Effects of Dietary Inclusion of Bilberry and Walnut Leaves Powder on the Digestive Performances and Health of Tetra SL Laying Hens
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Antioxidant Capacity
2.2. Hens and Experimental Treatments
2.3. Sample Collection for Analyses
2.4. Light Microscopy Examination
2.5. Enzymatic Analysis
2.5.1. Maltase Activity Assay
2.5.2. Invertase Activity Assay
2.5.3. Alpha-Amylase Assay
2.5.4. Trypsin Assay
2.6. Protein Determination
2.7. Microbiota Characterization
2.8. Statistical Analysis
3. Results
3.1. Composition Analysis of Vegetable Additives
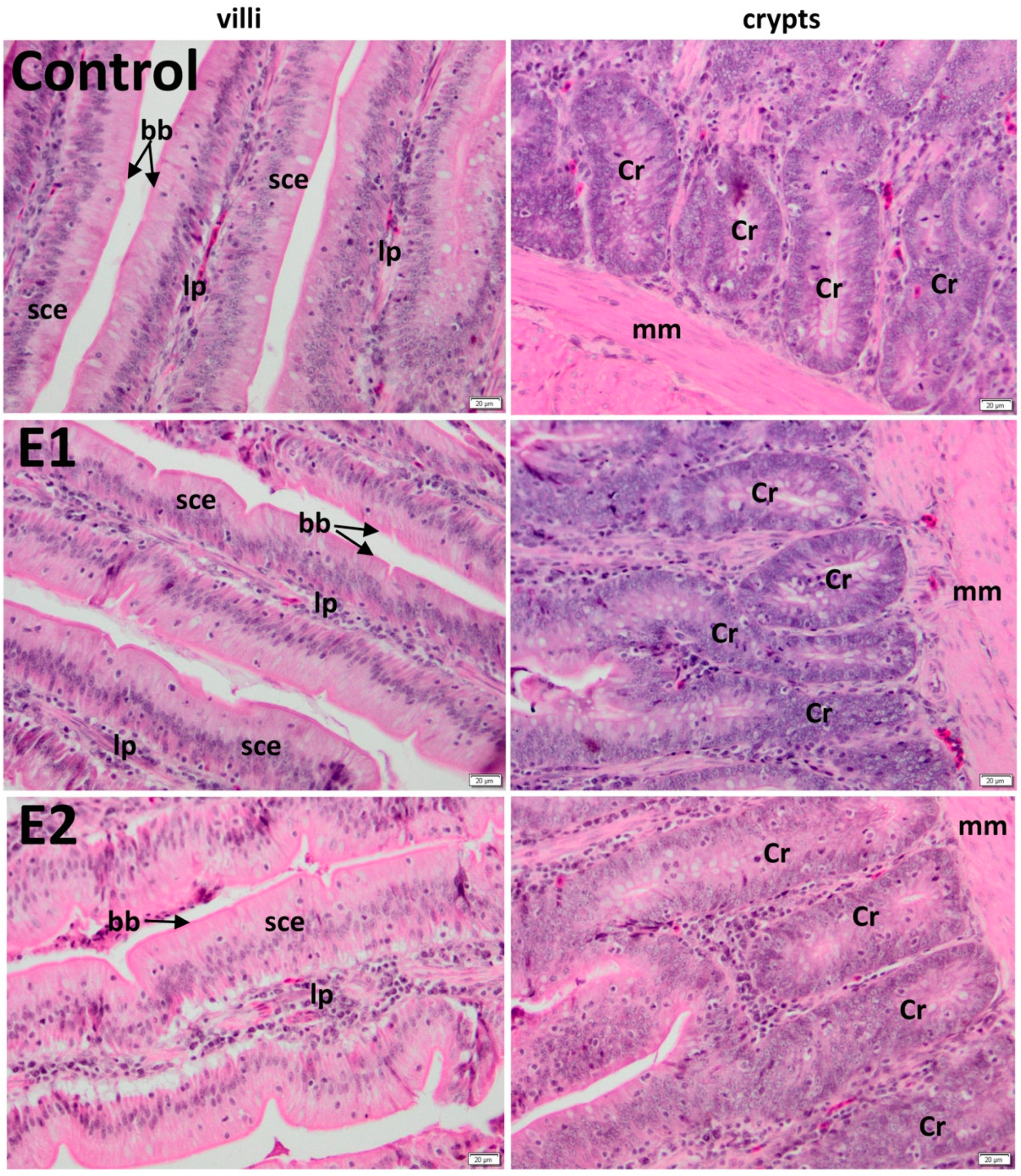
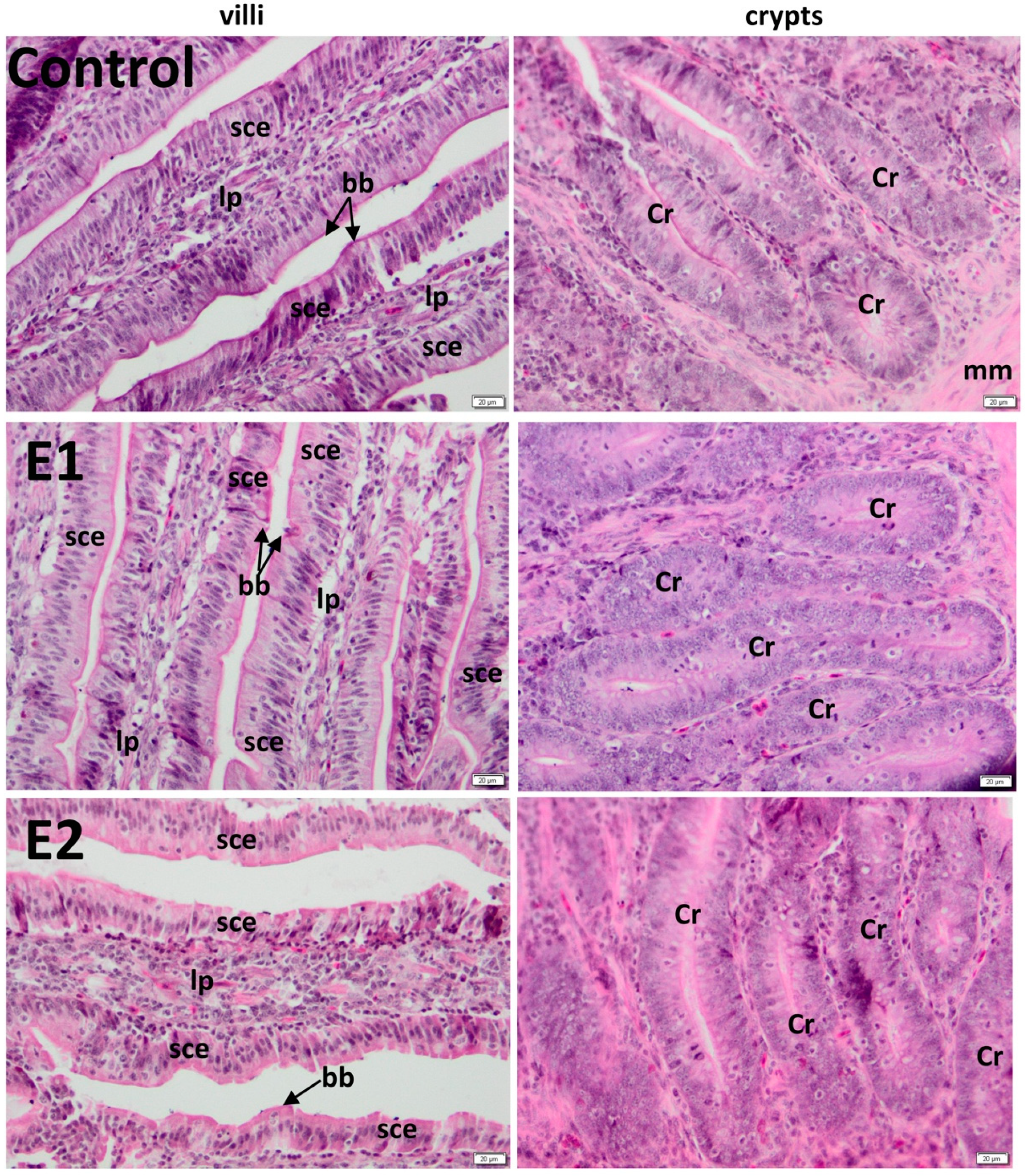
3.2. Histology of the Duodenum
3.3. Histology of the Jejunum
3.4. Intestinal Enzymes Activities
3.5. Intestinal Microbiota
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ahiwe, E.U.; Omede, A.A.; Abdallh, M.B.; Iji, P.A. Chapter 6: Managing dietary energy intake by broiler chickens to reduce production costs and improve product quality. In Animal Husbandry and Nutrition; Yücel, B., Taşkin, T., Eds.; IntechOpen: London, UK, 2018; pp. 115–145. [Google Scholar]
- Alagawany, M.; El-Hack, M.A. The effect of rosemary herb as a dietary supplement on performance, egg quality, serum biochemical parameters, and oxidative status in laying hens. J. Anim. Feed. Sci. 2015, 24, 341–347. [Google Scholar] [CrossRef]
- Abou-Elkhair, R.; Selim, S.; Hussein, E. Effect of supplementing layer hen diet with phytogenic feed additives on laying performance, egg quality, egg lipid peroxidation and blood biochemical constituents. Anim. Nutr. 2018, 4, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Untea, A.E.; Varzaru, I.; Panaite, T.D.; Gavris, T.; Lupu, A.; Ropota, M. The Effects of Dietary Inclusion of Bilberry and Walnut Leaves in Laying Hens’ Diets on the Antioxidant Properties of Eggs. Animals 2020, 10, 191. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Otárola, N.; Sabat, P. Are levels of digestive enzyme activity related to the natural diet in passerine birds? Biol. Res. 2011, 44, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Uni, Z.; Noy, Y.; Sklan, D. Post hatch development of small intestinal function in the poultry. Poult. Sci. 1999, 78, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.Q.; Zhao, F.; Tan, H.Z.; Zhao, J.T.; Zhang, J.Z.; Zhang, H.F. Effects of dietary protein source on the digestive enzyme activities and electrolyte composition in the small intestinal fluid of chickens. Poult. Sci. 2012, 91, 1641–1646. [Google Scholar] [CrossRef]
