RETRACTED: Glutamine Metabolism and Its Role in Immunity, a Comprehensive Review
Simple Summary
Abstract
1. Introduction
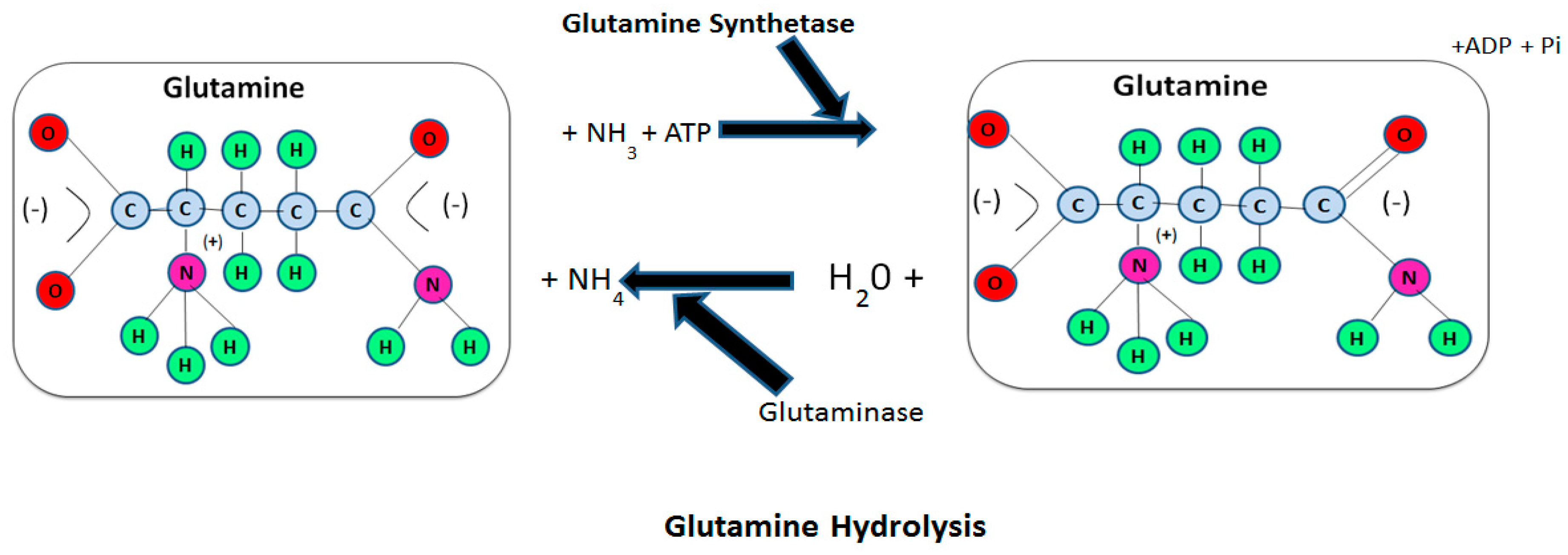
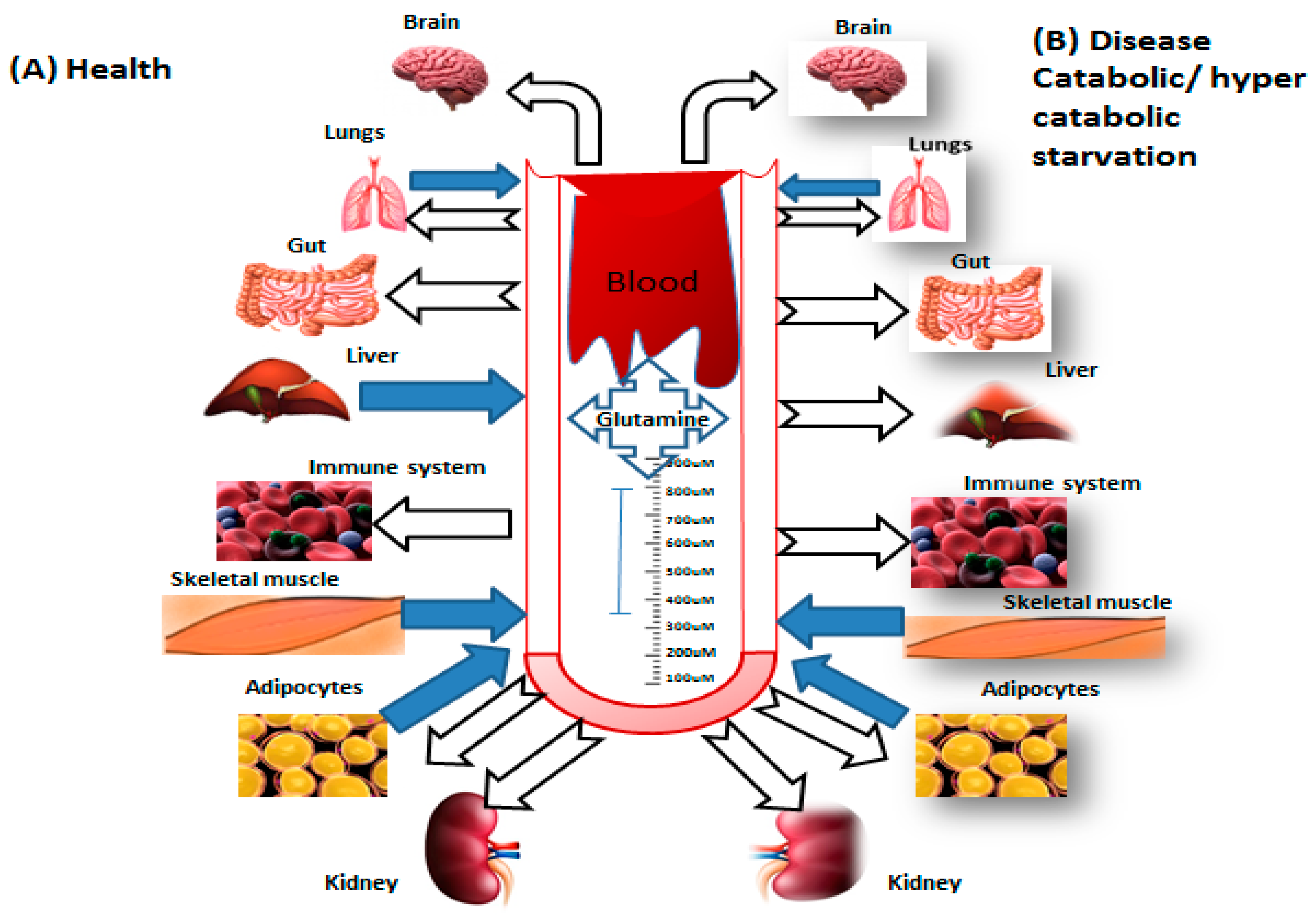
2. Glutamine Metabolism
3. Immune Cells Function and Glutamine
4. Neutrophils
5. Macrophages
6. Lymphocyte
7. Clinical Application of Glutamine Supplementation
8. Conclusions and Future Viewpoints
Author Contributions
Funding
Conflicts of Interest
References
- Ehrensvärd, G.; Fischer, A.; Stjernholm, R. Protein Metabolisiu of Tissue Cells in Vitro. 7. The Chemical Nature of Some Obligate Factors of Tissue Cell Nutrition. Act. Phys. Scandi 1949, 18, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Grohmann, U.; Mondanelli, G.; Belladonna, M.L.; Orabona, C.; Pallotta, M.T.; Iacono, A.; Puccetti, P.; Volpi, C. Amino-acid sensing and degrading pathways in immune regulation. Cytokine Growth Factor Rev. 2017, 35, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Cruzat, V.F.; Pantaleão, L.C.; Donato, J., Jr.; de Bittencourt, P.I.H., Jr.; Tirapegui, J. Oral supplementations with free and dipeptide forms of L-glutamine in endotoxemic mice: Effects on muscle glutamine-glutathione axis and heat shock proteins. J. Nutr. Biochem. 2014, 25, 345–352. [Google Scholar] [CrossRef]
- Newsholme, P. Why is L-glutamine metabolism important to cells of the immune system in health, postinjury, surgery or infection. J. Nutr. 2001, 131, 2515S–2522S. [Google Scholar] [CrossRef] [PubMed]
- Cruzat, V.F.; Krause, M.; Newsholme, P. Amino acid supplementation and impact on immune function in the context of exercise. J. Int. Soc. Sports Nutr. 2014, 11, 61. [Google Scholar] [CrossRef] [PubMed]
- Curi, R.; Newsholme, P.; Marzuca-Nassr, G.N.; Takahashi, H.K.; Hirabara, S.M.; Cruzat, V.; Krause, M.; de Bittencourt, P.I.H. Regulatory principles in metabolism—Then and now. Biochem. J. 2016, 473, 1845–1857. [Google Scholar] [CrossRef] [PubMed]
- Ardawi, M.S.M.; Newsholme, E.A. Maximum activities of some enzymes of glycolysis, the tricarboxylic acid cycle and ketone-body and glutamine utilization pathways in lymphocytes of the rat. Biochem. J. 1982, 208, 743–748. [Google Scholar] [CrossRef]
- Fläring, U.; Rooyackers, O.; Wernerman, J.; Hammarqvist, F. Glutamine attenuates post-traumatic glutathione depletion in human muscle. Clin. Sci. 2003, 104, 275–282. [Google Scholar] [CrossRef]
- Roth, E. Nonnutritive effects of glutamine. J. Nutr. 2008, 138, 2025S–2031S. [Google Scholar] [CrossRef]
- Rodas, P.C.; Rooyackers, O.; Hebert, C.; Norberg, Å.; Wernerman, J. Glutamine and glutathione at ICU admission in relation to outcome. Clin. Sci. 2012, 122, 591–597. [Google Scholar] [CrossRef]
