Metabolomics Approach Explore Diagnostic Biomarkers and Metabolic Changes in Heat-Stressed Dairy Cows
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Animal Management
2.2. Sampling and Analysis
2.3. Measurements
2.4. 1H Nuclear Magnetic Resonance (1H NMR) Spectroscopic Measurement
2.5. 1H NMR Data Handling
2.6. The Differential Metabolites Identification and the Analysis of the Metabolic Pathway
2.7. Calculation and Statistical Analysis
3. Results
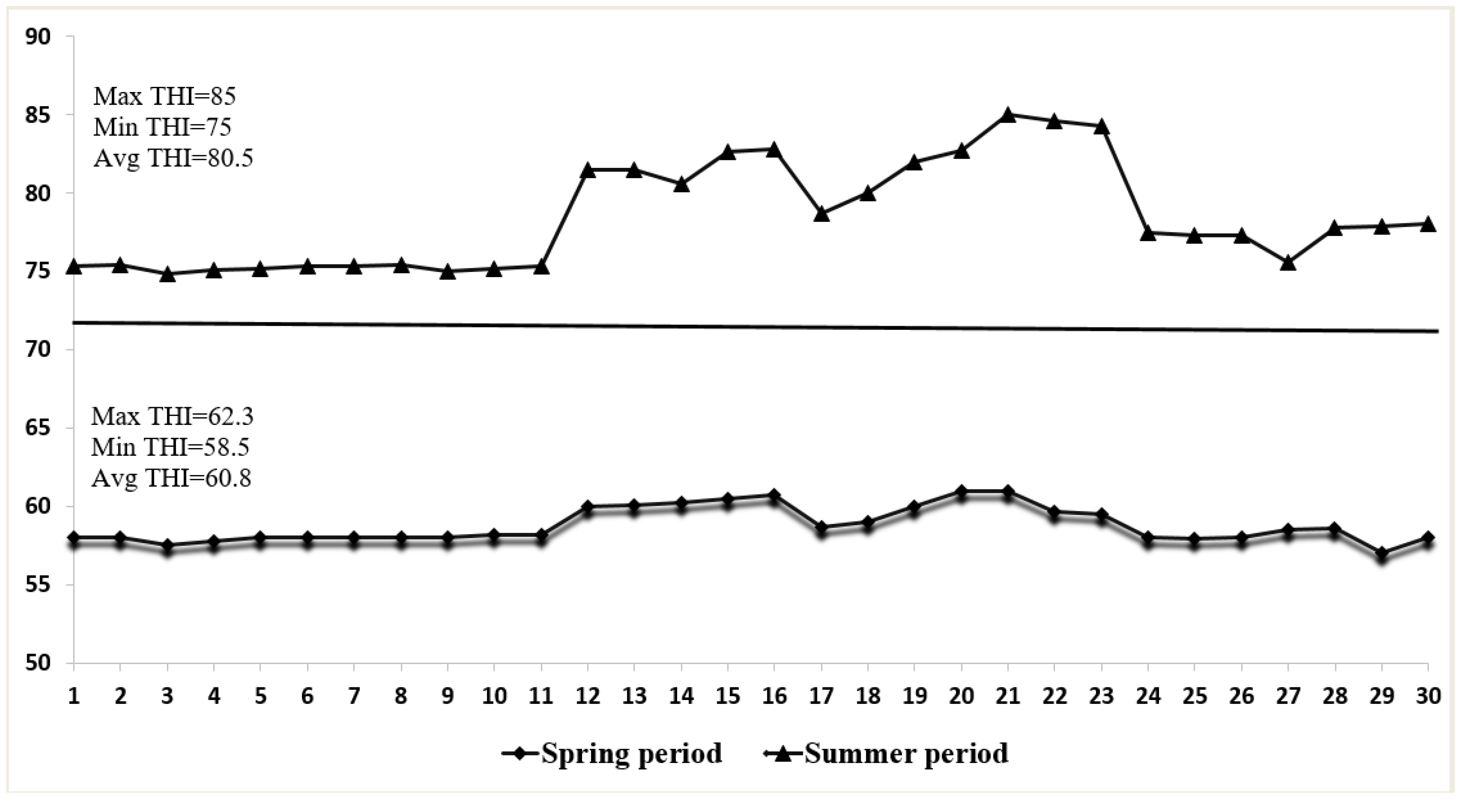
3.1. Environmental Conditions
3.2. Physiological Parameters and Animal Performance
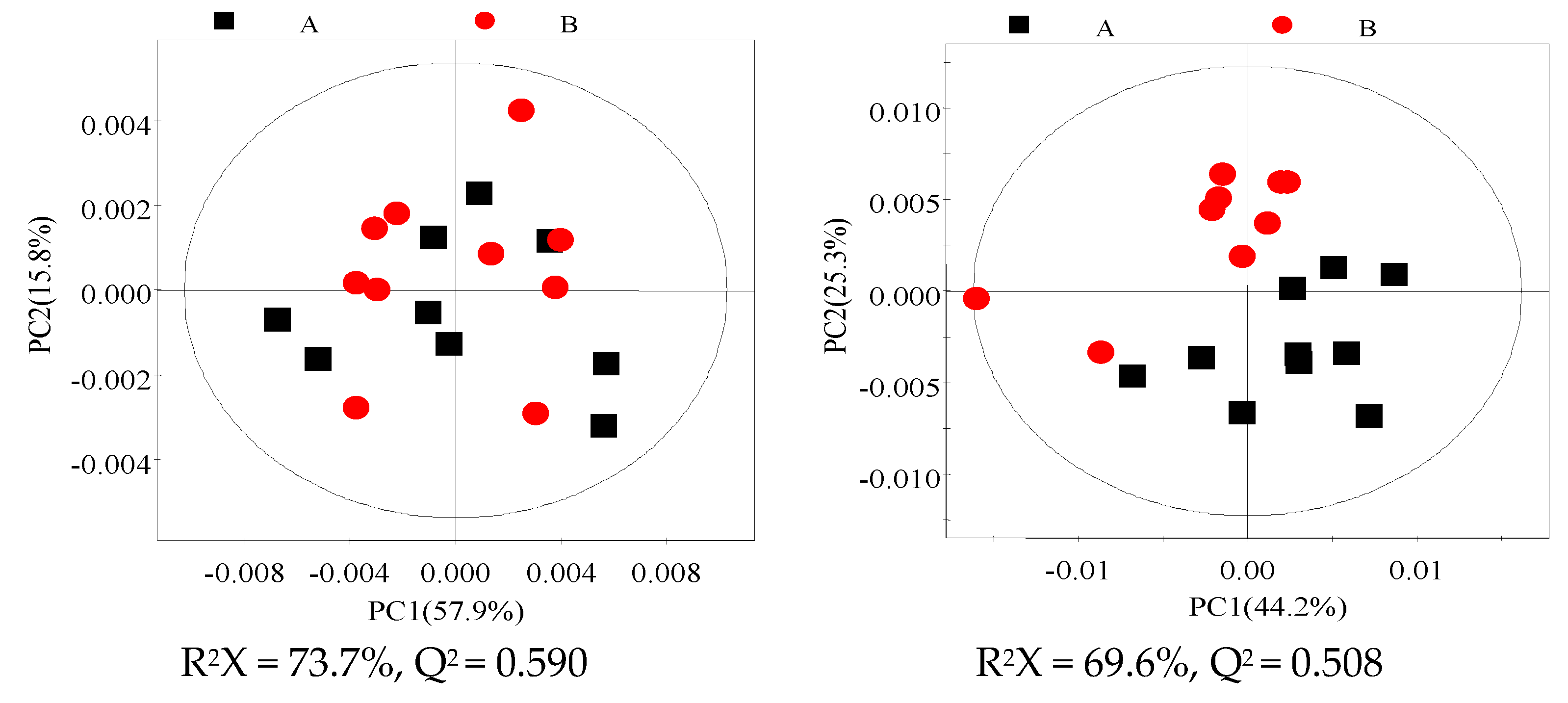
3.3. 1H NMR Spectra of Milk and Blood Plasma Samples
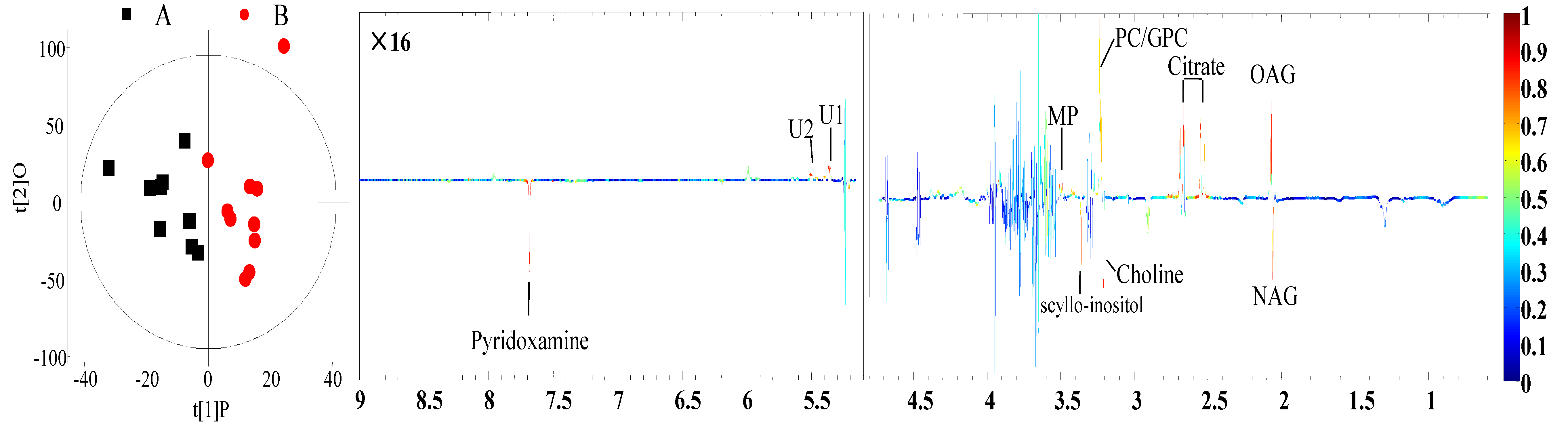
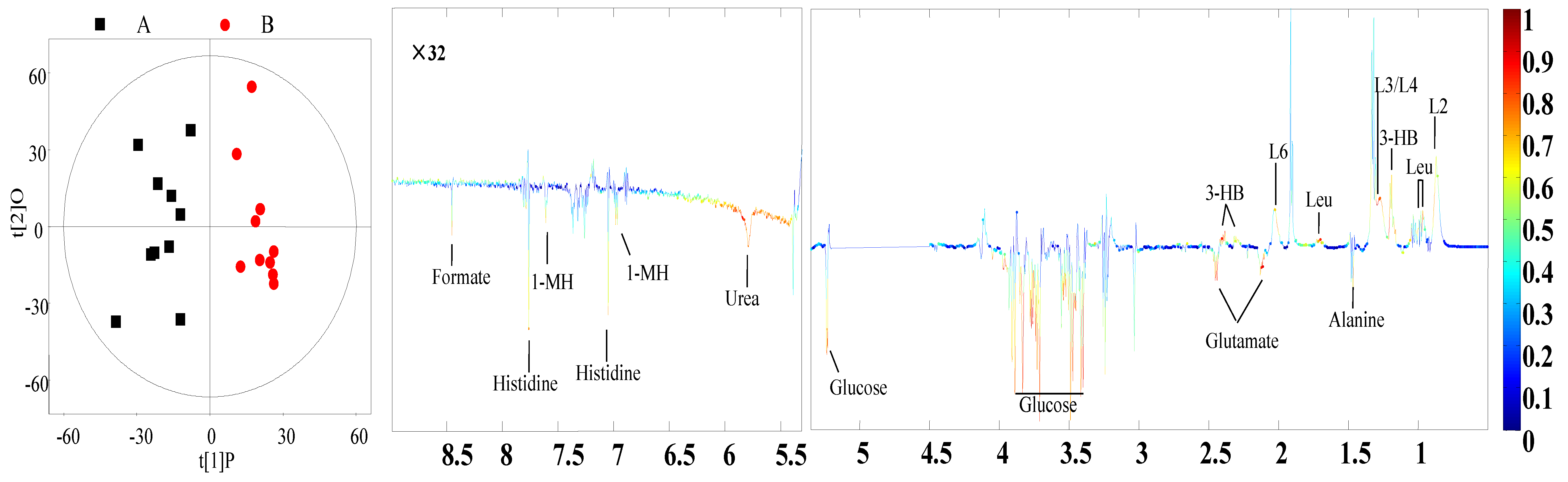
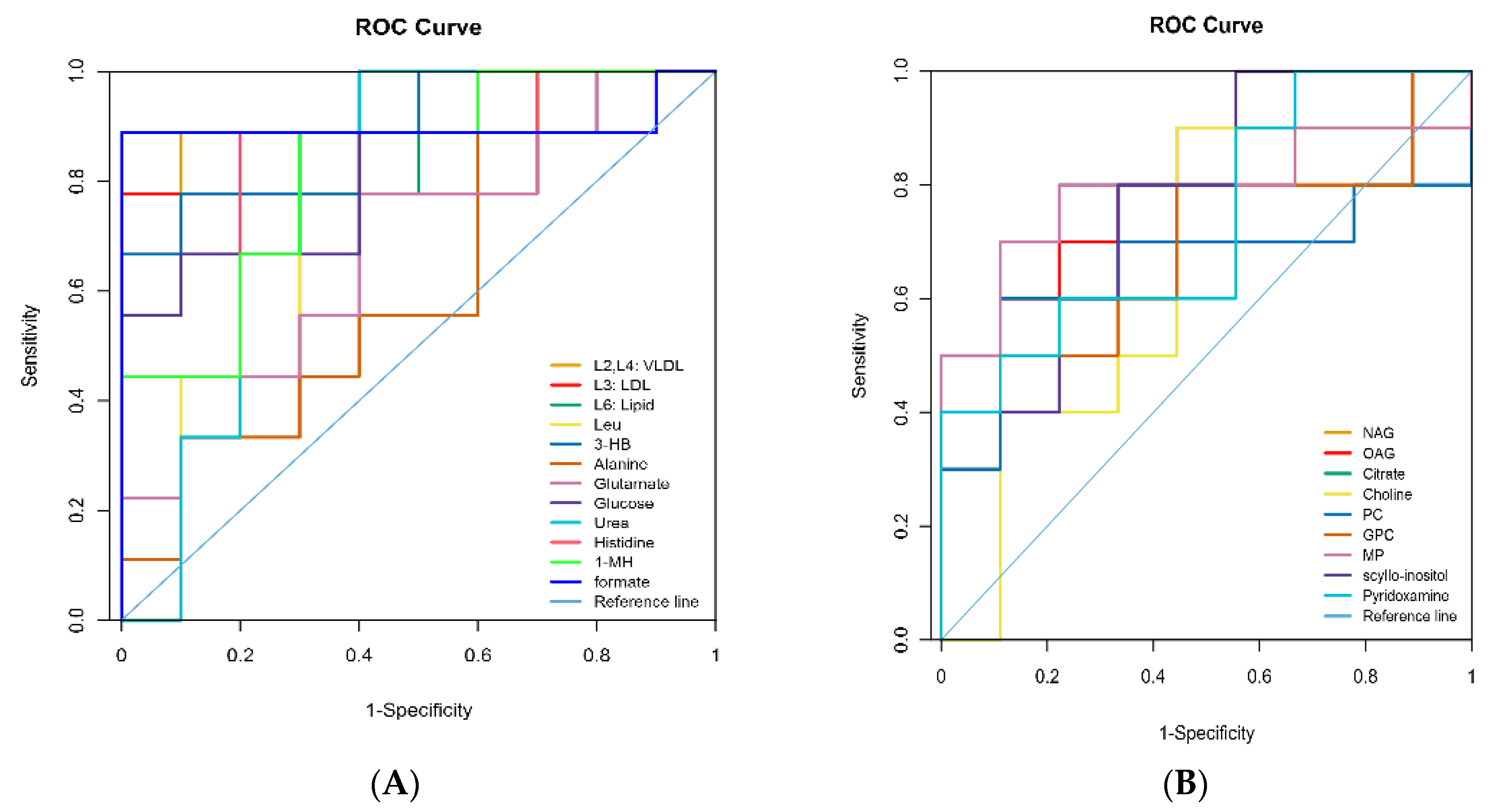
3.4. Identification of Different Metabolites
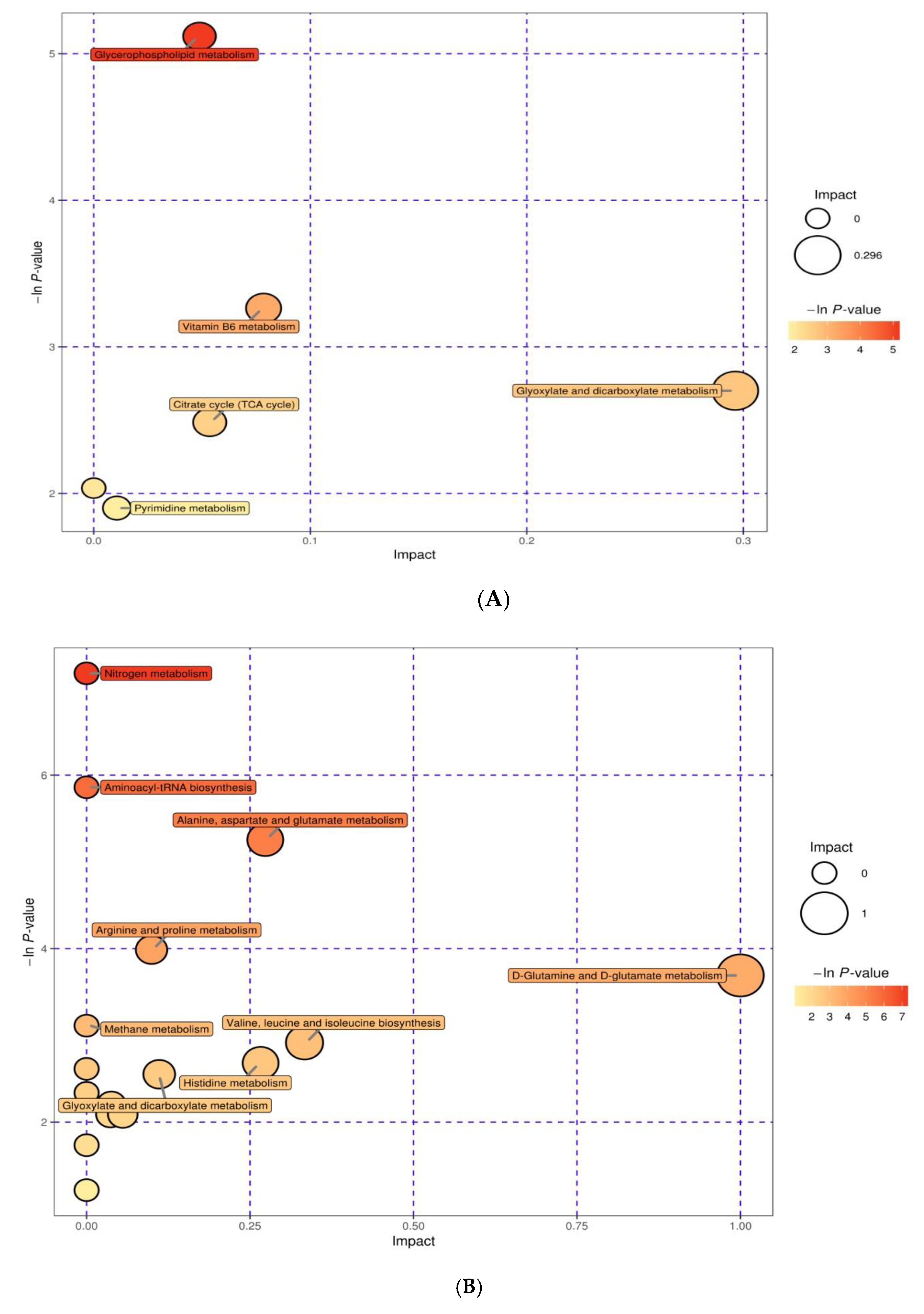
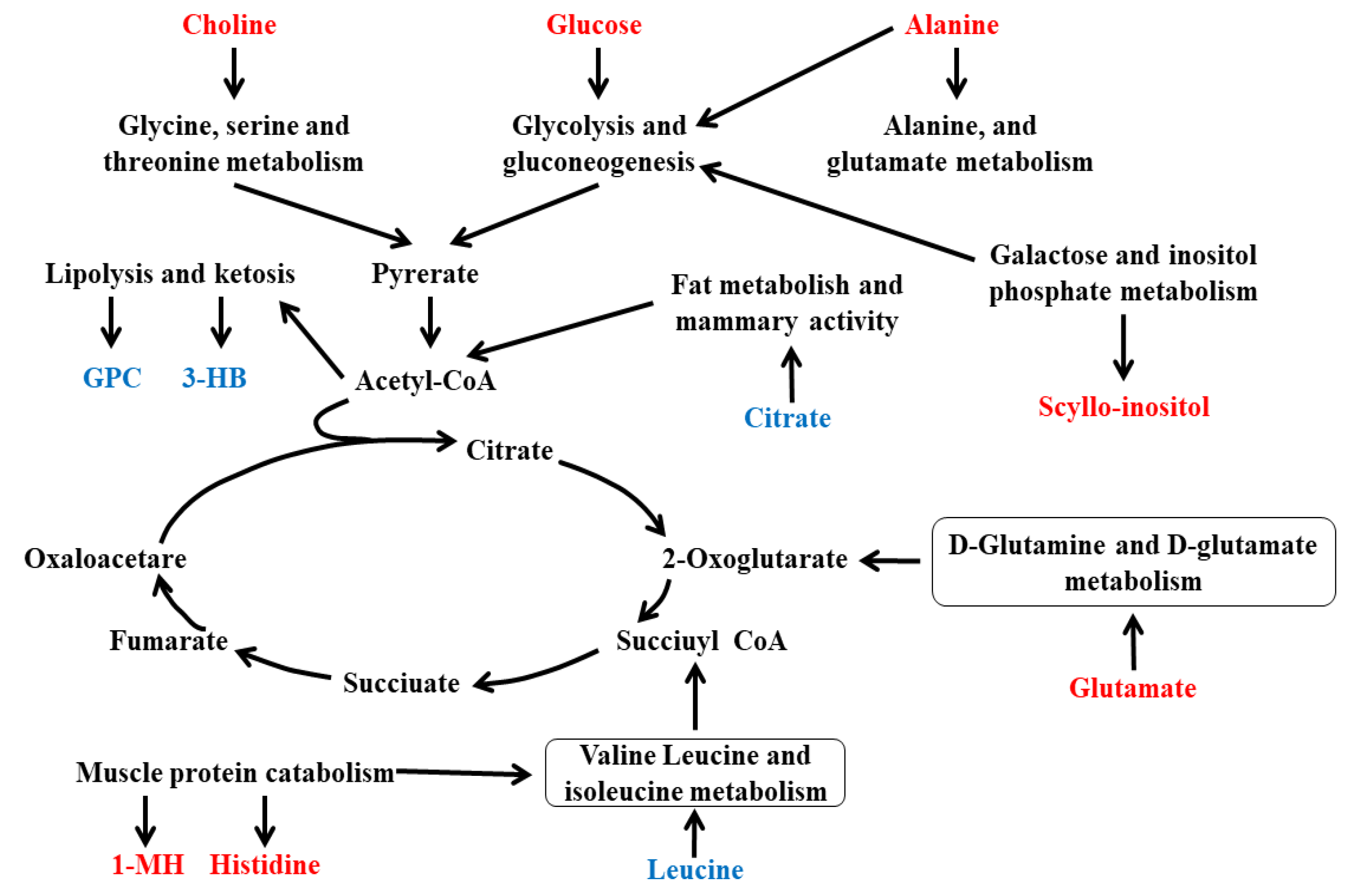
3.5. Integration of Key Different Metabolic Pathways
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bernabucci, U.; Biffani, S.; Buggiotti, L.; Vitali, A.; Lacetera, N.; Nardone, A. The effects of heat stress in Italian Holstein dairy cattle. J. Dairy Sci. 2014, 97, 471–486. [Google Scholar] [CrossRef] [PubMed]
- St-Pierre, N.; Cobanov, B.; Schnitkey, G. Economic losses from heat stress by US livestock industries. J. Dairy Sci. 2003, 86, E52–E77. [Google Scholar] [CrossRef] [Green Version]
- Nardone, A.; Ronchi, B.; Lacetera, N.; Ranieri, M.; Bernabucci, U. Effects of climate changes on animal production and sustainability of livestock systems. Livest. Sci. 2010, 130, 57–69. [Google Scholar] [CrossRef]
- Rhoads, M.L.; Kim, J.W.; Collier, R.J.; Crooker, B.A.; Boisclair, Y.R.; Baumgard, L.H.; Rhoads, R.P. Effects of heat stress and nutrition on lactating Holstein cows: II. Aspects of hepatic growth hormone responsiveness. J. Dairy Sci. 2010, 93, 170–179. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Li, L.; Chen, X.; Lu, Y.; Wang, D. Effects of heat stress on body temperature, milk production, and reproduction in dairy cows: A novel idea for monitoring and evaluation of heat stress—A review. Asian Australas. J. Anim. Sci. 2019, 32, 1332–1339. [Google Scholar] [CrossRef]
- Collier, R.J.; Doelger, S.G.; Head, H.H.; Thatcher, W.W.; Wilcox, C.J. Effects of heat stress during pregnancy on maternal hormone concentrations, calf birth weight and postpartum milk yield of holstein cows. J. Anim. Sci. 1982, 54, 309–319. [Google Scholar] [CrossRef]
- West, J. Effects of heat-stress on production in dairy cattle. J. Dairy Sci. 2003, 86, 2131–2144. [Google Scholar] [CrossRef]
