a4e73 (accessed on 18 April 2020).
- Dowding, C.V.; Harris, S.; Poulton, S.; Baker, P.J. Nocturnal ranging behaviour of urban hedgehogs, Erinaceus europaeus, in relation to risk and reward. Anim. Behav. 2010, 80, 13–21. [Google Scholar] [CrossRef]
- Met Office Integrated Data Archive System (MIDAS) Land and Marine Surface Stations Data (1853-Current). Available online: https://catalogue.ceda.ac.uk/uuid/220a65615218d5c9cc9e4785a3234bd0 (accessed on 10 February 2020).
- Reading, England, United Kingdom—Sunrise, Sunset, and Daylength. Available online: https://www.timeanddate.com/sun/uk/reading (accessed on 10 February 2020).
- Burnham, K.P.; Anderson, D.R. Model Selection and Inference, 2nd ed.; Springer: New York, NY, USA, 1998. [Google Scholar]
- Whittingham, M.J.; Stephens, P.A.; Bradbury, R.B.; Freckleton, R.P. Why do we still use stepwise modelling in ecology and behaviour? J. Anim. Ecol. 2006, 75, 1182–1189. [Google Scholar] [CrossRef]
- Mackenzie, D.I.; Bailey, L.L. Assessing the fit of site-occupancy models. JABES 2004, 9, 300–318. [Google Scholar] [CrossRef]
- Program MARK: A Gentle Introduction. Available online: http://www.phidot.org/software/mark/docs/book/ (accessed on 22 May 2020).
- Exercises in Occupancy Modelling and Estimation. Exercise 4: Single-Species, Single-Season Model with Site Level Covariates. Available online: http://www.uvm.edu/rsenr/vtcfwru/spreadsheets/occupancy/occupancy.htm (accessed on 22 May 2020).
- Mackenzie, D.I.; Nichols, J.D.; Royle, J.A.; Pollock, K.H.; Bailey, L.L.; Hines, J.E. Occupancy Estimation and Modeling Inferring Patterns and Dynamics of Species Occurrence; Academic Press: Cambridge, MA, USA, 2006. [Google Scholar]
- Baldwin, R.A.; Bender, L.C. Denning chronology of black bears in eastern rocky mountain national park, Colorado. West. North Am. Nat. 2010, 70, 48–54. [Google Scholar] [CrossRef]
- Haigh, A.; O’Riordan, R.M.; Butler, F. Nesting behaviour and seasonal body mass changes in a rural Irish population of the Western hedgehog (Erinaceus europaeus). Acta Theirol. 2012, 57, 321–331. [Google Scholar] [CrossRef]
- Turbill, C.; Smith, S.; Deimel, C.; Ruf, T. Daily torpor is associated with telomere length change over winter in Djungarian hamsters. Biol. Lett. 2012, 8, 304–307. [Google Scholar] [CrossRef] [PubMed]
- Turbill, C.; Ruf, T.; Smith, S.; Bieber, C. Seasonal variation in telomere length of a hibernating rodent. Biol. Lett. 2013, 9, 20121095. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hoelzl, F.; Cornils, J.S.; Smith, S.; Moodley, Y.; Ruf, T. Telomere dynamics in free-living edible dormice (Glis glis): The impact of hibernation and food supply. J. Exp. Biol. 2016, 219, 2469–2474. [Google Scholar] [CrossRef] [PubMed]
- Lyman, C.P.; Brien, R.C.O.; Greene, G.C.; Papafrangos, E.D. Hibernation and longevity in the Turkish hamster Mesocricetus brandi. Science 1981, 212, 668–670. [Google Scholar] [CrossRef]
- Blanco, M.B.; Zehr, S.M. Striking longevity in a hibernating lemur. J. Zool. 2015, 296, 177–188. [Google Scholar] [CrossRef]
- Wu, C.; Storey, K.B. Life in the cold: Links between mammalian hibernation and longevity. Biomol. Concepts 2016, 7, 41–52. [Google Scholar] [CrossRef]
| Covariate | Source | Description |
|---|---|---|
| FEEDHOG | Q | An ordinal measure of whether food was left out for hedgehogs during the study: 1 = never, 2 = less frequently (monthly or less), 3 = more frequently (nightly or weekly) |
| FEDBEFORE | Q | A binary measure of whether the participant usually left out food for hedgehogs prior to the commencement of the study |
| FEEDOTHERS | Q | A binary measure of whether food was left out by the participant for birds or other animals at some point during the study |
| NESTSITES | Q | The number of potential types of nest sites available in the participant’s garden as assessed by the participant. Tick-box options of possible nesting sites were listed on the questionnaire as “hedgehog house”, “under a shed or decking”, “under bushes or shrubs”, “under a compost heap” or “other (please provide more information)”. The total number of potential nest sites were converted to z-scores |
| CONNECTIVITY | Q | The proportion of front and back gardens neighbouring the participant’s household that is accessible for hedgehogs from the participant’s own gardens |
| FRONT2BACK | Q | A binary measure of whether a hedgehog could access the participant’s back garden from their front garden |
| GOODHABITAT | Q | The proportion of habitat in the participant’s back garden only that is considered ”good” for wildlife, including lawn, shrubs, flowerbeds and ponds |