- Newsholme, E.; Parry-Billings, M. Properties of glutamine release from muscle and its importance for the immune system. J. Parenteral Enteral Nutr. 1990, 14, 63S–67S. [Google Scholar] [CrossRef] [PubMed]
- Wernerman, J. Clinical use of glutamine supplementation. J. Nutr. 2008, 138, 2040S–2044S. [Google Scholar] [CrossRef] [PubMed]
- Berg, A.; Norberg, Å.; Martling, C.-R.; Gamrin, L.; Rooyackers, O.; Wernerman, J. Glutamine kinetics during intravenous glutamine supplementation in ICU patients on continuous renal replacement therapy. Intensive Care Med. 2007, 33, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Labow, B.I.; Souba, W.W.; Abcouwer, S.F. Mechanisms governing the expression of the enzymes of glutamine metabolism—Glutaminase and glutamine synthetase. J. Nutr. 2001, 131, 2467S–2474S. [Google Scholar] [CrossRef]
- Cruzat, V.F.; Newsholme, P. An introduction to glutamine metabolism. In Glutamine; CRC Press: Boca Raton, FL, USA, 2017; pp. 1–18. [Google Scholar]
- Cooney, G.; Curi, R.; Mitchelson, A.; Newsholme, P.; Simpson, M.; Newsholme, E.A. Activities of some key enzymes of carbohydrate, ketone body, adenosine and glutamine metabolism in liver, and brown and white adipose tissues of the rat. Biochem. Biophys. Res. Commun. 1986, 138, 687–692. [Google Scholar] [CrossRef]
- Tan, H.W.S.; Sim, A.Y.L.; Long, Y.C. Glutamine metabolism regulates autophagy-dependent mTORC1 reactivation during amino acid starvation. Nat. Commun. 2017, 8, 338. [Google Scholar] [CrossRef]
- Parry-Billings, M.; Dimitriadis, G.; Leighton, B.; Bond, J.; Bevan, S.; Opara, E.; Newsholme, E. Effects of hyperthyroidism and hypothyroidism on glutamine metabolism by skeletal muscle of the rat. Biochem. J. 1990, 272, 319–322. [Google Scholar] [CrossRef]
- Parry-Billings, M.; Dimitriadis, G.; Leighton, B.; Dunger, D.; Newsholme, E. The effects of growth hormone administration in vivo on skeletal muscle glutamine metabolism of the rat. Horm. Metab. Res. 1993, 25, 292–293. [Google Scholar] [CrossRef]
- Salleh, M.; Ardawi, M. Glutamine metabolism in the lungs of glucocorticoid-treated rats. Clin. Sci. 1991, 81, 37–42. [Google Scholar] [CrossRef]
- Krebs, H.A. Metabolism of amino-acids: The synthesis of glutamine from glutamic acid and ammonia, and the enzymic hydrolysis of glutamine in animal tissues. Biochem. J. 1935, 29, 1951. [Google Scholar] [CrossRef]
- Neu, J.; Shenoy, V.; Chakrabarti, R. Glutamine nutrition and metabolism: Where do we go from here? FASEB J. 1996, 10, 829–837. [Google Scholar] [CrossRef]
- Holeček, M. Branched-chain amino acids in health and disease: Metabolism, alterations in blood plasma, and as supplements. Nutr. Metab. 2018, 15, 33. [Google Scholar] [CrossRef]
