Effects of Wheat Bran and Clostridium butyricum Supplementation on Cecal Microbiota, Short-Chain Fatty Acid Concentration, pH and Histomorphometry in Broiler Chickens
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Treatments
2.2. Sampling
2.3. DNA Extraction, PCR Amplification of the 16S rRNA Genes, and Illumina MiSeq Sequencing
2.4. Chemical Analyses
2.5. Histomorphological Analysis
2.6. Feed Analyses
2.7. Statistical Analyses
3. Results
3.1. Growth Characteristics
3.2. Cecal Histology, pH and SCFA Composition
3.3. Microbiota Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ricke, S.C.; Lee, S.I.; Kim, S.A.; Park, S.H.; Shi, Z. Prebiotics and the poultry gastrointestinal tract microbiome. Poult. Sci. 2020, 99, 670–677. [Google Scholar] [CrossRef]
- Jeurissen, S.H.M.; Lewis, F.; van der Klis, J.D.; Mroz, Z.; Rebel, J.M.J.; ter Huurne, A.A.H.M. Parameters and techniques to determine intestinal health of poultry as constituted by immunity, integrity, and functionality. Curr. Issues Intest. Microbiol. 2002, 3, 1–14. [Google Scholar]
- Rinttilä, T.; Apajalahti, J. Intestinal microbiota and metabolites—Implications for broiler chicken health and performance. J. Appl. Poult Res. 2013, 22, 647–658. [Google Scholar] [CrossRef]
- Dhama, K.; Tiwari, R.; Khan, R.U.; Chakraborty, S.; Gopi, M.; Karthik, K.; Saminathan, M.; Desingu, P.A.; Sunkara, L.T. Growth promoters and novel feed additives improving poultry production and health, bioactive principles and beneficial applications: The trends and advances-a review. Int. J. Pharmacol. 2014, 10, 129–159. [Google Scholar] [CrossRef]
- Vermeulen, K.; Verspreet, J.; Courtin, C.M.; Haesebrouck, F.; Baeyen, S.; Haegeman, A.; Ducatelle, R.; Van Immerseel, F. Reduced-Particle-Size Wheat Bran Is Efficiently Colonized by a Lactic Acid-Producing Community and Reduces Levels of Enterobacteriaceae in the Cecal Microbiota of Broilers. Appl. Environ. Microbiol. 2018, 84. [Google Scholar] [CrossRef] [PubMed]
- Maes, C.; Delcour, J.A. Structural Characterisation of Water-extractable and Water-unextractable Arabinoxylans in Wheat Bran. J. Cereal Sci. 2002, 35, 315–326. [Google Scholar] [CrossRef]
- Courtin, C.M.; Swennen, K.; Broekaert, W.F.; Swennen, Q.; Buyse, J.; Decuypere, E.; Michiels, C.W.; De Ketelaere, B.; Delcour, J.A. Effects of dietary inclusion of xylooligosaccharides, arabinoxylooligosaccharides and soluble arabinoxylan on the microbial composition of caecal contents of chickens. J. Sci. Food Agric. 2008, 88, 2517–2522. [Google Scholar] [CrossRef]
