4.1. Growth Performance
Supplementation of POLM enhanced the growth performance of broiler chickens. In this study, compared to the control group, BWG was significantly increased (
p ˂ 0.05) in the Po8 group on day 21; however, FI and FCR were not affected (
p > 0.05) by dietary supplementation of POLM. Aroche et al. [
31] reported that the dietary inclusion of phytobiotics in the form of 0.5 % mixed powder of
M. citrifolia,
P. guajava, and
A. occidentale improved the feed efficiency, which resulted in increased BWG. The
P. guajava and M. citrifolia contain flavonoids and possess antioxidant and antimicrobial properties [
32]. The flavonoid contents of these phytobiotics are assumed to increase the growth performance in supplemented broiler chickens [
33,
34]. The secondary metabolites of herbs such as alkaloids, tannins, and flavonoids positively influence the birds’ health, as they possess antimicrobial, anti-inflammatory, and antioxidant properties [
35]. Thus, the inclusion of phytobiotics as a dietary supplement help to improve the growth in chickens [
36,
37,
38].
On day 42, compared to the control group, BWG was increased (
p ˂ 0.05) and FCR was significantly decreased in POLM-supplemented groups (Po2, Po4, and Po8). Additionally, The BWG showed a linear increase and FCR showed a linear decrease (
p ˂ 0.05) with increasing POLM dosage. Supplementation of POLM resulted in enhanced BWG in broiler chickens, which may have been due to the flavonoids and the secondary bioactive compound quercetin, which has a primary role and was successfully quantified from POLM. Quercetin is a flavone that should improve growth in birds by upregulating growth hormone, triggering the hepatic growth hormone receptor; this stimulus increases the concentration of insulin-like growth factor-1 [
39]. Kim et al. [
40] reported increased broiler growth by supplementing quercetin in broilers. Quercetin can limit the effects of oxidative stress [
41] and pro-inflammatory cytokines such as TNF-α, interleukin, and cyclooxygenase-2 [
42], thus modulating the gut environment to better utilise the nutrients, improving the growth in birds.
The overall growth performance (1–42 days) of broiler chickens showed that compared to the control group, BWG was maximumly increased (
p ˂ 0.05) and FCR was significantly decreased (
p ˂ 0.05) in the Po8 dietary group. In addition, the BWG showed a linear increase (
p ˂ 0.01) and FCR showed a linear decrease (
p ˂ 0.01) with increasing POLM dosage. Salami et al. [
43] identified that the incorporation of medicinal herbs as feed additives in the broiler chickens’ diets improved the FCR in the last growth phase of the birds. Other studies also suggested the roles of flavonoids in the growth performance of broiler chickens [
34,
44]. The present study results are in agreement with Mpofu et al. [
45], where inclusion of
L. javanica at the rate of 5 g/kg in broiler chickens’ diets had a positive impact on overall growth. In another study, Paraskeuas et al. [
36] reported that inclusion of phytobiotics such as eugenol, menthol, and anethol at the rate of 100 to 150 mg/kg in feed improved the nutrient digestibility, thus enhancing the growth measures in broiler chickens.
Conclusively, the supplementation of POLM in broiler chickens showed a positive effect on growth performance, hence effectively increasing the BWG and feed efficiency with decreased FCR. These findings are in agreement with the positive results of the previous studies, where in-feed phytobiotics were tested in broiler chickens [
31,
45,
46]. Thus, the present results show that POLM effectively enhanced the growth performance of broiler chickens, even at the supplementation rate of 8 g/kg.
4.2. Haematological Blood Indicators
Haematology blood tests of experimental animals are very significant when evaluating the toxic effects of a supplemented compound or plant extract. Haematology blood tests are also tools that can be used to determine the physiological and pathological statuses of the organisms [
47]. The haematological blood indicators in this study were found to be within normal ranges [
48]. The normal blood haematology values in this study indicated the adequacy of nutrients and better immune status of the broiler chickens supplemented with POLM.
The current study findings indicated significant increases in RBC and WBC counts, and in Hb and PCV values. These outcomes are comparable with the results of Reis et al. [
49], who indicated that inclusion of phytobiotics such as cinnamic aldehyde, thymol, and carvacrol in broiler chickens significantly increased erythrocyte counts and haemoglobin in comparison with the control. Similar findings in another study were reported by Krauze et al. [
50], who studied the dietary effects of probiotic
Bacillus subtilis (0.25 g/L)
Enterococcus faecium (0.25 g/L), and phytobiotics containing cinnamon oil (0.25 mL/L) in broiler chickens and found improvements in the immune system and parameters such as RBCs and Hb. In another experiment, Gilani et al. [
11] examined the efficacy of organic acids and phytobiotics (possessing flavonoids) in poultry feed as alternatives to AGPs, observing significant increases in RBC and WBC counts, as well as an increase in PCV in broiler chickens. Similarly, broiler chickens fed Garden cress (
Lepidium satvium) seed powder [
51], cayenne pepper (
Capsicum frutescens) and turmeric (
Curcuma longa) powders [
52], and pawpaw leaf and seed meal [
53] showed increased values of Hb, PCV, and RBCs.