- Babarykin, D.; Smirnova, G.; Krumina, G.; Vasiljeva, S.; Krumina, Z.; Basova, N.; Fedotova, A. Stimulating Effect of Red Beetroot (Beta vulgaris) Juice, Fractioned by Membrane Ultrafiltration, on Iron Absorption in Chicken Intestines. JBSM 2018, 6, 37–49. [Google Scholar] [CrossRef]
- Dawood, M.A.O. Evaluation of Yeast Fermented Poultry By-Product Meal in Nile Tilapia (Oreochromis niloticus) Feed: Effects on Growth Performance, Digestive Enzymes Activity, Innate Immunity, and Antioxidant Capacity. Front. Vet. Sci. 2020, 6, 1–9. [Google Scholar] [CrossRef]
- Bird, F.H. Distribution of trypsin and α—Amylase activities in the duodenum of the domestic fowl. Br. Poult. 1971, 12, 373–378. [Google Scholar] [CrossRef]
- Duke, G.E. Alimentary canal: Secretion and digestion, special digestive functions, and absorption. In Avian Physiology, 4th ed.; Sturkie, P.D., Ed.; Springer: New York, NY, USA, 1986; pp. 289–302. [Google Scholar]
- Alshamy, Z.; Richardson, K.C.; Hu, H.; Hafez, H.M. Comparison of the gastrointestinal tract of a dual-purpose to a broiler chicken line: A qualitative and quantitative macroscopic and microscopic study. PLoS ONE 2018, 13, e0204921. [Google Scholar] [CrossRef]
- Raza, A.; Bashir, S.; Tabassum, R. An update on carbohydrases: Growth performance and intestinal health of poultry. Heliyon 2019, 5, e01437. [Google Scholar] [CrossRef]
- Shang, Y.; Kumar, S.; Oakley, B.; Kim, W.K. Chicken Gut Microbiota: Importance and Detection Technology. Front. Vet. Sci. 2018, 5, 254. [Google Scholar] [CrossRef] [PubMed]
- Maki, J.J.; Klima, C.L.; Sylte, M.J. The Microbial Pecking Order: Utilization of Intestinal Microbiota for Poultry Health. Microorganisms 2019, 7, 376. [Google Scholar] [CrossRef] [PubMed]
- Grond, K.; Sandercock, B.K.; Jumpponen, A.; Zeglin, L.H. The Avian Gut Microbiota: Community, Physiology and Function in Wild Birds. J. Avian Biol. 2018, 49, 1–19. [Google Scholar] [CrossRef]
- Giannenas, I.; Bonos, E.; Skoufos, I.; Tzora, A.; Stylianaki, I.; Lazari, D.; Tsinas, A.; Christaki, E.; Florou-paneri, P. Effect of herbal feed additives on performance parameters, intestinal microbiota, intestinal morphology and meat lipid oxidation of broiler chickens. Br. Poult. Sci. 2018, 59, 545–553. [Google Scholar] [CrossRef]
- Franz, C.M.; Baser, K.H.C.; Hahn-Ramssl, I. Chapter 3: Herbs and aromatic plants as feed additives: Aspects of composition, safety, and registration rules. In Feed Additives—Aromatic Plants and Herbs in Animal Nutrition and Health; Florou-Paneri, P., Christaki, E., Giannenas, I., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 35–56. [Google Scholar]