- Such, N.; Molnár, A.; Farkas, V.; Pál, L.; Husvéth, F.; Koltay, I.A.; Rawash, M.A.; Mezőlaki, Á.; Dublecz, K. Feeding two single strain probiotic bacteria and wheat bran failed to modify the production traits but altered some gut characteristics in broiler chickens. J. Cent. Eur. Agric. 2020, 21, 499–507. [Google Scholar] [CrossRef]
- Chen, Y.; Wen, C.; Zhou, Y. Dietary synbiotic incorporation as an alternative to antibiotic improves growth performance, intestinal morphology, immunity and antioxidant capacity of broilers. J. Sci. Food Agric. 2018, 98, 3343–3350. [Google Scholar] [CrossRef]
- Cassir, N.; Benamar, S.; La Scola, B. Clostridium butyricum: From beneficial to a new emerging pathogen. Clin. Microbiol. Infect. 2016, 22, 37–45. [Google Scholar] [CrossRef]
- Abdel-Latif, M.A.; El-Hack, M.E.A.; Swelum, A.A.; Saadeldin, I.M.; Elbestawy, A.R.; Shewita, R.S.; Ba-Awadh, H.A.; Alowaimer, A.N.; El-Hamid, H.S.A. Single and combined effects of Clostridium butyricum and Saccharomyces cerevisiae on growth indices, intestinal health, and immunity of broilers. Animals 2018, 8, 184. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Wang, Y.; Song, D.; Lu, Z.; Dong, Z.; Miao, H.; Wang, W.; He, J.; Li, A. Effects of Clostridium butyricum and Lactobacillus plantarum on growth performance, immune function and volatile fatty acid level of caecal digesta in broilers. Food Agric. Immunol. 2018, 29, 797–807. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, X.; Guo, Y.; Long, F. Effects of dietary lipids and Clostridium butyricum on the performance and the digestive tract of broiler chickens. Arch. Anim. Nutr. 2011, 65, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wang, L.; Khan, A.; Zhao, R.; Wei, S.; Jing, X. Fermented wheat bran by xylanase-producing Bacillus cereus boosts the intestinal microflora of broiler chickens. Poult. Sci. 2020, 99, 263–271. [Google Scholar] [CrossRef]
- Shang, Q.H.; Liu, S.J.; He, T.F.; Liu, H.S.; Mahfuz, S.; Ma, X.K.; Piao, X.S. Effects of wheat bran in comparison to antibiotics on growth performance, intestinal immunity, barrier function, and microbial composition in broiler chickens. Poult. Sci. 2020, 99, 4929–4938. [Google Scholar] [CrossRef]
- Lin, W.C.; Lee, T.T. Effects of Laetiporus sulphureus-fermented wheat bran on growth performance, intestinal microbiota and digesta characteristics in broiler chickens. Animals 2020, 10, 1457. [Google Scholar] [CrossRef]
- Aviagen Group. Aviagen Ross 308 Broiler Nutrition Specifications; Aviagen Group: Huntsville, AL, USA, 2014. [Google Scholar]
- Aviagen Group. Aviagen Ross 308 Broiler Management Handbook; Aviagen Group: Huntsville, AL, USA, 2014. [Google Scholar]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef]