- Kao, C.; Hsu, J.; Bandi, V.; Jahoor, F. Alterations in glutamine metabolism and its conversion to citrulline in sepsis. Am. J. Phys. Endocrinol. Metab. 2013, 304, E1359–E1364. [Google Scholar] [CrossRef] [PubMed]
- Rogero, M.M.; Borges, M.C.; De Oliveira Pires, I.S.; Borelli, P.; Tirapegui, J. Effect of glutamine supplementation and in vivo infection with Mycobacterium bovis (bacillus calmette-guerin) in the function of peritoneal macrophages in early weaned mice. Ann. Nutr. Metab. 2007, 51, 173–174. [Google Scholar]
- Karinch, A.M.; Pan, M.; Lin, C.-M.; Strange, R.; Souba, W.W. Glutamine metabolism in sepsis and infection. J. Nutr. 2001, 131, 2535S–2538S. [Google Scholar] [CrossRef] [PubMed]
- Cruzat, V.F.; Rogero, M.M.; Tirapegui, J. Effects of supplementation with free glutamine and the dipeptide alanyl-glutamine on parameters of muscle damage and inflammation in rats submitted to prolonged exercise. Cell Biochem. Funct. 2010, 28, 24–30. [Google Scholar] [CrossRef]
- Curi, R.; Lagranha, C.; Doi, S.; Sellitti, D.; Procopio, J.; Pithon-Curi, T. Glutamine-dependent changes in gene expression and protein activity. Cell Biochem. Funct. 2005, 23, 77–84. [Google Scholar] [CrossRef]
- Djoko, K.Y.; Phan, M.-D.; Peters, K.M.; Walker, M.J.; Schembri, M.A.; McEwan, A.G. Interplay between tolerance mechanisms to copper and acid stress in Escherichia coli. Proc. Nat. Acad. Sci. USA 2017, 114, 6818–6823. [Google Scholar]
- Eagle, H.; Oyama, V.I.; Levy, M.; Horton, C.L.; Fleischman, R. The growth response of mammalian cells in tissue culture to L-glutamine and L-glutamic acid. J. Biol. Chem. 1956, 218, 607–616. [Google Scholar]
- Newsholme, P.; Curi, R.; Gordon, S.; Newsholme, E.A. Metabolism of glucose, glutamine, long-chain fatty acids and ketone bodies by murine macrophages. Biochem. J. 1986, 239, 121–125. [Google Scholar] [CrossRef]
- Curi, T.C.P.; de Melo, M.P.; de Azevedo, R.B.; Curi, R. Glutamine utilisation by rat neutrophils. Biochem. Soc. Trans. 1997, 25, 249S. [Google Scholar] [CrossRef]
- Curi, T.C.P.; De Melo, M.P.; De Azevedo, R.B.; Zorn, T.M.; Curi, R. Glutamine utilization by rat neutrophils: Presence of phosphate-dependent glutaminase. Am. J. Phys.-Cell Phys. 1997, 273, C1124–C1129. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, E.A.; Newsholme, P.; Curi, R. The role of the citric acid cycle in cells of the immune system and its importance in sepsis, trauma and burns. Biochem. Soc. Symp. 1987, 54, 145–162. [Google Scholar]
- Curi, R.; Newsholme, P.; Newsholme, E. Intracellular distribution of some enzymes of the glutamine utilisation pathway in rat lymphocytes. Biochem. Biophys. Res. Commun. 1986, 138, 318–322. [Google Scholar] [CrossRef]