| HOUSETYPE | Q | A binary measure of whether houses were: (i) semi-detached, link-detached or detached; or (ii) other (e.g., terraced) |
| GARDENSIZE | E | The area of each garden (m2) converted to z-scores |
| NEARESTOTHER | D | Distance from each site to the next nearest site (m) converted to z-scores |
| NEAREST + VE | D | Distance from each site to the next nearest hedgehog-positive site (m) per season (autumn, winter or spring) converted to z-scores |
| ARABLEDIST | E | Distance from each site to the nearest area of arable land (m) converted to z-scores |
| ARABLE 500 m | E | The area of arable land (m2) within a 500 m radius of each site converted to z-scores (Note: As only 4 sites fell within 250 m of arable land, the potential variable ARABLE250 m was not considered for analyses) |
| WOODDIST | E | Distance from each site to the nearest area of woodland (m) converted to z-scores |
| WOOD 250 m and WOOD 500 m | E | The area of woodland (m2) within 250 and 500 m radii of each site converted to z-scores |
| GRASSDIST | E | Distance from each site to the nearest area of grassland (m) converted to z-scores |
| GRASS 250 m and GRASS 500 m | E | The area of grassland (m2) within 250 and 500 m radii of each site converted to z-scores |
| URBAN 250 m and URBAN 500 m | E | The area of urban and suburban habitat (m2) within 250 and 500 m radii of each site converted to z-scores (Note: As all sites fell within the urban habitat classification, the straight-line distance from each site to urban habitat was not considered for analysis) |
| DAYTIME | E | Mean daylength (time between sunrise and sunset) per week, converted to z-scores |
| AIRTEMP | E | Minimum air temperature (°C) averaged per survey week based on hourly recordings taken between 21:00 and 09:00, converted to z-scores |
| GRASSTEMP | E | Minimum grass temperature (°C) averaged per survey week based on daily recordings taken at 09:00, converted to z-scores |
| Season | Model | QAIC | ΔQAIC | AIC Weight | Model Likelihood | K | Detection Rate | Naïve Ψ | True Ψ |
|---|---|---|---|---|---|---|---|---|---|
| Autumn | Ψ(.), p(survey-specific) | 270.59 | 0.00 | 1 | 1.0000 | 8 | 0.8234 | 0.5397 | 0.5403 |
| 0.8225 | |||||||||
| 0.8225 | |||||||||
| 0.6756 | |||||||||
| 0.6756 | |||||||||
| 0.2938 | |||||||||
| 0.2938 | |||||||||
| Ψ(.), p(.) | 294.26 | 23.67 | 0.0000 | 0.0000 | 2 | 0.6138 | 0.5397 | 0.5411 | |
| Winter | Ψ(.), p(.) | 213.63 | 0.00 | 0.9852 | 1.0000 | 2 | 0.2626 | 0.3016 | 0.3224 |
| Ψ(.), p(survey-specific) | 222.02 | 8.39 | 0.0148 | 0.0151 | 10 | 0.2481 | 0.3016 | 0.3198 | |
| 0.2481 | |||||||||
| 0.2481 | |||||||||
| 0.397 | |||||||||
| 0.1489 | |||||||||
| 0.1489 | |||||||||
| 0.3474 | |||||||||
| 0.1489 | |||||||||
| 0.4467 | |||||||||
| Spring | Ψ(.), p(.) | 64.13 | 0.00 | 0.8006 | 1.0000 | 2 | 0.6459 | 0.3810 | 0.3870 |
| Ψ(.), p(survey-specific) | 66.91 | 2.78 | 0.1994 | 0.2491 | 5 | 0.5377 | 0.3810 | 0.3838 | |
| 0.5377 | |||||||||
| 0.6204 | |||||||||
| 0.9100 |
| Season | Model | QAIC | ΔQAIC | AIC Weight | Model Likelihood | K |
|---|---|---|---|---|---|---|
| Autumn | Ψ(FEDBEFORE + WOOD500 m), p(survey + NESTSITES) | 283.85 | 0.00 | 0.8966 | 1.0000 | 11 |
| Winter | Ψ(FEDBEFORE), p(FEEDHOG + FEEDOTHERS) | 214.42 | 0.00 | 0.4561 | 1.0000 | 5 |
| Ψ(FEDBEFORE), p(FEEDHOG + GRASSTEMP) | 215.41 | 0.99 | 0.2780 | 0.6096 | 5 | |
| Ψ(FEDBEFORE), p(FEEDHOG + AIRTEMP) | 215.51 | 1.09 | 0.2645 | 0.5798 | 5 | |
| Spring | Ψ(FEEDHOG), p(DAYTIME + FEEDOTHERS) | 71.83 | 0.00 | 0.2413 | 1.0000 | 5 |
| Ψ(FEDBEFORE), p(.) | 71.97 | 0.14 | 0.2250 | 0.9324 | 3 | |
| Ψ(FEEDHOG), p(.) | 72.82 | 0.99 | 0.1471 | 0.6096 | 3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gazzard, A.; Baker, P.J. Patterns of Feeding by Householders Affect Activity of Hedgehogs (Erinaceus europaeus) during the Hibernation Period. Animals 2020, 10, 1344. https://doi.org/10.3390/ani10081344
Gazzard A, Baker PJ. Patterns of Feeding by Householders Affect Activity of Hedgehogs (Erinaceus europaeus) during the Hibernation Period. Animals. 2020; 10(8):1344. https://doi.org/10.3390/ani10081344
Chicago/Turabian StyleGazzard, Abigail, and Philip J. Baker. 2020. "Patterns of Feeding by Householders Affect Activity of Hedgehogs (Erinaceus europaeus) during the Hibernation Period" Animals 10, no. 8: 1344. https://doi.org/10.3390/ani10081344
APA StyleGazzard, A., & Baker, P. J. (2020). Patterns of Feeding by Householders Affect Activity of Hedgehogs (Erinaceus europaeus) during the Hibernation Period. Animals, 10(8), 1344. https://doi.org/10.3390/ani10081344