- Illumina Inc. 16S Metagenomic Sequencing Library Preparation-Preparing 16S Ribosomal RNA Gene Amplicons for the Illumina MiSeq System; Illumina Inc.: San Diego, CA, USA, 2013. [Google Scholar]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pẽa, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Molnár, A.; Dublecz, F.; Pál, L.; Wágner, L.; Hess, C.; Hess, M.; Husvéth, F.; Dublecz, K. Soluble nondigestible carbohydrates improve intestinal function and increase caecal coliform load in broiler chickens. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1615–1624. [Google Scholar] [CrossRef] [PubMed]
- Rakszegi, M.; Molnár, I.; Lovegrove, A.; Darkó, É.; Farkas, A.; Láng, L.; Bedő, Z.; Doležel, J.; Molnár-Láng, M.; Shewry, P. Addition of Aegilops U and M Chromosomes Affects Protein and Dietary Fiber Content of Wholemeal Wheat Flour. Front. Plant Sci. 2017, 8, 1529. [Google Scholar] [CrossRef] [PubMed]
- Dhariwal, A.; Chong, J.; Habib, S.; King, I.L.; Agellon, L.B.; Xia, J. MicrobiomeAnalyst: A web-based tool for comprehensive statistical, visual and meta-analysis of microbiome data. Nucleic Acids Res. 2017, 45, W180–W188. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Leblois, J.; Taminiau, B.; Schroyen, M.; Beckers, Y.; Bindelle, J.; Everaert, N. The effect of inulin and wheat bran on intestinal health and microbiota in the early life of broiler chickens. Poult. Sci. 2018, 97, 3156–3165. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; McCartney, E.; Knox, A.; Francesch, M.; Oka, K.; Wada, K.; Ideno, M.; Uno, K.; Kozłowski, K.; Jankowski, J.; et al. Effects of the butyric acid-producing strain Clostridium butyricum MIYAIRI 588 on broiler and piglet zootechnical performance and prevention of necrotic enteritis. Anim. Sci. J. 2018, 89, 895–905. [Google Scholar] [CrossRef]
- Zhao, X.; Shen, Y.; Shao, D.; Shi, S.R.; Ding, X.; Yang, Z.B. Effects of Clostridium butyricum on growth performance, lipid metabolism and the caecal microecological environment of broilers. Eur. Poult. Sci. 2017, 81. [Google Scholar] [CrossRef]
- Yang, C.M.; Cao, G.T.; Ferket, P.R.; Liu, T.T.; Zhou, L.; Zhang, L.; Xiao, Y.P.; Chen, A.G. Effects of probiotic, Clostridium butyricum, on growth performance, immune function, and cecal microflora in broiler chickens. Poult. Sci. 2012, 91, 2121–2129. [Google Scholar] [CrossRef]
- Svihus, B.; Choct, M.; Classen, H.L. Function and nutritional roles of the avian caeca: A review. Worlds. Poult. Sci. J. 2013, 69, 249–264. [Google Scholar] [CrossRef]