- Shwartz, G.; Rhoads, M.L.; Vanbaale, M.J.; Rhoads, R.P.; Baumgard, L.H. Effects of a supplemental yeast culture on heat-stressed lactating Holstein cows. J. Dairy Sci. 2009, 92, 935–942. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.P.; Bu, D.P.; Wang, J.Q.; Huo, X.K.; Guo, T.J.; Wei, H.Y.; Zhou, L.L.; Rastani, R.R.; Baumgatd, L.H.; Li, F.D. Effect of saturated fatty acid supplementation on production and metabolism indices in heat-stressed mid-lactation dairy cows. J. Dairy Sci. 2010, 93, 4121–4127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, J.B.; Wang, W.Y.; Zheng, N.; Li, S.L.; Zhang, Y.D.; Zhang, X.K. Natural Period Change of Heat Stress Reveals Unique “Heat-stressed Milk Protein Decrease Syndrome” in Mid-lactation Dairy Cows. China Anim. Husb. Vet. Med. 2014, 41, 73–84. (In Chinese) [Google Scholar]
- Shahid, R.; Qureshi, Z.I.; Rahman, Z.U.; Ahmad, N. Effect of recombinant bovine somatotropin (rbST) and oxytocin on health biomarkers, reproductive performance and milk composition of Nili-Ravi buffaloes (Bubalus bubalis). Pak. Vet. J. 2019, 39, 553–557. [Google Scholar] [CrossRef]
- Cowley, F.C.; Barber, D.G.; Houlihan, A.V.; Poppi, D.P. Immediate and residual effects of heat stress and restricted intake on milk protein and casein composition and energy metabolism. J. Dairy Sci. 2015, 98, 2356–2368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuquay, J.W. Heat stress as it affects animal production. J. Anim. Sci. 1981, 52, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Zheng, N.; Wang, W.; Cheng, J.; Li, S.; Zhang, Y.; Wang, J.Q. Integrated Metabolomics Study of the Milk of Heat-stressed Lactating Dairy Cows. Sci. Rep. 2016, 6, 24208. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.Z.; Wang, D.M.; Wang, B.; Wang, J.K.; Liu, H.Y.; Guan, L.L.; Liu, J.X. Metabolomics of Four Biofluids from Dairy Cows: Potential Biomarkers for Milk Production and Quality. J. Proteome Res. 2015, 14, 1287–1298. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Zheng, N.; Wang, W.; Cheng, J.; Li, S.; Zhang, Y.; Wang, J.Q. Identification of diagnostic biomarkers and metabolic pathway shifts of heat-stressed lactating dairy cows. J. Proteom. 2015, 125, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Abeni, F.; Calamari, L.; Stefanini, L. Metabolic conditions of lactating Friesian cows during the hot season in the Po valley. 1. Blood indicators of heat stress. Int. J. Biometeorol. 2007, 52, 87–96. [Google Scholar] [CrossRef]
- Calamari, L.; Abeni, F.; Calegari, F.; Stefanini, L. Metabolic conditions of lactating Friesian cows during the hot season in the Po valley. 2. Blood minerals and acid-base chemistry. Int. J. Biometeorol. 2007, 52, 97–107. [Google Scholar] [CrossRef]
- Calamari, L.; Petrera, F.; Stefanini, L.; Abeni, F. Effects of different feeding time and frequency on metabolic conditions and milk production in heat-stressed dairy cows. Int. J. Biometeorol. 2013, 57, 785–796. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Dairy Cattle, 7th ed.; National Academy Press: Washington, DC, USA, 2001. [Google Scholar]