- Tuyen, P.T.; Xuan, T.D.; Khang, D.T.; Ahmad, A.; Quan, N.V.; Tu Anh, T.T.; Anh, H.; Minh, T.N. Phenolic Compositions and Antioxidant Properties in Bark, Flower, Inner Skin, Kernel and Leaf Extracts of Castanea crenata Sieb. et Zucc. Antioxidants 2017, 6, 31. [Google Scholar] [CrossRef]
- Karcheva-bahchevanska, D.P.; Lukova, P.K.; Nikolova, M.M.; Mladenov, R.D.; Iliev, I.N. Effect of Extracts of Bilberries (Vaccinium myrtillus L.) on Amyloglucosidase and α-Glucosidase Activity. Folia Med. 2017, 59, 197–202. [Google Scholar] [CrossRef]
- Jepson, R.G.; Craig, J.C. A systematic review of the evidence for cranberries and blueberries in UTI prevention. Mol. Nutr. Food Res. 2007, 51, 738–745. [Google Scholar] [CrossRef]
- Wang, Y.; Chang, C.; Chou, J.; Chen, H.; Deng, X.; Harvey, B.K.; Lud, J.; Bickford, P.C. Dietary supplementation with blueberries, spinach, or spirulina reduces ischemic brain damage. Exp. Neurol. 2005, 193, 75–84. [Google Scholar] [CrossRef]
- Malin, D.H.; Lee, D.R.; Goyarzu, P.; Chang, Y.H.; Ennis, L.J.; Beckett, E.; Shukitt-Hale, B.; Joseph, J.A. Short-term blueberry-enriched diet prevents and reverses object recognition memory loss in aging rats. Nutrition 2011, 27, 338–342. [Google Scholar] [CrossRef]
- Wang, L.; Wu, J.; Wang, H.; Li, S. Composition of phenolic compounds and antioxidant activity in the leaves of blueberry cultivars. J. Funct. Foods 2015, 16, 295–304. [Google Scholar] [CrossRef]
- Feng, C.; Wang, W.; Ye, J.; Li, S.; Wu, Q. Polyphenol profile and antioxidant activity of the fruit and leaf of Vaccinium glaucoalbum from the Tibetan Himalayas. Food Chem. 2016, 219, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Sakaida, H.S.; Agao, K.N.; Iga, K.H.; Hirouchi, B.S.; Noue, N.I.; Idaka, F.H.; Ai, T.K.; Anagita, T.Y. Effect of Vaccinium ashei reade Leaves on Angiotensin Converting Enzyme Activity in Vitro and on Systolic Blood Pressure of Spontaneously Hypertensive Rats in Vivo. Biosci. Biotechnol. Biochem. 2007, 71, 2335–2337. [Google Scholar] [CrossRef] [PubMed]
- Nagao, K.N.; Iga, K.H.; Hirouchi, B.S.; Omura, S.N.; Noue, N.I. Effect of Vaccinium ashei reade Leaves on Lipid Metabolism in Otsuka Long-Evans Tokushima Fatty Rats. Biosci. Biotechnol. Biochem. 2008, 72, 1619–1622. [Google Scholar] [CrossRef]
- Labuckaset, D.O.; Maestri, D.M.; Perello, M.; Martı, M.L.; Labuckas, D.O.; Lamarque, A.L. Phenolics from walnut (Juglans regia L.) kernels: Antioxidant activity and interactions with proteins. Food Chem. 2008, 107, 607–612. [Google Scholar] [CrossRef]
- Pereira, A.; Oliveira, I.; Sousa, A.; Andrade, P.B.; Ferreira, I.C.F.R.; Ferreres, F.; Bento, A.; Seabra, R. Walnut (Juglans regia L.) leaves: Phenolic compounds, antibacterial activity and antioxidant potential of different cultivars. Food Chem. Toxicol. 2007, 45, 2287–2295. [Google Scholar] [CrossRef]
