Chopping Roughage Length Improved Rumen Development of Weaned Calves as Revealed by Rumen Fermentation and Bacterial Community
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Treatments, Animals, and Experimental Design
2.2. Chemical Analysis
2.3. Rumen Fermentation Parameters
2.4. Plasma Biochemical Parameters
2.5. DNA Extraction and 16S rRNA Pyrosequencing
2.6. Statistical Analysis
3. Results
3.1. Growth Performance and Nutrient Apparent Digestibility
3.2. Rumen Fermentation Characteristics
3.3. Plasma Parameters
3.4. Sequencing Depth and Diversity Estimates
3.5. Rumen Bacteria Composition
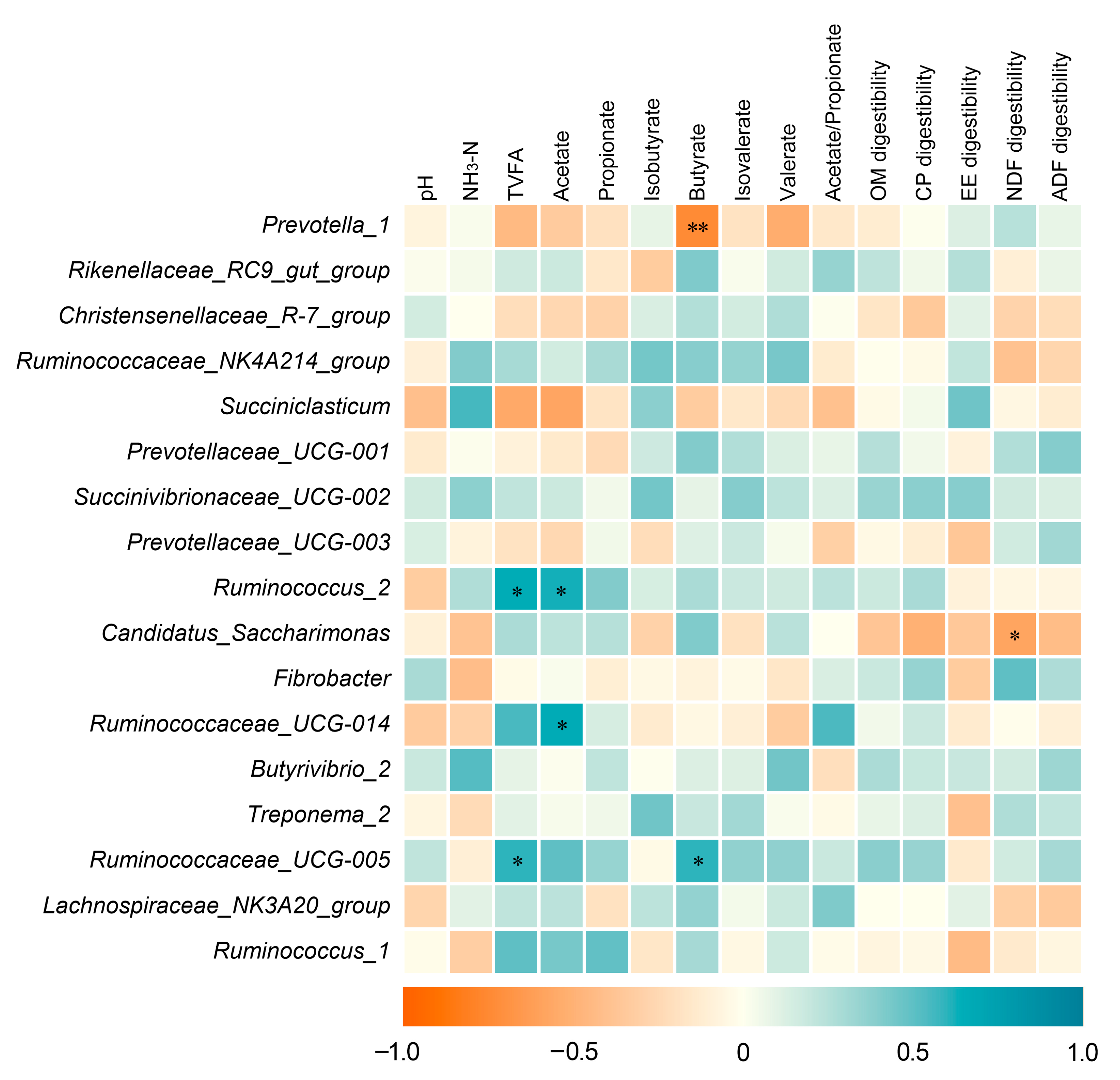
3.6. Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Weary, D.M.; Jasper, J.; Hötzel, M.J. Understanding weaning distress. Appl. Anim. Behav. Sci. 2008, 110, 24–41. [Google Scholar] [CrossRef]
- Beharka, A.; Nagaraja, T.; Morrill, J.; Kennedy, G.; Klemm, R. Effects of form of the diet on anatomical, microbial, and fermentative development of the rumen of neonatal calves. J. Dairy Sci. 1998, 81, 1946–1955. [Google Scholar] [CrossRef]
- Klein, R.; Kincaid, R.; Hodgson, A.; Harrison, J.; Hillers, J.; Cronrath, J. Dietary fiber and early weaning on growth and rumen development of calves. J. Dairy Sci. 1987, 70, 2095–2104. [Google Scholar] [CrossRef]
- Beiranvand, H.; Ghorbani, G.; Khorvash, M.; Nabipour, A.; Dehghan-Banadaky, M.; Homayouni, A.; Kargar, S. Interactions of alfalfa hay and sodium propionate on dairy calf performance and rumen development. J. Dairy Sci. 2014, 97, 2270–2280. [Google Scholar] [CrossRef] [PubMed]
- Galyean, M.; Defoor, P. Effects of roughage source and level on intake by feedlot cattle. J. Anim. Sci. 2003, 81, E8–E16. [Google Scholar]
- Terre, M.; Pedrals, E.; Dalmau, A.; Bach, A. What do preweaned and weaned calves need in the diet: A high fiber content or a forage source? J. Dairy Sci. 2013, 96, 5217–5225. [Google Scholar] [CrossRef]
- Martz, F.; Belyea, R. Role of particle size and forage quality in digestion and passage by cattle and sheep. J. Dairy Sci. 1986, 69, 1996–2008. [Google Scholar] [CrossRef]
- Badurdeen, A.; Ibrahim, M.; Ranawana, S. Methods to improve utilization of rice straw III. Effect of urea ammonia treatment and urea molasses blocks supplementation on intake, digestibility, rumen and blood parameters. Asian-Australas. J. Anim. Sci. 1994, 7, 363–372. [Google Scholar] [CrossRef]
- Beauchemin, K.; Yang, W.; Rode, L.M. Effects of particle size of alfalfa-based dairy cow diets on chewing activity, ruminal fermentation, and milk production. J. Dairy Sci. 2003, 86, 630–643. [Google Scholar] [CrossRef]