- AOAC International. Official Methods of Analysis, 18th ed.; AOAC international: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy. Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Cloarec, O.; Dumas, M.E.; Trygg, J.; Craig, A.; Barton, R.H.; Lindon, J.C.; Holmes, E. Evaluation of the Orthogonal Projection on Latent Structure Model Limitations Caused by Chemical Shift Variability and Improved Visualization of Biomarker Changes in 1H NMR Spectroscopic Metabonomic Studies. Anal. Chem. 2005, 77, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Trygg, J.; Wold, S. Orthogonal projections to latent structures (O-PLS). J. Chemom. 2002, 16, 119–128. [Google Scholar] [CrossRef]
- Armstrong, D. Heat stress interaction with shade and cooling. J. Dairy Sci. 2002, 77, 2044–2050. [Google Scholar] [CrossRef]
- Dikmen, S.; Alava, E.; Pontes, E.; Fear, J.M.; Dikmen, B.Y.; Olson, T.A.; Hansen, P.J. Differences in thermoregulatory ability between slick-haired and wild-type lactating holstein cows in response to acute heat stress. J. Dairy Sci. 2008, 91, 3395–3402. [Google Scholar] [CrossRef] [PubMed]
- Dikmen, S.; Hansen, P. Is the temperature-humidity index the best indicator of heat stress in lactating dairy cows in a subtropical environment? J. Dairy Sci. 2009, 92, 109–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimbelman, R.; Rhoads, R.; Rhoads, M.; Duff, G.; Baumgard, L.; Collier, R. A re-evaluation of the impact of temperature humidity index (THI) and black globe humidity index (BGHI) on milk production in high producing dairy cows. In Proceedings of the 24th Southwest Nutrition and Management Conference, 1111 N. Dunlap Ave, Savoy, IL, USA, 26–27 February 2009; pp. 158–169. [Google Scholar]
- Kadzere, C.; Murphy, M.; Silanikove, N.; Maltz, E. Heat stress in lactating dairy cows: A review. Livest. Prod. Sci. 2002, 77, 59–91. [Google Scholar] [CrossRef]
- Rhoads, M.; Rhoads, R.; VanBaale, M.; Collier, R.J.; Sanders, S.; Weber, W.J.; Crooker, B.A.; Baumgard, L. Effects of heat stress and plane of nutrition on lactating Holstein cows: I. Production, metabolism, and aspects of circulating somatotropin. J. Dairy Sci. 2009, 92, 1986–1997. [Google Scholar] [CrossRef] [Green Version]
- Belić, B.; Cincović, M.; Stevančević, M.; Toholj, B. Parameters of the negative energy balance (NEFA, BHB and glucose) and neutrophil to lymphocyte ratio in cows after calving- comparison of two indicators of stress. Vet. Stanica 2011, 42, 281–284. [Google Scholar]
- Harris, C.I.; Milne, G. The urinary excretion of N-methyl histidine by cattle: Validation as an index of muscle protein breakdown. Br. J. Nutr. 1981, 45, 411–422. [Google Scholar] [CrossRef]
- Hussain, R.; Ali, F.; Rafique, A.; Ghaffar, A.; Jabeen, G.; Rafay, M.; Liaqat, S.; Khan, I.; Malik, R.; Khan, M.K.; et al. Exposure to sub-acute concentrations of glyphosate induce clinico-hematological, serum biochemical and genotoxic damage in adult cockerels. Pak. Vet. J. 2019, 39, 181–186. [Google Scholar] [CrossRef]
- Tůma, P.; Samcova, E.; Balinova, P. Determination of 3-methylhistidine and 1-methylhistidine in untreated urine samples by capillary electrophoresis. J. Chromatogr. B 2005, 821, 53–59. [Google Scholar]
- Wang, C.; Guo, F. Branched chain amino acids and metabolic regulation. Sci. Bull. 2013, 58, 1228–1235. [Google Scholar] [CrossRef] [Green Version]