- Delaviz, H.; Mohammadi, J.; Ghalamfarsa, G.; Mohammadi, B.; Farhadi, N. A Review Study on Phytochemistry and Pharmacology Applications of Juglans Regia Plant. Pharmacogn. Rev. 2017, 11, 145–152. [Google Scholar]
- Muthusamy, N.; Sankar, V.; Sheep, M. Phytogenic compounds used as a feed additive in poultry production. Int. J. Sci. Environ. Technol. 2015, 4, 167–171. [Google Scholar]
- Mousavi, R.B.; Roostaei, A.M.M.; Mohiti, A.M. Effect of walnut green husk (Juglans regia) powder on immune repsonses of broiler chickens. Iran J. Vet. Res. 2017, 13, 86–95. [Google Scholar]
- Coșarcă, S.-L.; Moacă, E.-A.; Tanase, C.; Muntean, D.L.; Pavel, I.Z.; Dehelean, C.A. Spruce and beech batk aqueous extracts: Source of polyphenols, tannins and antioxidants correlated to in vitro antitumor potential on teo different cell lines. Wood Sci. Technol. 2019, 53, 313–333. [Google Scholar] [CrossRef]
- Huang, C.; Xiao, L.; Xing, S.; Chen, J.; Yang, Y.; Zhou, Y.; Chen, W.; Liang, J.; Mi, J.; Wang, Y.; et al. The microbiota structure in the cecum of laying hens contributes to dissimilar H2S production. BMC Genom. 2019, 20, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Olszewska, M.A.; Michel, P. Antioxidant activity of inflorescences, leaves and fruits of three Sorbus species in relation to their polyphenolic composition. Nat. Prod. Res. 2009, 23, 1507–1521. [Google Scholar] [CrossRef] [PubMed]
- Burits, M.; Bucar, F. Antioxidant activity of Nigella sativa essential oil. Phytother. Res. 2000, 14, 323–328. [Google Scholar] [CrossRef]
- Stan, M.S.; Voicu, S.N.; Caruntu, S.; Nica, I.C.; Olah, N.; Burtescu, R.; Balta, C.; Rosu, M.; Herman, H.; Hermenean, A.; et al. Antioxidant and Anti-Inflammatory Properties of a Thuja occidentalis Mother Tincture for the Treatment of Ulcerative Colitis. Antioxidants 2019, 8, 416. [Google Scholar] [CrossRef]
- Dávalos, A.; Gómez-Cordovés, C.; Bartolomé, B. Extending applicability of the oxygen radical absorbance capacity (ORAC-fluorescein) assay. J. Agric. Food Chem. 2004, 52, 48–54. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements for Poultry, 9th ed.; National Academy Press: Washington, DC, USA, 1994. [Google Scholar]
- Bernfeld, P. Amylases: Alpha and beta methods. Enzymol 1995, 1, 149–158. [Google Scholar]