- Nemati, M.; Amanlou, H.; Khorvash, M.; Moshiri, B.; Mirzaei, M.; Khan, M.A.; Ghaffari, M.H. Rumen fermentation, blood metabolites, and growth performance of calves during transition from liquid to solid feed: Effects of dietary level and particle size of alfalfa hay. J. Dairy Sci. 2015, 98, 7131–7141. [Google Scholar] [CrossRef]
- Mirzaei, M.; Khorvash, M.; Ghorbani, G.R.; Kazemi-Bonchenari, M.; Riasi, A.; Nabipour, A.; Van Den Borne, J.J.G.C. Effects of supplementation level and particle size of alfalfa hay on growth characteristics and rumen development in dairy calves. J. Anim. Physiol. Anim. Nutr. 2015, 99, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Mena, F.X.; Heinrichs, A.J.; Jones, C.M.; Hill, T.M.; Quigley, J.D. Straw particle size in calf starters: Effects on digestive system development and rumen fermentation. J. Dairy Sci. 2016, 99, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.; Amélia, C.S.; Jürgen, C.; Uwe, B.; Markus, R.; Jana, S. A structural and functional elucidation of the rumen microbiome influenced by various diets and microenvironments. Front. Microbiol. 2017, 8, 1605. [Google Scholar]
- Mentschel, J.; Leiser, R.; Mülling, C.; Pfarrer, C.; Claus, R. Butyric acid stimulates rumen mucosa development in the calf mainly by a reduction of apoptosis. Arch. Anim. Nutr. 2001, 55, 85–102. [Google Scholar] [CrossRef]
- Van Horn, H.; Harris, B., Jr.; Taylor, M.; Bachman, K.; Wilcox, C. By-product feeds for lactating dairy cows: Effects of cottonseed hulls, sunflower hulls, corrugated paper, peanut hulls, sugarcane bagasse, and whole cottonseed with additives of fat, sodium bicarbonate, and Aspergillus oryzae product on milk production. J. Dairy Sci. 1984, 67, 2922–2938. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 15th ed.; AOAC: Arlington, VA, USA, 1990. [Google Scholar]
- Van Soest, P.; Robertson, J.; Lewis, B. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Van Keulen, J.; Young, B. Evaluation of acid-insoluble ash as a natural marker in ruminant digestibility studies. J. Anim. Sci. 1977, 44, 282–287. [Google Scholar] [CrossRef]
- Zhong, R.Z.; Li, J.G.; Gao, Y.X.; Tan, Z.L.; Ren, G.P. Effects of substitution of different levels of steam-flaked corn for finely ground corn on lactation and digestion in early lactation dairy cows. J. Dairy Sci. 2008, 91, 3931–3937. [Google Scholar] [CrossRef]
- Weatherburn, M. Phenol-hypochlorite reaction for determination of ammonia. Anal. Chem. 1967, 39, 971–974. [Google Scholar] [CrossRef]
- Wang, H.; He, Y.; Li, H.; Wu, F.; Qiu, Q.; Niu, W.; Gao, Z.; Su, H.; Cao, B. Rumen fermentation, intramuscular fat fatty acid profiles and related rumen bacterial populations of Holstein bulls fed diets with different energy levels. Appl. Microbiol. Biotechnol. 2019, 103, 4931–4942. [Google Scholar] [CrossRef]