- Gao, S.T.; Guo, J.; Quan, S.Y.; Nan, X.M.; Fernandez, M.S.; Baumgard, L.H.; Bu, D.P. The effects of heat stress on protein metabolism in lactating Holstein cows. J. Dairy Sci. 2017, 100, 5040–5049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, J.; Gao, S.; Quan, S.; Zhang, Y.; Bu, D.; Wang, J. Blood amino acids profile responding to heat stress in dairy cows. Asian Australas. J. Anim. Sci. 2018, 31, 47–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meijer, A.J. Amino Acids as Regulators and Components of Nonproteinogenic Pathways. J. Nutr. 2003, 133, 2057–2062. [Google Scholar] [CrossRef]
- Ai, Y.; Cao, Y.; Xie., Z.L.; Zhang., Y.S.; Shen., X.Z. Relationship between Free Amino Acids in Cow’s Blood and Decreasing Milk Protein under Heat Stress (In Chinese). Food Sci. 2015, 36, 38–41. [Google Scholar] [CrossRef]
- Newsholme, P.; Lima, M.R.; Procopio, J.; Pithoncuri, T.C.; Doi, S.Q.; Bazotte, R.B.; Curi, R. Glutamine and glutamate as vital metabolites. Braz. J. Med. Biol. Res. 2003, 36, 153–163. [Google Scholar] [CrossRef] [Green Version]
- Brosnan, J.T. Glutamate, at the Interface between Amino Acid and Carbohydrate Metabolism. J. Nutr. 2000, 130, 988. [Google Scholar] [CrossRef]
- Salgado, M.C.; Meton, I.; Anemaet, I.G.; Baanante, I.V. Activating transcription factor 4 mediates up-regulation of alanine aminotransferase 2 gene expression under metabolic stress. Biochim. Biophys. Acta 2014, 1839, 288–296. [Google Scholar] [CrossRef]
- Stahel, P.; Purdie, N.G.; Cant, J.P. Use of dietary feather meal to induce histidine deficiency or imbalance in dairy cows and effects on milk composition. J. Dairy Sci. 2014, 97, 439–445. [Google Scholar] [CrossRef] [Green Version]
- Lamarre, S.G.; Morrow, G.P.; Macmillan, L.; Brosnan, M.E.; Brosnan, J.T. Formate: An essential metabolite, a biomarker, or more? Clin. Chem. Lab. Med. 2013, 51, 571–578. [Google Scholar] [CrossRef]
- Morgavi, D.P.; Forano, E.; Martin, C.; Newbold, C.J. Microbial ecosystem and methanogenesis in ruminants. Animal 2010, 4, 1024–1036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barceloux, D.G.; Bond, G.R.; Krenzelok, E.P.; Cooper, H.; Vale, J.A. American academy of clinical toxicology practice guidelines on the treatment of methanol poisoning. J. Toxicol. Clin. Toxicol. 2002, 40, 415–446. [Google Scholar] [PubMed]
- Medinsky, M.A.; Dorman, D.C.; Bond, J.A.; Moss, O.R.; Janszen, D.B. Pharmacokinetics of methanol and formate in female cynomolgus monkeys exposed to methanol vapors. Res. Rep. Health Eff. Inst. 1997, 77, 1–30. [Google Scholar]
- Hovda, K.E.; Andersson, K.S.; Urdal, P.; Jacobsen, D. Methanol and Formate Kinetics during Treatment with Fomepizole. Clin. Toxicol. 2005, 43, 221–227. [Google Scholar] [CrossRef]