The present study results were not significant (
p > 0.05) for MCV, MCH, or MCHC in experimental broiler chickens. These results affirm the findings of Oghenebrorhie and Oghenesuvwe, [
54], who reported no significant results for MCV, MCH, or MCHC among broilers supplemented with
Moringa oleifera leaf meal (MOLM)
.In conclusion, the dietary supplementation of POLM improved the RBCs, WBCs, PCV, and Hb, suggesting better utilisation of the dietary nutrients.
4.3. Serum Biochemistry
Serum biochemical parameters show the metabolism of nutrients in the body and highlight the possible changes resulting from intrinsic and extrinsic factors [
55,
56]. The liver is one of the largest and most vital organs of living organisms, and it has a pivotal role in detoxification, metabolism, and elimination of endogenous and exogenous substances [
57]. The activity levels of ALP, AST, and ALT are considered as diagnostic tools that may be used to evaluate hepatotoxicity [
58]. Any pathological manifestation or toxicity results in enhanced activity levels of AST and ALT [
59]. Moreover, their activity levels are considered as specific indicators of liver injury or impairment [
60]. The current study results showed decreased (
p < 0.05) serum activity of AST and ALT by increasing the POLM supplementation dosage. However, the serum activity of ALP was not influenced by (
p > 0.05) POLM supplementation in experimental broiler chickens. The decreased activity of ALT and AST indicated the hepatoprotective nature of the POLM. The POLM possesses a significant concentration of flavonoids and secondary metabolites, including quercetin, which is believed to be responsible for hepatoprotective activity [
17,
61]. Farag and El-Rayes [
62] revealed the hepatoprotective effect of quercetin from bee pollen in broilers, which has an ability to restrict oxidative damage to the liver. Another study by Odetola et al. [
12] showed that the graded supplementation of
Petiveria alliacea root meal in broiler chickens significantly decreased the activity of AST. In a previous study, Oloruntola et al. [
63] found that the dietary inclusion of pawpaw and bamboo leaf meal significantly decreased the activity of ALT in broiler chickens.
Serum proteins are primarily synthesised in the liver and their concentrations reflect the functional status of hepatocytes. Any decline in the levels of serum proteins (TP, albumin, and globulin) may be the result of hepatic insufficiency, malnutrition, and active inflammation, which may be due to the recurrent infections and immune deficiency [
64]. Furthermore, the serum protein levels of birds are considered important indicators for the determination of their health status. The fattening period of broiler chickens is very short, and there is a rapid accumulation of building proteins in the body tissues, which may significantly influence the concentrations of proteins in the blood, as well as their composition [
65]. This rapid growth trend requires intensive erythropoiesis and haemoglobin synthesis, which can result in increased globulin production, potentially affecting the concentrations of serum protein levels in growing chickens [
66,
67]. The current study results showed that the inclusion of POLM significantly increased the levels of TP, albumin, and globulin compared to the control group. In addition, TP, albumin, and globulin showed linear increases with increasing supplementation of POLM. The present study results are in accordance with the results of Goerge et al. [
68], who noted higher serum TP levels in broilers fed a ginger-powder-supplemented diet at starting and finishing phases. Abudabos et al. [
69] reported trends for serum TP and globulin for broilers fed anise and thyme essential oils that were in agreement with the current study.
In birds, the normal reference range of serum glucose is 200 to 500 mg/dL [
48]. The present study showed that the serum glucose concentrations were not influenced by POLM in the experimental chickens; however, numerically high values were recorded in the control group compared to supplemented groups. The current findings are in line with the study by Abudabos et al. [
69], where serum glucose did not differ significantly in experimental broilers supplemented with phytogenic feed additives.