- Curi, R.; Lagranha, C.J.; Doi, S.Q.; Sellitti, D.; Procópio, J.; Pithon-Curi, T.C.; Corless, M.; Newsholme, P. Molecular mechanisms of glutamine action. J. Cell. Physiol. 2005, 204, 392–401. [Google Scholar] [CrossRef]
- Roth, E.; Oehler, R.; Manhart, N.; Exner, R.; Wessner, B.; Strasser, E.; Spittler, A. Regulative potential of glutamine—Relation to glutathione metabolism. Nutrition 2002, 18, 217–221. [Google Scholar] [CrossRef]
- Hiscock, N.; Petersen, E.W.; Krzywkowski, K.; Boza, J.; Halkjaer-Kristensen, J.; Pedersen, B.K. Glutamine supplementation further enhances exercise-induced plasma IL-6. J. Appl. Phys. 2003, 95, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.L.; Kelly, B.; O’Neill, L.A. Mitochondria are the powerhouses of immunity. Nat. Immunol. 2017, 18, 488. [Google Scholar] [CrossRef] [PubMed]
- Pithon-Curi, T.C.; De Melo, M.P.; Curi, R. Glucose and glutamine utilization by rat lymphocytes, monocytes and neutrophils in culture: A comparative study. Cell Biochem. Funct. 2004, 22, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Branzk, N.; Lubojemska, A.; Hardison, S.E.; Wang, Q.; Gutierrez, M.G.; Brown, G.D.; Papayannopoulos, V. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat. Immunol. 2014, 15, 1017–1025. [Google Scholar] [CrossRef]
- Pithon-Curi, T.C.; Levada, A.C.; Lopes, L.R.; Rui, C. Glutamine plays a role in superoxide production and the expression of p47phox, p22phox and gp91phox in rat neutrophils. Clin. Sci. 2002, 103, 403–408. [Google Scholar] [CrossRef]
- Garcia, C.; Pithon-Curi, T.C.; Firmano, M.D.L.; De Melo, M.P.; Newsholme, P.; Rui, C. Effects of adrenaline on glucose and glutamine metabolism and superoxide production by rat neutrophils. Clin. Sci. 1999, 96, 549–555. [Google Scholar] [CrossRef]
- Newsholme, P.; Rosa, L.C.; Newsholme, E.; Curi, R. The importance of fuel metabolism to macrophage function. Cell Biochem. Funct. 1996, 14, 1–10. [Google Scholar] [CrossRef]
- Rosa, L.C.; Safi, D.; Curi, R. Effect of thioglycollate and BCG stimuli on glucose and glutamine metabolism in rat macrophages. J. Leukoc. Biol. 1994, 56, 10–14. [Google Scholar]
- Langston, P.K.; Shibata, M.; Horng, T. Metabolism supports macrophage activation. Front. Immunol. 2017, 8, 61. [Google Scholar] [CrossRef] [PubMed]
- Vergadi, E.; Ieronymaki, E.; Lyroni, K.; Vaporidi, K.; Tsatsanis, C. Akt signalling pathway in macrophage activation and M1/M2 polarization. J. Immunol. 2017, 198, 1006–1014. [Google Scholar] [CrossRef]
- Martinez, F.O.; Sica, A.; Mantovani, A.; Locati, M. Macrophage activation and polarization. Front. Biosci. 2008, 13, 453–461. [Google Scholar] [CrossRef]
- Gordon, S.; Martinez, F.O. Alternative activation of macrophages: Mechanism and functions. Immune 2010, 32, 593–604. [Google Scholar] [CrossRef]