- Hummel, B.C.W. A modified spectrophotometric determination of chymotrypsin, trypsin and thrombin. Can. J. Biochem. Physiol. 1959, 37, 1393–1399. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Rinttila, T.; Kassinen, A.; Malinen, E.; Krogius, L.; Palva, A. Development of an extensive set of 16S rDNA-targeted primers for quantification of pathogenic and indigenous bacteria in faecal samples by real-time PCR. J. Appl. Microbiol. 2004, 97, 1166–1177. [Google Scholar] [CrossRef]
- McAuley, J.L.; Linden, S.K.; Png, C.W.; King, R.M.; Pennington, H.L.; Gendler, S.J.; Florin, T.H.; Hill, G.R.; Korolik, V.; McGuckin, M.A. MUC1 cell surface mucin is a critical element of the mucosal barrier to infection. J. Clin. Invest. 2007, 117, 2313–2324. [Google Scholar] [CrossRef]
- Matsuki, T.; Watanabe, K.; Fujimoto, J.; Takada, T.; Tanaka, R. Use of 16S rRNA gene-targeted group-specific primers for real-time PCR analysis of predominant bacteria in human feces. Appl. Env. Microbiol. 2004, 70, 7220–7228. [Google Scholar] [CrossRef] [PubMed]
- Gradisteanu, G.P.; Stoica, R.A.; Petcu, L.; Picu, A.; Suceveanu, A.P.; Salmen, T.; Stefan, D.S.; Serafinceanu, C.; Chifiriuc, M.C.; Stoian, A.P. Microbiota signatures in type-2 diabetic patients with chronic kidney disease—A Pilot Study. J. Mind Med. Sci. 2019, 6, 130–136. [Google Scholar] [CrossRef]
- Mahowald, M.A.; Rey, F.E.; Seedorf, H.; Turnbaugh, P.J.; Fulton, R.S.; Wollam, A.; Shah, N.; Wang, C.; Magrini, V.; Wilson, R.K.; et al. Characterizing a model human gut microbiota composed of members of its two dominant bacterial phyla. Proc. Natl. Acad. Sci. USA 2009, 106, 5859–5864. [Google Scholar] [CrossRef]
- Yadav, S.; Jha, R. Strategies to modulate the intestinal microbiota and their effects on nutrient utilization, performance, and health of poultry. J. Anim. Sci. Biotechnol. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pyner, A.; Nyambe-silavwe, H.; Williamson, G. Inhibition of Human and Rat Sucrase and Maltase Activities to Assess Antiglycemic Potential: Optimization of the Assay Using Acarbose and Polyphenols. J. Agric. Food Chem. 2017, 65, 8643–8651. [Google Scholar] [CrossRef] [PubMed]
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
- Takao, L.K.; Imatomi, M.; Gualtieri, S.C.J. Antioxidant activity and phenolic content of leaf infusions of Myrtaceae species from Cerrado (Brazilian Savanna). Braz. J. Biol. 2015, 75, 948–952. [Google Scholar] [CrossRef]
- Yamauchi, K.; Buwjoom, T.; Koge, K.; Ebashi, T. Histological intestinal recovery in chickens refed dietary sugar cane extract. Poult. Sci. 2006, 85, 645–651. [Google Scholar] [CrossRef]
- Zijlstra, R.T.; Whang, K.Y.; Easter, R.A.; Odle, J. Effect of feeding a milk replacer to early-weaned pigs on growth, body composition, and small intestinal morphology, compared with suckled littermates. J. Anim. Sci. 1996, 74, 2948–2959. [Google Scholar] [CrossRef]