The serum concentrations of cholesterol and triglycerides are considered to be indicators of lipid metabolism [
70]. The current study findings showed that the serum levels of triglycerides and cholesterol were not affected by POLM supplementation at day 21, however significant decreases in the levels of triglyceride and cholesterol were noted in POLM-supplemented groups relative to control on day 42. Furthermore, it was observed that the increasing dosages of POLM linearly decreased the serum levels of triglycerides and cholesterol. The current study results are endorsed by Vispute et al. [
13], who reported that dill and hemp seed (possessing flavonoids) significantly decreased the serum levels of triglycerides in the final growth phase. Similarly, our results are in agreement with Zhang et al. [
71], who specified that the supplementation of
Chinese bayberry leaves in chickens’ diets significantly decreased the serum concentrations of triglycerides and cholesterol. Our outcomes are affirmed by Zhou et al. [
72] and Niu et al. [
73], who reported that dietary supplementation of broilers with fermented
Ginkgo biloba rations and fermented
Ginkgo biloba leaves can significantly decrease the serum levels of triglycerides and cholesterol. Similar results were shared by Gilani et al. [
11], who revealed that phytobiotics, organic acids, and their combinations resulted in significantly reduced serum levels of cholesterol and triglycerides in broiler chickens.
Electrolyte balance plays an important role in acid–base balance and ultimately modifies the performance of broiler birds. Any alteration in the acid–base balance results in malfunction of the biochemical and metabolic pathways, which results in an inability to maintain the physiological status of the birds. The minerals Na, K, and Cl are essential for acid–base and osmotic balance, as well as transport of substances across the cell membranes. Thus, they play vital roles in the metabolisms of living organisms. Any imbalance in these minerals can directly alter the acid–base balance, metabolic functions, and ultimately the performance of broiler chickens [
74]. In the present study, the Na, K, and Cl values were within normal ranges. These results are in accordance with Malahubban and Ab Aziz, [
75], who reported the graded supplementation of Misai Kucing (
Orthosiphon stamineus) in broiler chicken.
The kidneys are considered the second target organs that may be injured due to metabolic dysfunctions. Kidney function plays a key role in measuring the possible toxicity of any compound. The status of kidney function can be measured via the increase or decrease in serum levels of urea and creatinine. Higher creatinine levels result from reduced glomerular filtration, which reflects kidney impairment [
76], while an elevated serum urea level indicates cardiac and renal tissue injuries. The current study findings showed that serum levels of creatinine and urea were significantly decreased as the POLM dosage increased. These findings indicated that POLM had no deleterious effects on kidney function. Various studies using phytobiotics supplementation in broiler chickens have supported our present study results, including the work by Rubio et al. [
77], Ahmad et al. [
78], and Adegoke et al. [