- Srikandakumar, A.; Johnson, E.H. Effect of heat stress on milk production, rectal temperature, respiratory rate and blood chemistry in Holstein, Jersey and Australian Milking Zebu cows. Trop. Anim. Health Prod. 2004, 36, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Garriga, C.; Hunter, R.R.; Amat, C.; Planas, J.M.; Mitchell, M.A.; Moreto, M. Heat stress increases apical glucose transport in the chicken jejunum. Am. J. Physiol.-Regul. I 2006, 290, 195–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikari, A.; Nakano, M.; Suketa, Y.; Harada, H.; Takagi, K. Reorganization of ZO-1 by sodium-dependent glucose transporter activation after heat stress in LLC-PK1 cells. J. Cell. Physiol. 2005, 203, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Febbraio, M.A. Alterations in Energy Metabolism during Exercise and Heat Stress. Sports Med. 2001, 31, 47–59. [Google Scholar] [CrossRef]
- Akram, M. A Focused Review of the Role of Ketone Bodies in Health and Disease. J. Med. Food 2013, 16, 965–967. [Google Scholar] [CrossRef]
- Zammit, V.A. Ketogenesis in the liver of ruminants—Adaptations to a challenge. J. Agric Sci. 1990, 115, 155–162. [Google Scholar] [CrossRef]
- Belhadj Slimen, I.; Najar, T.; Ghram, A.; Abdrrabba, M. Heat stress effects on livestock: Molecular, cellular and metabolic aspects, a review. J. Anim. Physiol. Anim. Nutr. 2016, 100, 401–412. [Google Scholar] [CrossRef] [Green Version]
- Qu, M.; Wei, S.; Chen, Z.; Wang, G.; Zheng, Y.; Yan, P. Differences of hormones involved in adipose metabolism and lactation between high and low producing Holstein cows during heat stress. Anim. Nutr. 2015, 1, 339–343. [Google Scholar] [CrossRef] [PubMed]
- DeFrain, J.; Hippen, A.; Kalscheur, K.; Schingoethe, D. Feeding lactose increases ruminal butyrate and plasma β-hydroxybutyrate in lactating dairy cows. J. Dairy Sci. 2004, 87, 2486–2494. [Google Scholar] [CrossRef]
- Fried, S.K.; Watford, M. Leucing weight with a futile cycle. Cell Metab. 2007, 6, 155–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blomstrand, E.; Celsing, F.; Newsholme, E. Changes in plasma concentrations of aromatic and branched-chain amino acids during sustained exercise in man and their possible role in fatigue. Acta Physiol. Scand. 1988, 133, 115–121. [Google Scholar] [CrossRef]
- Harris, R.A.; Zhang, B.; Goodwin, G.W.; Kuntz, M.J.; Shimomura, Y.; Rougraff, P.; Dexter, P.; Zhao, Y.; Gibson, R.; Crabb, D.W. Regulation of the branched-chain α-ketoacid dehydrogenase and elucidation of a molecular basis for maple syrup urine disease. Adv. Enzym. Regul. 1990, 30, 245–256. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, M.; Sun, H.; Wang, Y. Branched-chain amino acid metabolism in heart disease: An epiphenomenon or a real culprit? Cardiovasc. Res. 2011, 90, 220–223. [Google Scholar] [CrossRef] [Green Version]
- Taegtmeyer, H.; Harinstein, M.E.; Gheorghiade, M. More than bricks and mortar: Comments on protein and amino acid metabolism in the heart. Am. J. Cardiol. 2008, 101, S3–S7. [Google Scholar] [CrossRef]
- Bell, A.W. Lipid metabolism in liver and selected tissues and in the whole body of ruminant animals. Prog. Lipid Res. 1979, 18, 117–164. [Google Scholar] [CrossRef]
- Gruffat, D.; Durand, D.; Graulet, B.; Bauchart, D. Regulation of VLDL synthesis and secretion in the liver. Reprod. Nutr. Dev. 1996, 36, 375–389. [Google Scholar] [CrossRef] [PubMed]