- Ehle, F.; Murphy, M.; Clark, J. In situ particle size reduction and the effect of particle size on degradation of crude protein and dry matter in the rumen of dairy steers. J. Dairy Sci. 1982, 65, 963–971. [Google Scholar] [CrossRef]
- Susmel, P.; Spanghero, M.; Stefanon, B.; Mills, C.; Cargnelutti, C. Effect of NDF concentration and physical form of fescue hay on rumen degradability, intake and rumen turn-over of cows. Anim. Sci. 1991, 53, 305–313. [Google Scholar] [CrossRef]
- Castells, L.; Bach, A.; Araujo, G.; Montoro, C.; Terré, M. Effect of different forage sources on performance and feeding behavior of Holstein calves. J. Dairy Sci. 2012, 95, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Aschenbach, J.; Penner, G.; Stumpff, F.; Gäbel, G. Ruminant nutrition symposium: Role of fermentation acid absorption in the regulation of ruminal pH. J. Anim. Sci. 2011, 89, 1092–1107. [Google Scholar] [CrossRef] [PubMed]
- Kononoff, P.; Heinrichs, A.J.; Lehman, H. The effect of corn silage particle size on eating behavior, chewing activities, and rumen fermentation in lactating dairy cows. J. Dairy Sci. 2003, 86, 3343–3353. [Google Scholar] [CrossRef]
- Wang, W.; Li, C.; Li, F.; Wang, X.; Zhang, X.; Liu, T.; Nian, F.; Yue, X.; Li, F.; Pan, X.; et al. Effects of early feeding on the host rumen transcriptome and bacterial diversity in lambs. Sci. Rep. 2016, 6, 32479. [Google Scholar] [CrossRef]
- Biddle, A.; Stewart, L.; Blanchard, J.; Leschine, S. Untangling the genetic basis of fibrolytic specialization by Lachnospiraceae and Ruminococcaceae in diverse gut communities. Diversity 2013, 5, 627–640. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, C.; Zhang, Y.L.; Pei, C.X.; Zhang, S.L.; Wang, Y.X.; Zhang, Z.W.; Yang, W.Z.; Wang, H.; Guo, G.; et al. Effects of isovalerate supplementation on growth performance and ruminal fermentation in pre- and post-weaning dairy calves. J. Agric. Sci. 2016, 154, 1499–1508. [Google Scholar] [CrossRef]
- Cline, J.H.; Hershberger, T.; Bentley, O.G. Utilization and/or synthesis of valeric acid during the digestion of glucose, starch and cellulose by rumen micro-organisms in vitro. J. Anim. Sci. 1958, 17, 284–292. [Google Scholar] [CrossRef]
- Khan, M.; Lee, H.; Lee, W.; Kim, H.; Kim, S.; Ki, K.; Park, S.; Ha, J.; Choi, Y. Starch source evaluation in calf starter: I. Feed consumption, body weight gain, structural growth, and blood metabolites in Holstein calves. J. Dairy Sci. 2007, 90, 5259–5268. [Google Scholar] [CrossRef]
- Perino, L.J.; Sutherland, R.L.; Woollen, N.E. Serum gamma-glutamyltransferase activity and protein concentration at birth and after suckling in calves with adequate and inadequate passive transfer of immunoglobulin G. Am. J. Vet. Res. 1993, 54, 56. [Google Scholar] [PubMed]