- Awad, W.A.; Mann, E.; Dzieciol, M.; Hess, C.; Schmitz-Esser, S.; Wagner, M.; Hess, M. Age-Related Differences in the Luminal and Mucosa-Associated Gut Microbiome of Broiler Chickens and Shifts Associated with Campylobacter jejuni Infection. Front. Cell. Infect. Microbiol. 2016, 6, 154. [Google Scholar] [CrossRef]
- Mohd Shaufi, M.A.; Sieo, C.C.; Chong, C.W.; Gan, H.M.; Ho, Y.W. Deciphering chicken gut microbial dynamics based on high-throughput 16S rRNA metagenomics analyses. Gut Pathog. 2015, 7, 4. [Google Scholar] [CrossRef]
- Oakley, B.B.; Buhr, R.J.; Ritz, C.W.; Kiepper, B.H.; Berrang, M.E.; Seal, B.S.; Cox, N.A. Successional changes in the chicken cecal microbiome during 42 days of growth are independent of organic acid feed additives. BMC Vet. Res. 2014, 10, 282. [Google Scholar] [CrossRef] [PubMed]
- Siegerstetter, S.-C.; Schmitz-Esser, S.; Magowan, E.; Wetzels, S.U.; Zebeli, Q.; Lawlor, P.G.; O’Connell, N.E.; Metzler-Zebeli, B.U. Intestinal microbiota profiles associated with low and high residual feed intake in chickens across two geographical locations. PLoS ONE 2017, 12, e0187766. [Google Scholar] [CrossRef] [PubMed]
- Crisol-Martínez, E.; Stanley, D.; Geier, M.S.; Hughes, R.J.; Moore, R.J. Understanding the mechanisms of zinc bacitracin and avilamycin on animal production: Linking gut microbiota and growth performance in chickens. Appl. Microbiol. Biotechnol. 2017, 101, 4547–4559. [Google Scholar] [CrossRef] [PubMed]
- Waite, D.W.; Taylor, M.W. Exploring the avian gut microbiota: Current trends and future directions. Front. Microbiol. 2015, 6, 673. [Google Scholar] [CrossRef]
- Xiao, Y.; Xiang, Y.; Zhou, W.; Chen, J.; Li, K.; Yang, H. Microbial community mapping in intestinal tract of broiler chicken. Poult. Sci. 2017, 96, 1387–1393. [Google Scholar] [CrossRef]
- Tan, H.; Zhai, Q.; Chen, W. Investigations of Bacteroides spp. towards next-generation probiotics. Food Res. Int. 2019, 116, 637–644. [Google Scholar] [CrossRef]
- Wei, S.; Morrison, M.; Yu, Z. Bacterial census of poultry intestinal microbiome. Poult. Sci. 2013, 92, 671–683. [Google Scholar] [CrossRef]
- Pandit, R.J.; Hinsu, A.T.; Patel, N.V.; Koringa, P.G.; Jakhesara, S.J.; Thakkar, J.R.; Shah, T.M.; Limon, G.; Psifidi, A.; Guitian, J.; et al. Microbial diversity and community composition of caecal microbiota in commercial and indigenous Indian chickens determined using 16s rDNA amplicon sequencing. Microbiome 2018, 6, 115. [Google Scholar] [CrossRef]
- Gophna, U.; Konikoff, T.; Nielsen, H.B. Oscillospira and related bacteria—From metagenomic species to metabolic features. Environ. Microbiol. 2017, 19, 835–841. [Google Scholar] [CrossRef]
- Miquel, S.; Martín, R.; Rossi, O.; Bermúdez-Humarán, L.G.; Chatel, J.M.; Sokol, H.; Thomas, M.; Wells, J.M.; Langella, P. Faecalibacterium prausnitzii and human intestinal health. Curr. Opin. Microbiol. 2013, 16, 255–261. [Google Scholar] [CrossRef]