- Fast, D.G.; Vance, D.E. Nascent VLDL phospholipid composition is altered when phosphatidylcholine biosynthesis is inhibited: Evidence for a novel mechanism that regulates VLDL secretion. Biocheim. Biophys. Acta. 1995, 1258, 159–168. [Google Scholar] [CrossRef]
- Cheng, X.; Luming, W.; Yongxue, L.; Jianzhong, Z.; Baozhang, L.Q. Effect of acute heat stress on the contents of phospholipids and the fluidity of cell membrane in rat lung. Chin. J. Appl. Physiol. 1992, 8, 321–324. [Google Scholar]
- Sundekilde, U.K.; Frederiksen, P.D.; Clausen, M.R.; Larsen, L.B.; Bertram, H.C. Relationship between the metabolite profile and technological properties of bovine milk from two dairy breeds elucidated by NMR-based metabolomics. J. Agric. Food. Chem. 2011, 59, 7360–7367. [Google Scholar] [CrossRef]
- Moremen, K.W.; Tiemeyer, M.; Nairn, A.V. Vertebrate protein glycosylation: Diversity, synthesis and function. Nat. Rev. Mol. Cell Biol. 2012, 13, 448–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiete, D.; Srivastava, V.; Hindsgaul, O.; Baenziger, J.U. A hepatic reticuloendothelial cell receptor specific for SO4-4GalNAcβ1, 4GlcNAcβ1, 2Manα that mediates rapid clearance of lutropin. Cell 1991, 67, 1103–1110. [Google Scholar] [CrossRef]
- Do Amaral, B.; Connor, E.; Tao, S.; Hayen, M.; Bubolz, J.; Dahl, G. Heat stress abatement during the dry period influences metabolic gene expression and improves immune status in the transition period of dairy cows. J. Dairy Sci. 2011, 94, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Dahl, G. Invited review: Heat stress effects during late gestation on dry cows and their calves. J. Dairy Sci. 2013, 96, 4079–4093. [Google Scholar] [CrossRef]
- Thompson, I.; Dahl, G. Dry-period seasonal effects on the subsequent lactation. Prof. Anim. Sci. 2012, 28, 628–631. [Google Scholar] [CrossRef]
- Tao, S.; Monteiro, A.; Thompson, I.; Hayen, M.; Dahl, G. Effect of late-gestation maternal heat stress on growth and immune function of dairy calves. J. Dairy Sci. 2012, 95, 7128–7136. [Google Scholar] [CrossRef] [Green Version]
- Nardone, A.; Lacetera, N.; Bernabucci, U.; Ronchi, B. Composition of colostrum from dairy heifers exposed to high air temperatures during late pregnancy and the early postpartum period. J. Dairy Sci. 1997, 80, 838–844. [Google Scholar] [CrossRef]
- Stott, G.; Wiersma, F.; Menefee, B.; Radwanski, F. Influence of environment on passive immunity in calves. J. Dairy Sci. 1976, 59, 1306–1311. [Google Scholar] [CrossRef]
- Holub, B.J. Advances in Nutritional Research; Springer: Boston, MA, USA, 1982. [Google Scholar]
- Steer, T.E.; Gee, J.N.; Johnson, I.T.; Gibson, G.R. Biodiversity of human faecal bacteria isolated from phytic acid enriched chemostat fermenters. Curr. Issues Intest. Microbiol. 2004, 5, 23–39. [Google Scholar] [PubMed]