- Lohakare, J.; Pattanaik, A.; Khan, S. Effect of dietary protein levels on the performance, nutrient balances, metabolic profile and thyroid hormones of crossbred calves. Asian Australas. J. Anim. Sci. 2006, 19, 1588–1596. [Google Scholar] [CrossRef]
- Vanhatalo, A.; Kuoppala, K.; Ahvenjärvi, S.; Rinne, M. Effects of feeding grass or red clover silage cut at two maturity stages in dairy cows. 1. Nitrogen metabolism and supply of amino acids. J. Dairy Sci. 2009, 92, 5620–5633. [Google Scholar] [CrossRef] [PubMed]
- Vi, R.B.; McLeod, K.; Klotz, J.; Heitmann, R. Rumen development, intestinal growth and hepatic metabolism in the pre- and postweaning ruminant. J. Dairy Sci. 2004, 87, E55–E65. [Google Scholar]
- Tajima, K.; Nonaka, I.; Higuchi, K.; Takusari, N.; Kurihara, M.; Takenaka, A.; Mitsumori, M.; Kajikawa, H.; Aminov, R.I. Influence of high temperature and humidity on rumen bacterial diversity in Holstein heifers. Anaerobe 2007, 13, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Lesmeister, K.; Heinrichs, A.J.; Gabler, M. Effects of supplemental yeast (Saccharomyces cerevisiae) culture on rumen development, growth characteristics, and blood parameters in neonatal dairy calves. J. Dairy Sci. 2004, 87, 1832–1839. [Google Scholar] [CrossRef]
- Tucker, C.M.; Cadotte, M.W.; Carvalho, S.B.; Davies, T.J.; Ferrier, S.; Fritz, S.A.; Grenyer, R.; Helmus, M.R.; Jin, L.S.; Mooers, A.O. A guide to phylogenetic metrics for conservation, community ecology and macroecology. Biol. Rev. 2017, 92, 698–715. [Google Scholar] [CrossRef] [PubMed]
- Henderson, G.; Cox, F.; Ganesh, S.; Jonker, A.; Young, W.; Janssen, P.H. Rumen microbial community composition varies with diet and host, but a core microbiome is found across a wide geographical range. Sci. Rep. 2015, 5, 14567. [Google Scholar] [CrossRef]
- Li, R.W.; Connor, E.E.; Li, C.; Baldwin, R.L., VI; Sparks, M.E. Characterization of the rumen microbiota of pre-ruminant calves using metagenomic tools. Environ. Microbiol. 2012, 14, 129–139. [Google Scholar] [CrossRef]
- Abecia, L.; Jiménez, E.; Martinez-Fernandez, G.; Martín-García, A.I.; Ramos-Morales, E.; Pinloche, E.; Denman, S.E.; Newbold, C.J.; Yáñez-Ruiz, D.R. Natural and artificial feeding management before weaning promote different rumen microbial colonization but not differences in gene expression levels at the rumen epithelium of newborn goats. PLoS ONE 2017, 12, e0182235. [Google Scholar] [CrossRef]
- Rey, M.; Enjalbert, F.; Combes, S.; Cauquil, L.; Bouchez, O.; Monteils, V. Establishment of ruminal bacterial community in dairy calves from birth to weaning is sequential. J. Appl. Microbiol. 2014, 116, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Hespell, R.; Paster, B.; Dewhirst, F. The Genus Selenomonas. Prokaryotes 2006, 4, 982–990. [Google Scholar]