- O’neill, L.A.; Pearce, E.J. Immunometabolism governs dendritic cell and macrophage function. J. Exp. Med. 2016, 213, 15–23. [Google Scholar] [CrossRef]
- Namgaladze, D.; Brüne, B. Fatty acid oxidation is dispensable for human macrophage IL-4-induced polarization. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2014, 1841, 1329–1335. [Google Scholar] [CrossRef]
- O’Neill, L.A. A broken krebs cycle in macrophages. Immune 2015, 42, 393–394. [Google Scholar] [CrossRef] [PubMed]
- Palsson-McDermott, E.M.; Curtis, A.M.; Goel, G.; Lauterbach, M.A.; Sheedy, F.J.; Gleeson, L.E.; van den Bosch, M.W.; Quinn, S.R.; Domingo-Fernandez, R.; Johnston, D.G. Pyruvate kinase M2 regulates Hif-1α activity and IL-1β induction and is a critical determinant of the warburg effect in LPS-activated macrophages. Cell Metab. 2015, 21, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Oren, R.; Farnham, A.E.; Saito, K.; Milofsky, E.; Karnovsky, M.L. Metabolic patterns in three types of phagocytizing cells. J. Cell Biol. 1963, 17, 487–501. [Google Scholar] [CrossRef] [PubMed]
- Tannahill, G.; Curtis, A.; Adamik, J.; Palsson-McDermott, E.; McGettrick, A.; Goel, G.; Frezza, C.; Bernard, N.; Kelly, B.; Foley, N. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 2013, 496, 238. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.K.; Huang, S.C.-C.; Sergushichev, A.; Lampropoulou, V.; Ivanova, Y.; Loginicheva, E.; Chmielewski, K.; Stewart, K.M.; Ashall, J.; Everts, B. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immune 2015, 42, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.C.; Rice, C.M.; Palmieri, E.M.; Taylor, P.R.; Kuhns, D.B.; McVicar, D.W. Peritoneal tissue-resident macrophages are metabolically poised to engage microbes using tissue-niche fuels. Nat. Commun. 2017, 8, 2074. [Google Scholar] [CrossRef]
- Liu, P.-S.; Wang, H.; Li, X.; Chao, T.; Teav, T.; Christen, S.; Di Conza, G.; Cheng, W.-C.; Chou, C.-H.; Vavakova, M. α-ketoglutarate orchestrates macrophage activation through metabolic and epigenetic reprogramming. Nat. Immunol. 2017, 18, 985. [Google Scholar] [CrossRef]
- Nelson, V.L.; Nguyen, H.C.; Garcìa-Cañaveras, J.C.; Briggs, E.R.; Ho, W.Y.; DiSpirito, J.R.; Marinis, J.M.; Hill, D.A.; Lazar, M.A. PPARγ is a nexus controlling alternative activation of macrophages via glutamine metabolism. Gen. Dev. 2018, 32, 1035–1044. [Google Scholar] [CrossRef]
- Newsholme, E.A.; Crabtree, B.; Ardawi, M.S.M. Glutamine metabolism in lymphocytes: Its biochemical, physiological and clinical importance. Quart. J. Exp. Phys. 1985, 70, 473–489. [Google Scholar] [CrossRef]