- Shamoto, K.; Yamauchi, K. Recovery responses of chick intestinal villus morphology to different refeeding procedures. Poult. Sci. 2000, 79, 718–723. [Google Scholar] [CrossRef]
- Wu, Q.J.; Zhou, Y.M.; Wu, Y.N.; Wang, T. Intestinal development and function of broiler chickens on diets supplemented with clinoptilolite. Asian Australas J. Anim. Sci. 2013, 26, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Wawrzyniak, A.; Kapica, M.; Stepien-Pysniak, D.; Luszczewska-Sierakowska, I.; Szewerniak, R.; Jarosz, L. The effect of dietary supplementation of transcarpathian zeolite on intestinal morphology in female broiler chickens. J. Appl. Poult. Res. 2017, 26, 421–430. [Google Scholar] [CrossRef]
- Moghaddam, H.N.; Alizadeh-Ghamsari, A.H. Improved performance and small intestinal development of broiler chickens by dietary L-glutamine supplementation. J. Appl. Anim. Res. 2013, 41, 1–7. [Google Scholar] [CrossRef]
- Lambertz, J.; Weiskirchen, S.; Landert, S.; Weiskirchen, R. Fructose: A Dietary Sugar in Crosstalk with Microbiota Contributing to the Development and Progression of Non-Alcoholic Liver Disease. Front. Immunol. 2017, 8, 1159. [Google Scholar] [CrossRef]
- Houghton, D.; Stewart, C.J.; Day, C.P.; Trenell, M. Gut Microbiota and Lifestyle Interventions in NAFLD. Int. J. Mol. Sci. 2016, 17, 447. [Google Scholar] [CrossRef]
- Kiela, P.R.; Ghishan, F.K. Physiology of Intestinal Absorption and Secretion. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 145–159. [Google Scholar] [CrossRef]
- Rohn, S.; Rawel, H.M.; Kroll, J. Inhibitory Effects of Plant Phenols on the Activity of Selected Enzymes. J. Agric. Food Chem. 2002, 50, 3566–3571. [Google Scholar] [CrossRef]
- Srinivas, D.; Mital, B.K.; Garg, S.K. Utilization of sugars by Lactobacillus acidophilus strains. Int. J. Food Microiol. 1990, 10, 51–57. [Google Scholar] [CrossRef]
- Andreevskaya, M.; Johansson, P.; Jääskeläinen, E.; Rämö, T.; Ritari, J.; Paulin, L.; Björkroth, J.; Auvinen, P. Lactobacillus oligofermentans glucose, ribose and xylose transcriptomes show higher similarity between glucose and xylose catabolism-induced responses in the early exponential growth phase. BMC Genom. 2016, 17, 539. [Google Scholar] [CrossRef]
- Peters, V.J.; Prescott, J.M.; Snell, E.E. Peptides and bacterial growth. IV. Histidine peptides as growth factorsfor Lactobacillus delbrueckii 9469. J. Biol. Chem. 1953, 202, 521–532. [Google Scholar]
- Chen, X.; Sun, H.; Jiang, F.; Shen, Y.; Li, X.; Hu, X.; Shen, X.; Wei, P. Alteration of the gut microbiota associated with childhood obesity by 16S rRNA gene sequencing. Peer J. 2020, 8, e8317. [Google Scholar] [CrossRef] [PubMed]