- Fenili, D.; Brown, M.; Rappaport, R.; McLaurin, J. Properties of scyllo–inositol as a therapeutic treatment of AD-like pathology. J. Mol. Med. 2007, 85, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Snell, E.E. Chemical Structure in Relation to Biological Activities of Vitamin B6. In Vitamins & Hormones; Academic Press: Cambridge, MA, USA, 1958; pp. 77–125. [Google Scholar]
- Depeint, F.; Bruce, W.R.; Shangari, N.; Mehta, R.; O’Brien, P.J. Mitochondrial function and toxicity: Role of B vitamins on the one-carbon transfer pathways. Chem. Biol. Interact. 2006, 163, 113–132. [Google Scholar] [CrossRef]
- Shennan, D.B.; Peaker, M. Transport of Milk Constituents by the Mammary Gland. Physiol. Rev. 2000, 80, 925–951. [Google Scholar] [CrossRef]
- Firyal, S.; Tanveer, S.; Awan, A.R.; Tayyab, M.; Wasim, M.; Nawaz, M.; Sattar, H.; Nawaz, S.; Saeed, S.; Ali, M.M. Association of bovine tumor necrosis factor alpha gene polymorphism with mastitis in Nili Ravi buffaloes. Pak. Vet. J. 2019, 39, 128–131. [Google Scholar] [CrossRef]
- Dhillon, K.S.; Singh, J. A new horizon in the pathobiology, aetiology and novel treatment of mastitis in buffalo. Buffalo Bull. 2013, 32, 26–34. [Google Scholar]
- Banks, W.; Clapperton, J.L.; Girdler, A.K. Effect of dietary unsaturated fatty acids in various forms on the de novo synthesis of fatty acids in the bovine mammary gland. J. Dairy Res. 1990, 57, 179–185. [Google Scholar] [CrossRef]
- Linzell, J.; Mepham, T.; Peaker, M. The secretion of citrate into milk. J. Physiol. 1976, 260, 739–750. [Google Scholar] [CrossRef]
- Yue, S.; Wang, Z.; Wang, L.; Peng, Q.; Xue, B. Transcriptome Functional Analysis of Mammary Gland of Cows in Heat Stress and Thermoneutral Condition. Animals 2020, 10, 1015. [Google Scholar] [CrossRef] [PubMed]
- Salama, A.A.K.; Contreras-Jodar, A.; Love, S.; Mehaba, N.; Such, X.; Caja, G. Milk yield, milk composition, and milk metabolomics of dairy goats intramammary-challenged with lipopolysaccharide under heat stress conditions. Sci Rep. 2020, 10, 5055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Parameter | TN 1 (Thermal Neutral) | HS 2 (Heat Stress) | p-Value |
|---|---|---|---|
| Number of cows | 10 | 10 | |
| Parity | 3.0 ± 1.0 | 3.2 ± 1.1 | 0.78 |
| Lactation days | 146.2 ± 12 | 145 ± 25 | 0.95 |
| Milk yield (kg) | 26.8 ± 1.5 | 27.2 ± 2.1 | 0.95 |
| Average bodyweight (kg) | 604.4 ± 56.44 | 601.81 ± 78.97 | 0.95 |
| Item | % |
|---|---|
| Ingredient | |
| Silage (Corn silage) | 19.63 |
| Alfalfa hay | 5.82 |
| Hemarthriia altissima1 | 10.60 |
| Sweet potato vine | 13.95 |
| Corn meal | 23.50 |
| Wheat bran | 6.00 |
| Soybean meal | 9.70 |
| Rapeseed meal | 6.00 |
| Calcium fatty acids | 1.00 |
| Limestone | 1.20 |
| Calcium superphosphate | 1.00 |
| Salt | 0.60 |
| Premix 2 | 1.00 |
| Total | 100 |
| Roughage-to-concentrate | 50/50 |
| Nutrient levels 3 | |
| NEL 4 (MJ/kg) | 6.36 |
| NEL (Mcal/kg) | 1.51 |
| CP 5 | 16.18 |
| NDF 6 | 36.46 |
| ADF 7 | 22.96 |
| Ca 8 | 1.06 |
| P9 | 0.56 |
| Item | Thermoneutral Period | Heat Stress Period | p-Value |
|---|---|---|---|
| Respiration rate (breaths/min) | 35.4 ± 4.7 | 82.3 ± 10.5 | <0.01 |