- Curi, R.; Newsholme, P.; Newsholme, E.A. Metabolism of pyruvate by isolated rat mesenteric lymphocytes, lymphocyte mitochondria and isolated mouse macrophages. Biochem. J. 1988, 250, 383–388. [Google Scholar] [CrossRef]
- Maciolek, J.A.; Pasternak, J.A.; Wilson, H.L. Metabolism of activated T lymphocytes. Curr. Opin. Immunol. 2014, 27, 60–74. [Google Scholar] [CrossRef]
- Tripmacher, R.; Gaber, T.; Dziurla, R.; Häupl, T.; Erekul, K.; Grützkau, A.; Tschirschmann, M.; Scheffold, A.; Radbruch, A.; Burmester, G.R. Human CD4+ T cells maintain specific functions even under conditions of extremely restricted ATP production. Eur. J. Immunol. 2008, 38, 1631–1642. [Google Scholar] [CrossRef] [PubMed]
- Wieman, H.L.; Wofford, J.A.; Rathmell, J.C. Cytokine stimulation promotes glucose uptake via phosphatidylinositol-3 kinase/Akt regulation of Glut1 activity and trafficking. Mol. Biol. Cell 2007, 18, 1437–1446. [Google Scholar] [CrossRef] [PubMed]
- Delgoffe, G.M.; Kole, T.P.; Zheng, Y.; Zarek, P.E.; Matthews, K.L.; Xiao, B.; Worley, P.F.; Kozma, S.C.; Powell, J.D. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immune 2009, 30, 832–844. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Gudapati, P.; Dragovic, S.; Spencer, C.; Joyce, S.; Killeen, N.; Magnuson, M.A.; Boothby, M. Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signalling pathways. Immune 2010, 32, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Michalek, R.D.; Gerriets, V.A.; Jacobs, S.R.; Macintyre, A.N.; MacIver, N.J.; Mason, E.F.; Sullivan, S.A.; Nichols, A.G.; Rathmell, J.C. Cutting edge: Distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J. Immunol. 2011, 186, 3299–3303. [Google Scholar] [CrossRef]
- Hardie, D.G.; Hawley, S.A.; Scott, J.W. AMP-activated protein kinase–development of the energy sensor concept. J. Phys. 2006, 574, 7–15. [Google Scholar] [CrossRef]
- Matarese, G.; Colamatteo, A.; De Rosa, V. Metabolic fuelling of proper T cell functions. Immunol. Lett. 2014, 161, 174–178. [Google Scholar] [CrossRef]
- Chang, C.-H.; Curtis, J.D.; Maggi, L.B., Jr.; Faubert, B.; Villarino, A.V.; O’Sullivan, D.; Huang, S.C.-C.; van der Windt, G.J.; Blagih, J.; Qiu, J. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell 2013, 153, 1239–1251. [Google Scholar] [CrossRef]
- Zheng, Y.; Delgoffe, G.M.; Meyer, C.F.; Chan, W.; Powell, J.D. Anergic T cells are metabolically anergic. J. Immunol. 2009, 183, 6095–6101. [Google Scholar] [CrossRef]
- Geltink, R.I.K.; O’Sullivan, D.; Corrado, M.; Bremser, A.; Buck, M.D.; Buescher, J.M.; Firat, E.; Zhu, X.; Niedermann, G.; Caputa, G. Mitochondrial priming by CD28. Cell 2017, 171, 385–397.e311. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, S.E.; O’Neill, L.A. HIF1α and metabolic reprogramming in inflammation. J. Clin. Investig. 2016, 126, 3699–3707. [Google Scholar] [CrossRef] [PubMed]