- Braun, H.; Cogoli, A.; Semenza, G. Carboxyl Groups at the Two Active Centers of Sucrase-Isomaltase from Rabbit Small Intestine. Eur. J. Biochem. 1977, 442, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Farner, D.S. Digestion and the digestive system. In Biology and Comparative Physiology of Birds; Marshall, A.J., Ed.; Academic Press: Cambridge, MA, USA, 1960; pp. 411–467. [Google Scholar]
- Jacob, J.P.; Pescatore, A.J. Small poultry flock resources. In Proceedings of the Poultry Science Association 102nd Annual Meeting, San Diego, CA, USA, 22–25 July 2013; p. 100. [Google Scholar]
- Ciminari, E.; Chediack, J.G. Activity of Digestive Enzymes in Chicken’s Small Intestine and Caeca: Effect of Dietary Protein and Carbohydrate Content. Asian J. Poul. Sci. 2014, 8, 49–63. [Google Scholar]
- Montagne, L.; Pluske, J.R.; Hampson, D.J. A review of interactions between dietary fibre and the intestinal mucosa, and their consequences on digestive health in young non-ruminant animals. Anim. Feed Sci. Technol. 2003, 108, 95–117. [Google Scholar] [CrossRef]
- Heinritz, S.N.; Weiss, E.; Eklund, M.; Aumiller, T.; Louis, S. Intestinal Microbiota and Microbial Metabolites Are Changed in a Pig Model Fed a High-Fat/Low-Fiber or a Low-Fat/High-Fiber Diet. PLoS ONE 2016, 11, e0154329. [Google Scholar] [CrossRef] [PubMed]
- Leusink, G.; Rempel, H.; Skura, B.; Berkyto, M.; White, W.; Yang, Y.; Rhee, J.Y.; Xuan, S.Y.; Chiu, S.; Silversides, F.; et al. Growth performance, meat quality, and gut microflora of broiler chickens fed with cranberry extract. Poult. Sci. 2010, 89, 1514–1523. [Google Scholar] [CrossRef]
| Taxonomic Target | Primer Sequence |
|---|---|
| Eubacteria | UniF340 ACT CCT ACG GGA GGC AGC AGT UniR514 ATT ACC GCG GCT GCT GGC |
| Lactobacilli | LabF362 ACG AGT AGG GAA ATC TTC CA LabR677 CAC CGC TAC ACA TGG AG |
| Enterobacteriaceae | Uni515F GTG CCA GCM GCC GCG GTAA Ent826R GCC TCA AGG GCA CAA CCT CCA AG |
| Bacteroidetes | Bact934F GGAACATGTGGTTTAATTCGATGAT Bact1060R AGCTGACGACAACCATGCAG |
| Firmicutes | Firm934F GGAGCATGTGGTTTAATTCGAAGCA Firm 1060R AGCTGACGACAACCATGCAC |
| Parameters | Bilberry Leaves | Walnut Leaves | ||
|---|---|---|---|---|
| Average | SD | Average | SD | |
| TPC (µg GAE/g d.w.) | 392.644 | ±12.531 | 192.025 | ±10 |
| DPPH (% inhibition) | 84.807 | ±1.24 | 57.589 | ±3.17 |
| ORAC (µmol Trolox/g d.w.) | 328.908 | ±7.21 | 175.700 | ±4.5 |
| Duodenum | Jejunum | |||
|---|---|---|---|---|
| Group | Villus (µm) | Crypt (µm) | Villus (µm) | Crypt (µm) |