- D’hoe, K.; Conterno, L.; Fava, F.; Falony, G.; Vieira-Silva, S.; Vermeiren, J.; Tuohy, K.; Raes, J. Prebiotic Wheat Bran Fractions Induce Specific Microbiota Changes. Front. Microbiol. 2018, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Baeza, Y.; Callewaert, C.; Debelius, J.; Hyde, E.; Marotz, C.; Morton, J.T.; Swafford, A.; Vrbanac, A.; Dorrestein, P.C.; Knight, R. Impacts of the Human Gut Microbiome on Therapeutics. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 253–270. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K. Strategies to promote abundance of Akkermansia muciniphila, an emerging probiotics in the gut, evidence from dietary intervention studies. J. Funct. Foods 2017, 33, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Pruss, K.M.; Marcobal, A.; Southwick, A.M.; Dahan, D.; Smits, S.A.; Ferreyra, J.A.; Higginbottom, S.K.; Sonnenburg, E.D.; Kashyap, P.C.; Choudhury, B.; et al. Mucin-derived O-glycans supplemented to diet mitigate diverse microbiota perturbations. bioRxiv 2020. [Google Scholar] [CrossRef]
- Geerlings, S.; Kostopoulos, I.; de Vos, W.; Belzer, C. Akkermansia muciniphila in the Human Gastrointestinal Tract: When, Where, and How? Microorganisms 2018, 6, 75. [Google Scholar] [CrossRef]
- Jia, L.; Li, D.; Feng, N.; Shamoon, M.; Sun, Z.; Ding, L.; Zhang, H.; Chen, W.; Sun, J.; Chen, Y.Q. Anti-diabetic Effects of Clostridium butyricum CGMCC0313.1 through Promoting the Growth of Gut Butyrate-producing Bacteria in Type 2 Diabetic Mice. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef]
- Chen, H.; Ma, X.; Liu, Y.; Ma, L.; Chen, Z.; Lin, X.; Si, L.; Ma, X.; Chen, X. Gut Microbiota Interventions With Clostridium butyricum and Norfloxacin Modulate Immune Response in Experimental Autoimmune Encephalomyelitis Mice. Front. Immunol. 2019, 10, 1662. [Google Scholar] [CrossRef]
- Han, G.G.; Kim, E.B.; Lee, J.; Lee, J.Y.; Jin, G.; Park, J.; Huh, C.S.; Kwon, I.K.; Kil, D.Y.; Choi, Y.J.; et al. Relationship between the microbiota in different sections of the gastrointestinal tract, and the body weight of broiler chickens. Springerplus 2016, 5. [Google Scholar] [CrossRef]
- Yan, W.; Sun, C.; Yuan, J.; Yang, N. Gut metagenomic analysis reveals prominent roles of Lactobacillus and cecal microbiota in chicken feed efficiency. Sci. Rep. 2017, 7, 45308. [Google Scholar] [CrossRef]
- Lin, Y.; Xu, S.; Zeng, D.; Ni, X.; Zhou, M.; Zeng, Y.; Wang, H.; Zhou, Y.; Zhu, H.; Pan, K.; et al. Disruption in the cecal microbiota of chickens challenged with Clostridium perfringens and other factors was alleviated by Bacillus licheniformis supplementation. PLoS ONE 2017, 12. [Google Scholar] [CrossRef]