- Araujo, L.; Khim, P.; Mkhikian, H.; Mortales, C.-L.; Demetriou, M. Glycolysis and glutaminolysis cooperatively control T cell function by limiting metabolite supply to N-glycosylation. Elife 2017, 6, e21330. [Google Scholar] [CrossRef]
- Hesterberg, R.; Cleveland, J.; Epling-Burnette, P. Role of polyamines in immune cell functions. Med. Sci. 2018, 6, 22. [Google Scholar] [CrossRef]
- Lobley, G.E.; Hoskin, S.O.; McNeil, C.J. Glutamine in animal science and production. J. Nutr. 2001, 131, 2525S–2531S. [Google Scholar] [CrossRef] [PubMed]
- Soeters, P.B. Glutamine: The link between depletion and diminished gut function? J. Am. Coll. Nutr. 1996, 15, 195–196. [Google Scholar] [CrossRef]
- Windle, E.M. Glutamine Supplementation in Critical Illness: Evidence, Recommendations, and Implications for Clinical Practice in Burn Care. J. Burn Care Res. 2006, 27, 764–772. [Google Scholar] [CrossRef]
- Newsholme, P.; Lima, M.M.; Procopio, J.; Pithon-Curi, T.C.; Doi, S.Q.; Bazotte, R.B.; Curi, R. Glutamine and glutamate as vital metabolites. Braz. J. Med. Biol. Res. 2003, 36, 153–163. [Google Scholar] [CrossRef]
- Fuentes-Orozco, C.; Anaya-Prado, R.; González-Ojeda, A.; Arenas-Márquez, H.; Cabrera-Pivaral, C.; Cervantes-Guevara, G.; Barrera-Zepeda, L.M. L-alanyl-L-glutamine-supplemented parenteral nutrition improves infectious morbidity in secondary peritonitis. Clin. Nutr. 2004, 23, 13–21. [Google Scholar] [CrossRef]
- Alpers, D.H. Is glutamine a unique fuel for small intestinal cells? Curr. Opin. Gastroenterol. 2000, 16, 155. [Google Scholar] [CrossRef]
- Perna, S.; Alalwan, T.A.; Alaali, Z.; Alnashaba, T.; Gasparri, C.; Infantino, V.; Hammad, L.; Riva, A.; Petrangolini, G.; Allegrini, P. The Role of Glutamine in the Complex Interaction between Gut Microbiota and Health: A Narrative Review. Int. J. Mol. Sci. 2019, 20, 5232. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, A.M.; Wang, Z.; Ma, J. RETRACTED: Glutamine Metabolism and Its Role in Immunity, a Comprehensive Review. Animals 2020, 10, 326. https://doi.org/10.3390/ani10020326
Shah AM, Wang Z, Ma J. RETRACTED: Glutamine Metabolism and Its Role in Immunity, a Comprehensive Review. Animals. 2020; 10(2):326. https://doi.org/10.3390/ani10020326
Chicago/Turabian StyleShah, Ali Mujtaba, Zhisheng Wang, and Jian Ma. 2020. "RETRACTED: Glutamine Metabolism and Its Role in Immunity, a Comprehensive Review" Animals 10, no. 2: 326. https://doi.org/10.3390/ani10020326
APA StyleShah, A. M., Wang, Z., & Ma, J. (2020). RETRACTED: Glutamine Metabolism and Its Role in Immunity, a Comprehensive Review. Animals, 10(2), 326. https://doi.org/10.3390/ani10020326