52].
In conclusion, our findings showed decreased activity of AST and ALT with reduced serum levels of urea and creatinine. These results highlighted that POLM supplementation was useful in terms of liver and kidney function, and was safe even at 8 g/kg in broiler chickens. Additionally, the increased blood protein levels (TP, albumin, and globulin) in this study might be due to the antioxidant and immunomodulatory properties of the POLM supplementation [
5,
6].
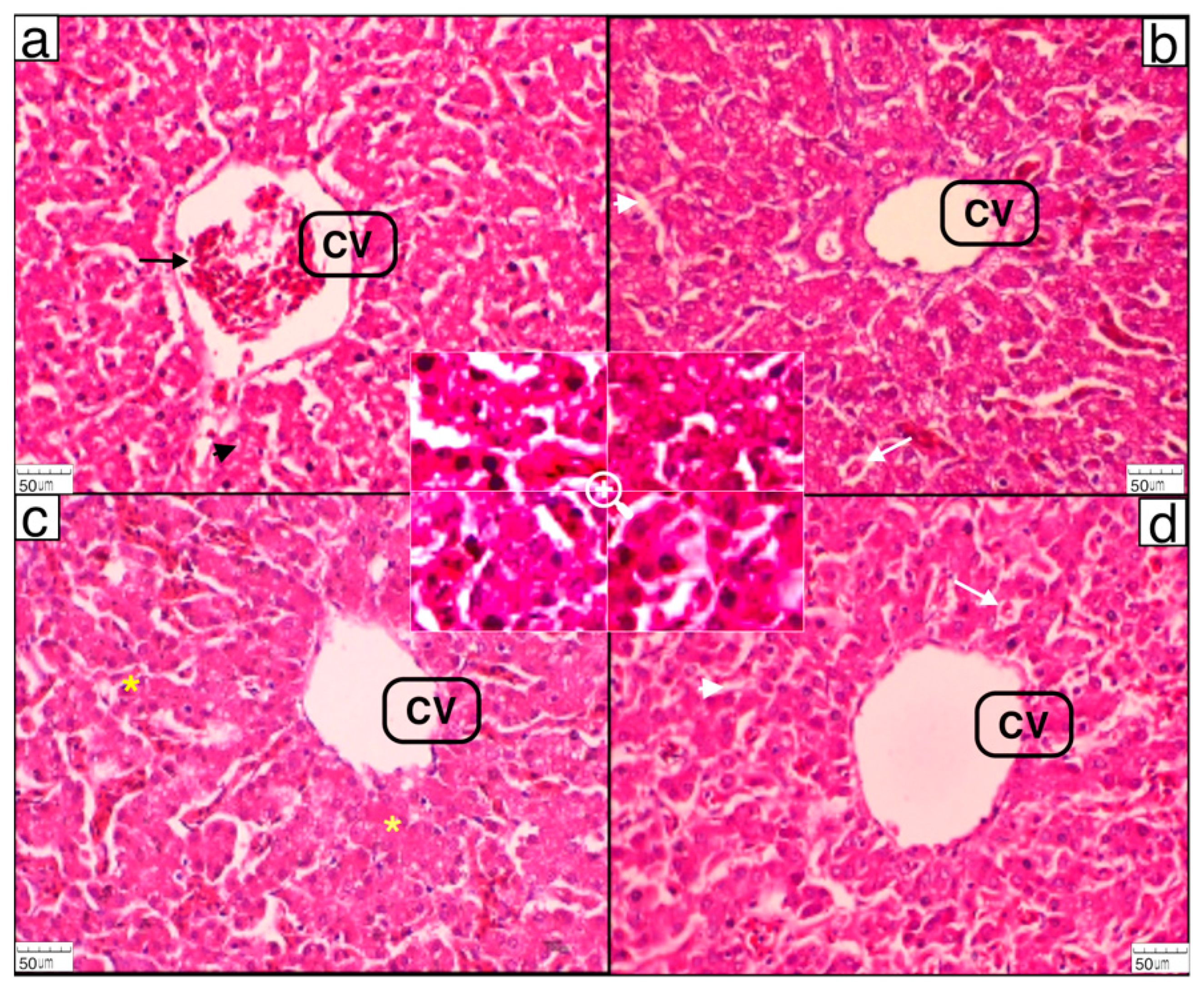
4.5. Histomorphological Analysis of the Liver
The histomorphological study of the liver revealed that POLM supplementation did not show deleterious effects on liver tissues. The vacuolar degeneration was more frequent in the control group compared to the hepatic tissue samples of all the POLM-supplemented groups. The microscopic characteristics of the hepatic tissues showed positive impacts on the histomorphologies of the livers, as seen in
Figure 1b–d compared to
Figure 1a (control group). The histomorphological changes in the present study were comparable to the previous study by Quereshi et al. [
80], in which the hepatoprotective effects of
fenugreek seeds and
dandelion leaves in broiler chickens resulted in normal architecture of the hepatic parenchyma. Additionally, the hepatoprotective effects of these phytobiotics were suggested to be due to the presence of flavonoids in
dandelion leaves and
fenugreek seeds. Klaric et al.’s [
15] results also supported the results of the present study, where supplementation of phytobiotics such as propolis and bee pollen, which possess flavonoids, ameliorated the liver morphology compared to the control group. Additionally, normal hepatocytes without regressive lesions were noticed in the supplemented groups compared to the control. The control group showed extensive regressive lesions in liver tissue sections. In another study, Farag and El-Rayes [
62] observed that the dietary supplementation of bee pollen in broiler chickens’ diets ameliorated the hepatic parenchyma and reduced tissue injury. Furthermore, flavonoids such as quercetin might have a protective effect against oxidative damage of the liver.
Inflammatory responses and oxidative stress are key factors that can damage the liver. Any substance that can diminish the oxidative stress and inflammation can produce hepatoprotective effects and reduce hepatic injury. In the present study, the POLM supplementation produced protective effects on the hepatocytes. These hepatoprotective effects were primarily due to quercetin, which produce effects by limiting oxidative stress [
41]; pro-inflammatory cytokines such as TNF-α, IL-6, and COX-2 [
42]; and nuclear factor NF-κB, probably via interference of the signalling of the toll-like receptor TLR
4 [
81]. Furthermore, quercetin increases the non-enzymatic and enzymatic antioxidants by stimulating the Nrf2–ARE signalling pathway in cells, which might positively influence the liver status and function [
82]. Conclusively, increasing the POLM supplementation levels has a gradual ameliorating histomorphological effect on hepatocytes. A predominantly healthy architecture of the liver parenchyma was noticed in POLM-supplemented group Po8 compared to the Po2 and control groups.