- Pan, X.; Xue, F.; Nan, X.; Tang, Z.; Wang, K.; Beckers, Y.; Jiang, L.; Xiong, B. Illumina sequencing approach to characterize thiamine metabolism related bacteria and the impacts of thiamine supplementation on ruminal microbiota in dairy cows fed high-grain diets. Front. Microbiol. 2017, 8, 1818. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, H.; Wu, F.; Qiu, X.; Yu, Z.; Niu, W.; He, Y.; Su, H.; Cao, B. Effects of dietary energy on growth performance, rumen fermentation and bacterial community, and meat quality of Holstein-Friesians bulls slaughtered at different ages. Animals 2019, 9, 1123. [Google Scholar] [CrossRef] [PubMed]
- Duncan, S.H.; Louis, P.; Flint, H.J. Lactate-utilizing bacteria, isolated from human feces, that produce butyrate as a major fermentation product. Appl. Environ. Microbiol. 2004, 70, 5810–5817. [Google Scholar] [CrossRef]
- Qian, W.; Ao, W.; Jia, C.; Li, Z. Bacterial colonisation of reeds and cottonseed hulls in the rumen of Tarim red deer (Cervus elaphus yarkandensis). Antonie Van Leeuwenhoek 2019, 112, 1283–1296. [Google Scholar] [CrossRef]

| Item | Starter Pellets | Oat Hay |
|---|---|---|
| Ingredient (g/kg, DM) | ||
| Ground corn | 350 | |
| Soybean meal | 50 | |
| Corn gluten meal | 80 | |
| DDGS | 150 | |
| Soybean hulls | 150 | |
| Premix | 50 | |
| Molasses | 20 | |
| Salt | 10 | |
| Wheat bran | 50 | |
| Corn germ meal | 40 | |
| Wheat flour | 50 | |
| Chemical composition (g/kg, DM) | ||
| OM | 900.2 | 939.3 |
| CP | 228.3 | 65.4 |
| EE | 10.4 | 27.1 |
| NDF | 253.3 | 712.1 |
| ADF | 71.2 | 437.1 |
| Item | Diet | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|
| SL | ML | LL | Linear | Quadratic | |||
| Growth performance | |||||||
| Initial body weight (kg) | 158.7 | 164.2 | 160.7 | 3.790 | 0.711 | 0.588 | |
| Final body weight (kg) | 196.8 | 200.0 | 196.0 | 4.514 | 0.896 | 0.805 | |
| ADG (kg/d) | 1.12 | 1.05 | 1.04 | 0.055 | 0.286 | 0.527 | |
| DMI (kg/d) | Starter pellets | 2.50 | 2.50 | 2.50 | |||
| Oat hay | 1.74 | 1.51 | 1.47 | 0.017 | 0.001 | <0.001 | |
| Roughage ratio (%) | 41.0 | 37.6 | 37.0 | 0.645 | 0.001 | <0.001 | |
| FCR | 3.78 | 3.81 | 3.81 | 0.021 | 0.280 | 0.495 | |
| Nutrient apparent digestibility (%) | |||||||
| OM | 86.13 | 81.76 | 83.59 | 1.147 | 0.149 | 0.037 | |
| CP | 86.25 | 83.30 | 84.00 | 1.245 | 0.213 | 0.232 | |
| EE | 83.39 | 82.07 | 85.26 | 1.861 | 0.483 | 0.485 | |
| NDF | 84.26 | 81.57 | 82.20 | 1.418 | 0.312 | 0.384 | |
| ADF | 84.04 | 80.99 | 82.54 | 1.282 | 0.421 | 0.256 | |
| Item | Diet | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| SL | ML | LL | Linear | Quadratic | ||
| pH | 6.41 | 6.65 | 6.58 | 0.095 | 0.215 | 0.198 |
| NH3-N (mg/dL) | 7.42 | 6.39 | 6.12 | 0.618 | 0.191 | 0.389 |