- Li, X.; Huang, Y.; Song, L.; Xiao, Y.; Lu, S.; Xu, J.; Li, J.; Ren, Z. Lactobacillus plantarum prevents obesity via modulation of gut microbiota and metabolites in high-fat feeding mice. J. Funct. Foods 2020, 73, 104103. [Google Scholar] [CrossRef]
- Suriano, F.; Bindels, L.B.; Verspreet, J.; Courtin, C.M.; Verbeke, K.; Cani, P.D.; Neyrinck, A.M.; Delzenne, N.M. Fat binding capacity and modulation of the gut microbiota both determine the effect of wheat bran fractions on adiposity. Sci. Rep. 2017, 7, 5621. [Google Scholar] [CrossRef] [PubMed]
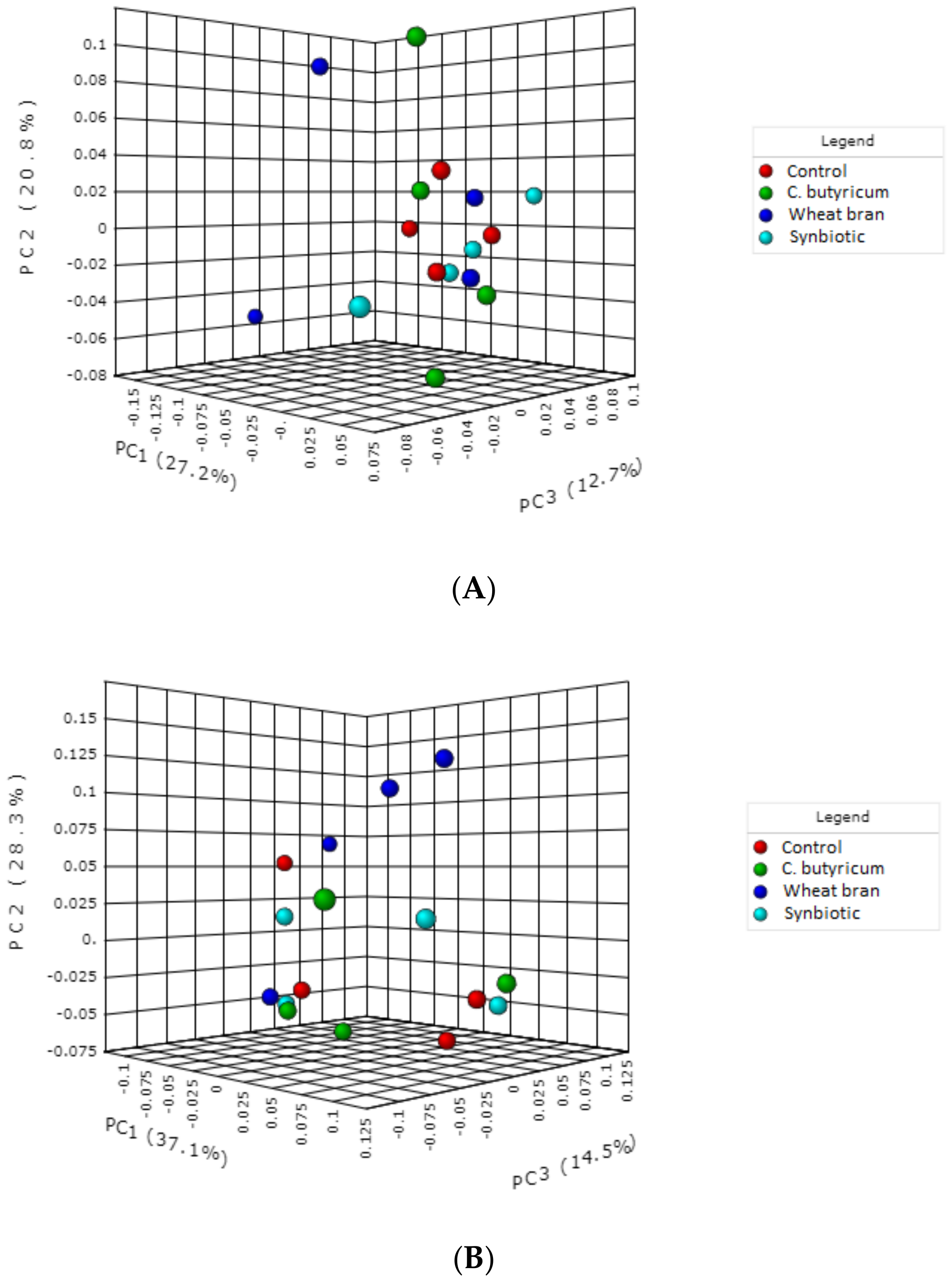
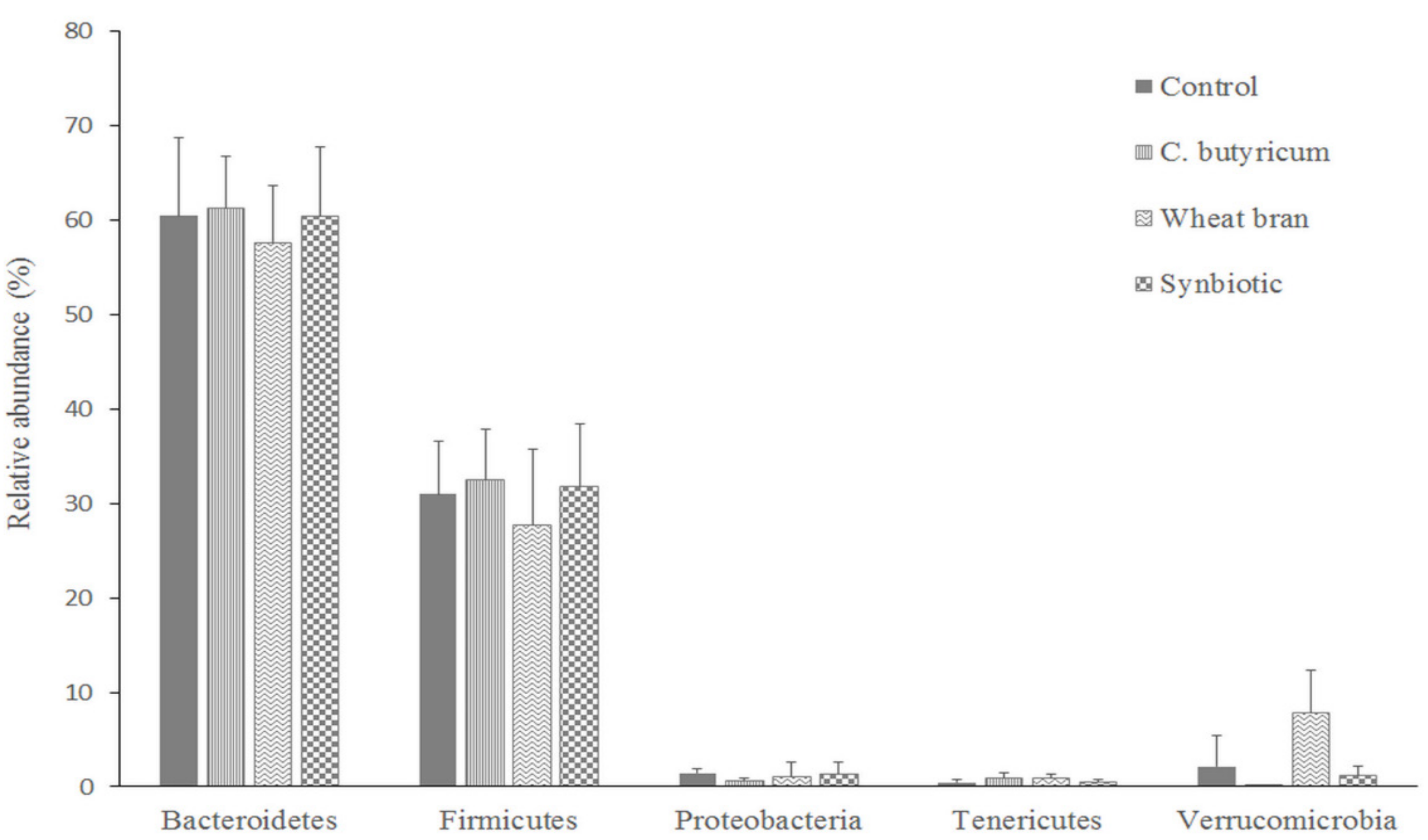
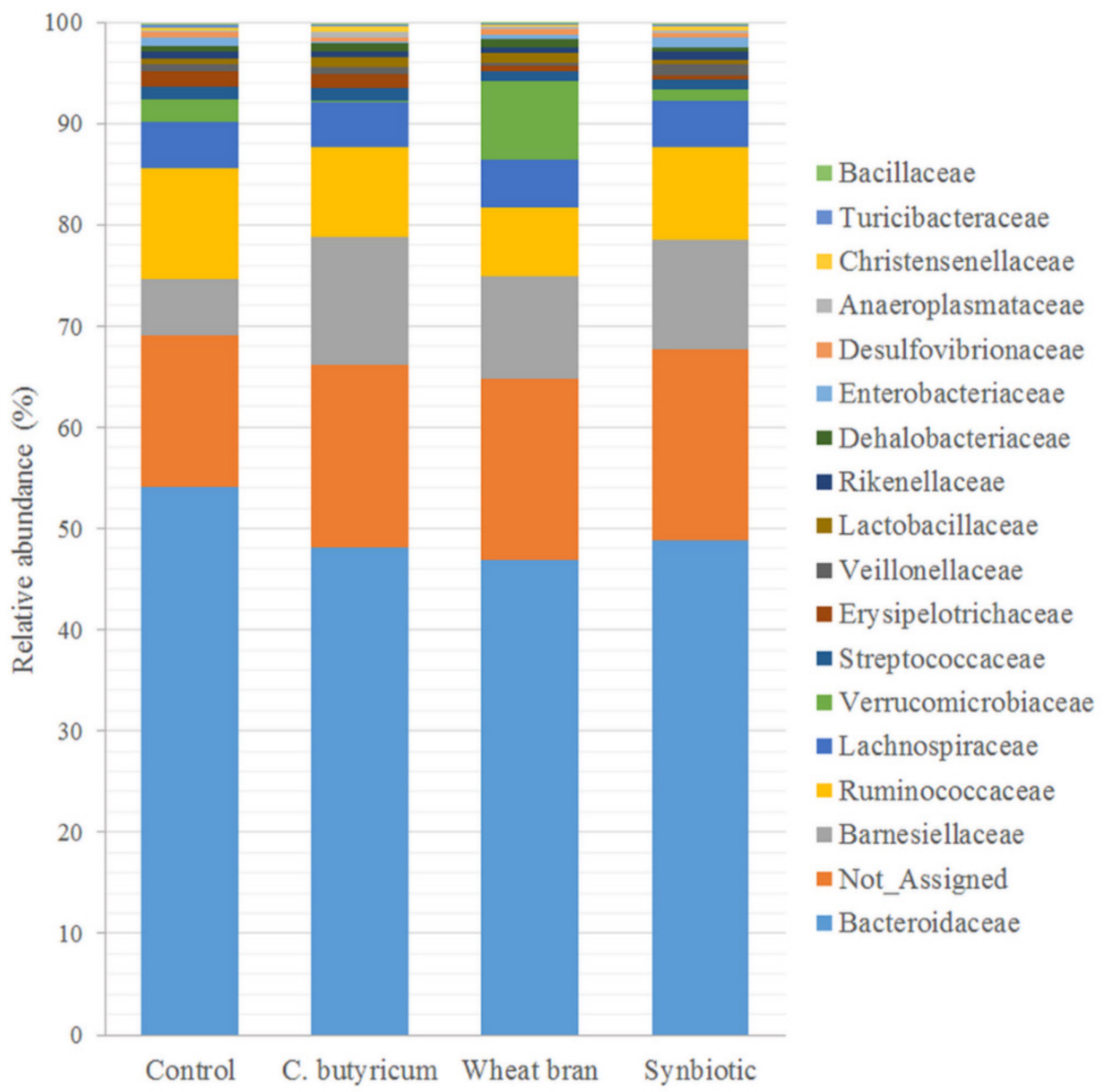
| Ingredient | Starter (Day 1 to 10 of Life) | Grower (Day 11 to 24 of Life) | Finisher (Day 25 to 37 of Life) | |||
|---|---|---|---|---|---|---|
| C | Wheat Bran | C | Wheat Bran | C | Wheat Bran | |
| Maize | 466 | 434 | 534 | 469 | 589 | 524 |
| Wheat bran | 0 | 30 | 0 | 60 | 0 | 60 |
| ESM | 338 | 333 | 361 | 352 | 310 | 300 |
| Sunflower oil | 63 | 70 | 62 | 76 | 60 | 74 |
| Limestone | 19 | 19 | 15 | 15 | 15 | 15 |
| Sunflower meal | 80 | 80 | 0 | 0 | 0 | 0 |
| MCP | 15 | 15 | 14 | 14 | 13 | 13 |
| L-LYS HCL | 5 | 5 | 2 | 2 | 2 | 2 |
| DL-MET | 4 | 4 | 3 | 3 | 3 | 3 |
| L-THR | 1 | 1 | 1 | 1 | 0 | 1 |
| Val | 1 | 1 | 0 | 0 | 0 | 0 |
| NaCl | 3 | 3 | 3 | 3 | 3 | 3 |
| NaHCO3 | 1 | 1 | 1 | 1 | 1 | 1 |
| Premix 1 | 4 | 4 | 4 | 4 | 3.5 | 3.5 |
| Phytase 2 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| NSP enzyme 3 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Total | 1000 | 1000 | 1000 | 1000 | 1000 | 1000 |
| Ingredient | Starter (Day 1 to 10 of Life) | Grower (Day 11 to 24 of Life) | Finisher (Day 25 to 37 of Life) | |||
|---|---|---|---|---|---|---|
| C | Wheat Bran | C | Wheat Bran | C | Wheat Bran | |
| AMEn (MJ/kg) 2 | 12.1 | 12.2 | 13.1 | 13.0 | 13.0 | 13.1 |
| Dry matter | 888 | 890 | 885 | 888 | 882 | 888 |
| Crude protein | 229 | 230 | 207 | 212 | 188 | 191 |
| Crude fat | 83 | 92 | 91 | 101 | 89 | 100 |
| Crude fiber | 40.2 | 45.8 | 37.7 | 41.8 | 36.3 | 43.3 |
| Crude ash | 66.9 | 68.3 | 56.1 | 59.6 | 54.3 | 56.9 |
| Ca | 10.7 | 10.8 | 9.4 | 9.4 | 8.9 | 8.9 |
| P | 8.0 | 8.1 | 6.7 | 7.1 | 6.6 | 7.0 |
| Starch | 305 | 294 | 369 | 336 | 387 | 364 |
| Dietary Treatments | Daily Gain (g) | Feed Intake (g) | Feed Conversion Ratio (FCR) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Starter | Grower | Finisher | Total | Starter | Grower | Finisher | Total | Starter | Grower | Finisher | Total | ||