| Rectal temperature (°C) | 38.6 ± 0.1 | 39.4 ± 0.3 | <0.01 |
| Initial 1 BW (kg) | 604.4 ± 56.4 | 601.8 ± 79.0 | 0.91 |
| Final BW (kg) | 612.2 ± 55.6 | 565.5 ± 74.6 | 0.17 |
| BW changes (kg) | 7.8 ± 8.3 | −36.4 ± 11.2 | <0.01 |
| 2 DMI (kg) | 18.1 ± 2.5 | 15.5 ± 2.0 | <0.05 |
| Milk yield (kg/d) | 28.6 ± 1.4 | 19.9 ± 2.1 | <0.05 |
| Protein (%) | 3.2 ± 0.2 | 2.9 ± 0.9 | <0.05 |
| Protein yield (g/d) | 914.5 ± 2.2 | 578.1 ± 1.8 | <0.01 |
| Fat (%) | 3.6 ± 0.7 | 3.8 ± 0.6 | 0.12 |
| Fat yield (g/d) | 1026.6 ± 3.2 | 753.8 ± 2.5 | <0.05 |
| Lactose (%) | 4.9 ± 0.2 | 4.7 ± 0.2 | 0.19 |
| Lactose yield(g/d) | 1407.2 ± 5.2 | 933.5 ± 3.8 | <0.05 |
| Somatic cells count 1000/mL | 286± 8.2 | 312± 9.5 | 0.03 |
| Metabolites 1 | Identification (ppm) and Multiplicity 2 | Correlation Coefficients (r) 3 | p-Value | |
|---|---|---|---|---|
| Milk | N-Acetyl glycoprotein | 2.06 (s) 4 | −0.73 | 0.02 |
| O-Acetyl glycoprotein | 2.07 (s) | 0.68 | 0.01 | |
| Citrate | 2.53 (d), 2.67 (d) 5 | 0.74 | <0.01 | |
| Choline | 3.20 (s) | −0.64 | 0.03 | |
| Glycerophosphorylcholine | 3.23 (s) | 0.63 | 0.01 | |
| Methyl phosphate | 3.49 (d) | 0.68 | 0.1 | |
| scyllo-Inositol | 3.36 (s) | −0.66 | 0.02 | |
| Pyridoxamine | 7.67 (s) | −0.68 | 0.02 | |
| Plasma | L2, L4: VLDL | 0.89 (br), 1.29 (br) 6 | 0.81 | <0.01 |
| L3: LDL | 1.27 (br) | 0.66 | <0.01 | |
| L6: Lipid | 2.01 (br) | 0.64 | <0.01 | |
| Leucine | 0.96 (t) 7, 1.69 (m) 8 | 0.75 | <0.01 | |
| 3-Hydroxybutyrate | 1.20 (d) | 0.74 | <0.01 | |
| Alanine | 1.48 (d) | −0.64 | <0.01 | |
| Glutamate | 2.13 (m), 2.46 (m) | −0.65 | <0.01 | |
| Glucose | 3.42 (t), 3.54 (dd) 3.71 (t), 3.73 (m) 3.84 (m), 5.23 (d) | −0.71 | <0.01 | |
| Urea | 5.78 (br) | −0.66 | 0.02 | |
| Histidine | 7.05 (s), 7.76(s) | −0.63 | <0.01 | |
| 1-Methylhistidine | 6.96 (s), 7.61(s) | −7.64 | <0.01 | |
| Formate | 8.45 (s) | −0.68 | <0.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, S.; Ding, S.; Zhou, J.; Yang, C.; Hu, X.; Zhao, X.; Wang, Z.; Wang, L.; Peng, Q.; Xue, B. Metabolomics Approach Explore Diagnostic Biomarkers and Metabolic Changes in Heat-Stressed Dairy Cows. Animals 2020, 10, 1741. https://doi.org/10.3390/ani10101741
Yue S, Ding S, Zhou J, Yang C, Hu X, Zhao X, Wang Z, Wang L, Peng Q, Xue B. Metabolomics Approach Explore Diagnostic Biomarkers and Metabolic Changes in Heat-Stressed Dairy Cows. Animals. 2020; 10(10):1741. https://doi.org/10.3390/ani10101741
Chicago/Turabian StyleYue, Shuangming, Siyan Ding, Jia Zhou, Chao Yang, Xiaofei Hu, Xiaonan Zhao, Zhisheng Wang, Lizhi Wang, Quanhui Peng, and Bai Xue. 2020. "Metabolomics Approach Explore Diagnostic Biomarkers and Metabolic Changes in Heat-Stressed Dairy Cows" Animals 10, no. 10: 1741. https://doi.org/10.3390/ani10101741
APA StyleYue, S., Ding, S., Zhou, J., Yang, C., Hu, X., Zhao, X., Wang, Z., Wang, L., Peng, Q., & Xue, B. (2020). Metabolomics Approach Explore Diagnostic Biomarkers and Metabolic Changes in Heat-Stressed Dairy Cows. Animals, 10(10), 1741. https://doi.org/10.3390/ani10101741