| C | 891.55 ± 15 | 189.94 ± 11 | 905.98 ± 27 | 209.51 ± 27 |
| E1 | 1158.84 ± 20 *** | 189.49 ± 11 ns | 1258.04 ± 21 *** | 210.26 ± 44 ns |
| E2 | 1263.44 ± 23 *** | 189.46 ± 14 ns | 1248.7 ± 18 *** | 211.39 ± 37 ns |
| Intestinal Segment | Group | Maltase (U/mg) | Invertase (U/mg) | Alpha-Amylase (U/mg) | Trypsin (U/mg) |
|---|---|---|---|---|---|
| Duodenum | C | 443.87 ± 92.65 | 8.68 ± 3.49 | 208.69 ± 23.63 | 131.33 ± 52.29 |
| E1 | 600.63 ± 306.37 ns | 7.98 ± 7.37 ns | 54.89 ± 10.91 * | 52.43 ± 8.95 *** | |
| E2 | 707.39 ± 245.02 ns | 20.29 ± 6.68 *** | 63.43 ± 23.74 ns | 42.95 ± 1.75 *** | |
| Jejunum | C | 559.18 ± 25.19 | 25.69 ± 2.22 | 18.14 ± 5.5 | 98.01 ± 8.17 |
| E1 | 473.00 ± 64.34 ns | 32.23 ± 5.96 ns | 14.06 ± 3.88 ** | 61.68 ± 28.34 * | |
| E2 | 889.09 ± 79.07 ns | 16.80 ± 3.43 *** | 15.37 ± 3.85 ns | 71.24 ± 16.66 * |
| Group | Bacteroidetes | Firmicutes | Lactobacillus sp. | Enterobacteriaceae |
|---|---|---|---|---|
| C | 1.15 ± 0.39 | 0.90 ± 0.41 | 1.11 ± 0.59 | 0.73 ± 0.33 |
| E1 | 1.09 ± 0.21 ns | 1.45 ± 0.52 ns | 1.26 ± 1.57 ** | 0.005 ± 0.01 ** |
| E2 | 0.97 ± 0.17 ns | 1.56 ± 0.35 ns | 4.55 ± 1.34 ** | 0.058 ± 0.05 ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popescu, R.G.; Voicu, S.N.; Gradisteanu Pircalabioru, G.; Ciceu, A.; Gharbia, S.; Hermenean, A.; Georgescu, S.E.; Panaite, T.D.; Dinischiotu, A. Effects of Dietary Inclusion of Bilberry and Walnut Leaves Powder on the Digestive Performances and Health of Tetra SL Laying Hens. Animals 2020, 10, 823. https://doi.org/10.3390/ani10050823
Popescu RG, Voicu SN, Gradisteanu Pircalabioru G, Ciceu A, Gharbia S, Hermenean A, Georgescu SE, Panaite TD, Dinischiotu A. Effects of Dietary Inclusion of Bilberry and Walnut Leaves Powder on the Digestive Performances and Health of Tetra SL Laying Hens. Animals. 2020; 10(5):823. https://doi.org/10.3390/ani10050823
Chicago/Turabian StylePopescu, Roua Gabriela, Sorina Nicoleta Voicu, Gratiela Gradisteanu Pircalabioru, Alina Ciceu, Sami Gharbia, Anca Hermenean, Sergiu Emil Georgescu, Tatiana Dumitra Panaite, and Anca Dinischiotu. 2020. "Effects of Dietary Inclusion of Bilberry and Walnut Leaves Powder on the Digestive Performances and Health of Tetra SL Laying Hens" Animals 10, no. 5: 823. https://doi.org/10.3390/ani10050823
APA StylePopescu, R. G., Voicu, S. N., Gradisteanu Pircalabioru, G., Ciceu, A., Gharbia, S., Hermenean, A., Georgescu, S. E., Panaite, T. D., & Dinischiotu, A. (2020). Effects of Dietary Inclusion of Bilberry and Walnut Leaves Powder on the Digestive Performances and Health of Tetra SL Laying Hens. Animals, 10(5), 823. https://doi.org/10.3390/ani10050823