| Control | 206 | 764 | 1457 | 2427 | 242 | 1410 | 2385 | 4036 | 1.171 | 1.848 | 1.639 | 1.665 | |
| C. butyricum | 208 | 781 | 1486 | 2474 | 242 | 1402 | 2498 | 4142 | 1.163 | 1.796 | 1.684 | 1.675 | |
| Wheat bran | 210 | 773 | 1458 | 2440 | 241 | 1368 | 2374 | 3982 | 1.150 | 1.771 | 1.630 | 1.633 | |
| SYN | 207 | 754 | 1515 | 2475 | 235 | 1436 | 2399 | 4070 | 1.140 | 1.904 | 1.588 | 1.646 | |
| Wheat bran | |||||||||||||
| No | 207 | 772 | 1471 | 2450 | 242 | 1406 | 2441 | 4089 | 1.167 | 1.822 | 1.662 | 1.670 | |
| Yes | 208 | 763 | 1486 | 2458 | 238 | 1402 | 2386 | 4026 | 1.145 | 1.838 | 1.609 | 1.639 | |
| C. butyricum | |||||||||||||
| No | 208 | 768 | 1458 | 2434 | 241 | 1389 | 2379 | 4009 | 1.161 | 1.810 | 1.635 | 1.649 | |
| Yes | 207 | 768 | 1500 | 2475 | 239 | 1419 | 2448 | 4105 | 1.152 | 1.850 | 1.636 | 1.660 | |
| Pooled SEM | 3.0 | 6.9 | 15.3 | 18.5 | 4.2 | 10.9 | 29.3 | 30.6 | 0.012 | 0.019 | 0.028 | 0.018 | |
| Wheat bran | 0.870 | 0.540 | 0.638 | 0.856 | 0.700 | 0.841 | 0.371 | 0.317 | 0.426 | 0.608 | 0.395 | 0.441 | |
| C. butyricum | 0.900 | 0.977 | 0.205 | 0.322 | 0.783 | 0.154 | 0.265 | 0.133 | 0.747 | 0.190 | 0.985 | 0.769 | |
| Wheat bran x C. butyricum | 0.753 | 0.234 | 0.672 | 0.875 | 0.783 | 0.082 | 0.472 | 0.887 | 0.974 | 0.008 | 0.483 | 0.976 | |
| Dietary Treatments | Cecal Crypt Depth 2 | Cecal pH | Acetate 3 | Propionate 3 | Butyrate 3 | Valerate 3 | Total SCFA 3 | Acetate/Butyrate Ratio | |
|---|---|---|---|---|---|---|---|---|---|
| Control | 65.5 | 6.58 | 38.9 | 8.90 | 11.6 | 1.03 | 61.5 | 3.71 | |
| C. butyricum | 74.4 | 6.57 | 41.1 | 8.07 | 12.8 | 1.06 | 64.0 | 3.37 | |
| Wheat bran | 93.7 | 6.50 | 45.1 | 9.93 | 10.3 | 1.03 | 67.4 | 4.23 | |
| SYN | 95.5 | 6.52 | 37.5 | 6.58 | 11.1 | 0.95 | 57.0 | 3.65 | |
| Wheat bran | |||||||||
| No | 69.7 b | 6.58 | 40.0 | 8.49 | 12.2 | 1.04 | 62.8 | 3.54 | |
| Yes | 94.6 a | 6.51 | 41.9 | 8.49 | 10.6 | 0.99 | 62.9 | 3.96 | |
| C. butyricum | |||||||||
| No | 79.7 | 6.54 | 42.2 | 9.45 | 10.9 | 1.03 | 64.7 | 3.97 | |
| Yes | 85.7 | 6.55 | 39.5 | 7.39 | 12.0 | 1.01 | 60.8 | 3.50 | |
| Pooled SEM | 3.9 | 0.05 | 2.2 | 0.55 | 1.0 | 0.07 | 3.3 | 0.19 | |
| p-Values | |||||||||
| Wheat bran | 0.001 | 0.535 | 0.778 | 0.830 | 0.464 | 0.701 | 0.306 | 0.325 | |
| C. butyricum | 0.428 | 0.960 | 0.566 | 0.063 | 0.616 | 0.872 | 0.856 | 0.255 | |
| Wheat bran x C. butyricum | 0.280 | 0.883 | 0.294 | 0.251 | 0.915 | 0.720 | 0.353 | 0.768 | |
| Dietary Treatments | Observed | ACE | Shannon | Simpson |
|---|---|---|---|---|
| Control | 296 | 297 | 3.13 | 0.82 |
| C. butyricum | 300 | 300 | 3.12 | 0.82 |
| Wheat bran | 274 | 275 | 3.05 | 0.84 |