| VFA (mmol/L) | ||||||
| TVFA | 71.42 | 62.41 | 63.47 | 3.539 | 0.123 | 0.160 |
| Acetate | 48.21 | 42.22 | 43.37 | 2.455 | 0.175 | 0.202 |
| Propionate | 13.45 | 12.12 | 11.96 | 0.663 | 0.118 | 0.231 |
| Isobutyrate | 0.95 | 0.89 | 0.95 | 0.091 | 0.941 | 0.875 |
| Butyrate | 6.77 | 5.54 | 5.54 | 0.383 | 0.031 | 0.043 |
| Isovalerate | 1.31 | 1.10 | 1.08 | 0.084 | 0.062 | 0.116 |
| Valerate | 0.73 | 0.55 | 0.56 | 0.054 | 0.034 | 0.038 |
| A/P | 3.58 | 3.49 | 3.63 | 0.070 | 0.633 | 0.371 |
| Item | Diet | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| SL | ML | LL | Linear | Quadratic | ||
| Glucose (mmol/L) | 4.864 | 4.703 | 4.462 | 0.137 | 0.043 | 0.129 |
| Triglyceride (mmol/L) | 0.304 | 0.314 | 0.281 | 0.025 | 0.508 | 0.626 |
| Cholesterol (mmol/L) | 3.214 | 2.803 | 2.452 | 0.159 | 0.002 | 0.007 |
| Total protein (g/L) | 69.74 | 64.13 | 65.54 | 1.314 | 0.039 | 0.013 |
| Urea nitrogen (mmol/L) | 5.057 | 5.409 | 5.487 | 0.201 | 0.135 | 0.285 |
| INS (mIU/L) | 26.81 | 33.59 | 19.99 | 4.417 | 0.300 | 0.109 |
| GH (ng/mL) | 5.576 | 5.407 | 6.737 | 0.485 | 0.103 | 0.124 |
| IGF1 (ng/mL) | 85.35 | 78.74 | 99.25 | 9.877 | 0.329 | 0.338 |
| Item | Diet | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| SL | ML | LL | Linear | Quadratic | ||
| Chao1 | 1844.9 | 1671.2 | 1654.7 | 35.92 | 0.007 | 0.008 |
| Observed_species | 1440.5 | 1269.1 | 1304.5 | 41.20 | 0.070 | 0.038 |
| PD_whole_tree | 132.7 | 120.6 | 122.3 | 2.75 | 0.042 | 0.025 |
| Shannon | 8.60 | 8.22 | 8.31 | 0.152 | 0.214 | 0.224 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Wu, F.; Guan, T.; Zhu, Y.; Yu, Z.; Zhang, D.; Zhang, S.; Su, H.; Cao, B. Chopping Roughage Length Improved Rumen Development of Weaned Calves as Revealed by Rumen Fermentation and Bacterial Community. Animals 2020, 10, 2149. https://doi.org/10.3390/ani10112149
Wang H, Wu F, Guan T, Zhu Y, Yu Z, Zhang D, Zhang S, Su H, Cao B. Chopping Roughage Length Improved Rumen Development of Weaned Calves as Revealed by Rumen Fermentation and Bacterial Community. Animals. 2020; 10(11):2149. https://doi.org/10.3390/ani10112149
Chicago/Turabian StyleWang, Haibo, Fei Wu, Tianci Guan, Yangxiang Zhu, Zhantao Yu, Depeng Zhang, Siyu Zhang, Huawei Su, and Binghai Cao. 2020. "Chopping Roughage Length Improved Rumen Development of Weaned Calves as Revealed by Rumen Fermentation and Bacterial Community" Animals 10, no. 11: 2149. https://doi.org/10.3390/ani10112149
APA StyleWang, H., Wu, F., Guan, T., Zhu, Y., Yu, Z., Zhang, D., Zhang, S., Su, H., & Cao, B. (2020). Chopping Roughage Length Improved Rumen Development of Weaned Calves as Revealed by Rumen Fermentation and Bacterial Community. Animals, 10(11), 2149. https://doi.org/10.3390/ani10112149