| SYN | 297 | 299 | 3.14 | 0.84 |
| Pooled SEM | 4.66 | 4.62 | 0.05 | 0.01 |
| Genus 2 | C | C. butyricum | Wheat Bran | SYN | Pooled SEM | p-Value | q-Value 2 |
|---|---|---|---|---|---|---|---|
| Bacteroides | 54.1 | 48.1 | 46.8 | 48.9 | 2.37 | 0.865 | 0.956 |
| Oscillospira | 2.57 | 1.91 | 1.43 | 2.12 | 0.201 | 0.521 | 0.956 |
| Akkermansia | 2.17 a,b | 0.02 c | 7.77 a | 1.17 b | 1.071 | <0.001 | 0.004 |
| Faecalibacterium | 2.09 | 1.88 | 0.77 | 1.72 | 0.250 | 0.936 | 0.956 |
| Ruminococcus | 1.83 | 1.50 | 1.33 | 1.78 | 0.143 | 0.667 | 0.956 |
| Streptococcus | 1.24 | 1.37 | 0.90 | 0.91 | 0.181 | 0.813 | 0.956 |
| Lactobacillus | 0.55 | 0.89 | 0.94 | 0.42 | 0.142 | 0.469 | 0.956 |
| Dehalobacterium | 0.52 | 0.71 | 0.77 | 0.51 | 0.100 | 0.606 | 0.956 |
| Anaeroplasma | 0.11 | 0.60 | 0.24 | 0.31 | 0.086 | 0.016 | 0.106 |
| Clostridium | 0.20 | 0.17 | 0.31 | 0.32 | 0.040 | 0.895 | 0.956 |
| Coprococcus | 0.24 | 0.16 | 0.22 | 0.22 | 0.022 | 0.430 | 0.956 |
| Butyricicoccus | 0.31 | 0.16 | 0.18 | 0.19 | 0.027 | 0.133 | 0.663 |
| Turicibacter | 0.27 | 0.23 | 0.14 | 0.17 | 0.028 | 0.856 | 0.956 |
| Anaerotruncus | 0.41 | 0.06 | 0.20 | 0.09 | 0.065 | 0.007 | 0.072 |
| Blautia | 0.20 | 0.19 | 0.12 | 0.15 | 0.019 | 0.956 | 0.956 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molnár, A.; Such, N.; Farkas, V.; Pál, L.; Menyhárt, L.; Wágner, L.; Husvéth, F.; Dublecz, K. Effects of Wheat Bran and Clostridium butyricum Supplementation on Cecal Microbiota, Short-Chain Fatty Acid Concentration, pH and Histomorphometry in Broiler Chickens. Animals 2020, 10, 2230. https://doi.org/10.3390/ani10122230
Molnár A, Such N, Farkas V, Pál L, Menyhárt L, Wágner L, Husvéth F, Dublecz K. Effects of Wheat Bran and Clostridium butyricum Supplementation on Cecal Microbiota, Short-Chain Fatty Acid Concentration, pH and Histomorphometry in Broiler Chickens. Animals. 2020; 10(12):2230. https://doi.org/10.3390/ani10122230
Chicago/Turabian StyleMolnár, Andor, Nikoletta Such, Valéria Farkas, László Pál, László Menyhárt, László Wágner, Ferenc Husvéth, and Károly Dublecz. 2020. "Effects of Wheat Bran and Clostridium butyricum Supplementation on Cecal Microbiota, Short-Chain Fatty Acid Concentration, pH and Histomorphometry in Broiler Chickens" Animals 10, no. 12: 2230. https://doi.org/10.3390/ani10122230
APA StyleMolnár, A., Such, N., Farkas, V., Pál, L., Menyhárt, L., Wágner, L., Husvéth, F., & Dublecz, K. (2020). Effects of Wheat Bran and Clostridium butyricum Supplementation on Cecal Microbiota, Short-Chain Fatty Acid Concentration, pH and Histomorphometry in Broiler Chickens. Animals, 10(12), 2230. https://doi.org/10.3390/ani10122230




