The Cooperation of Bifidobacterium longum and Active Vitamin D3 on Innate Immunity in Salmonella Colitis Mice via Vitamin D Receptor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. 1,25(OH)2D3 Treatment
2.3. Animal Experiments
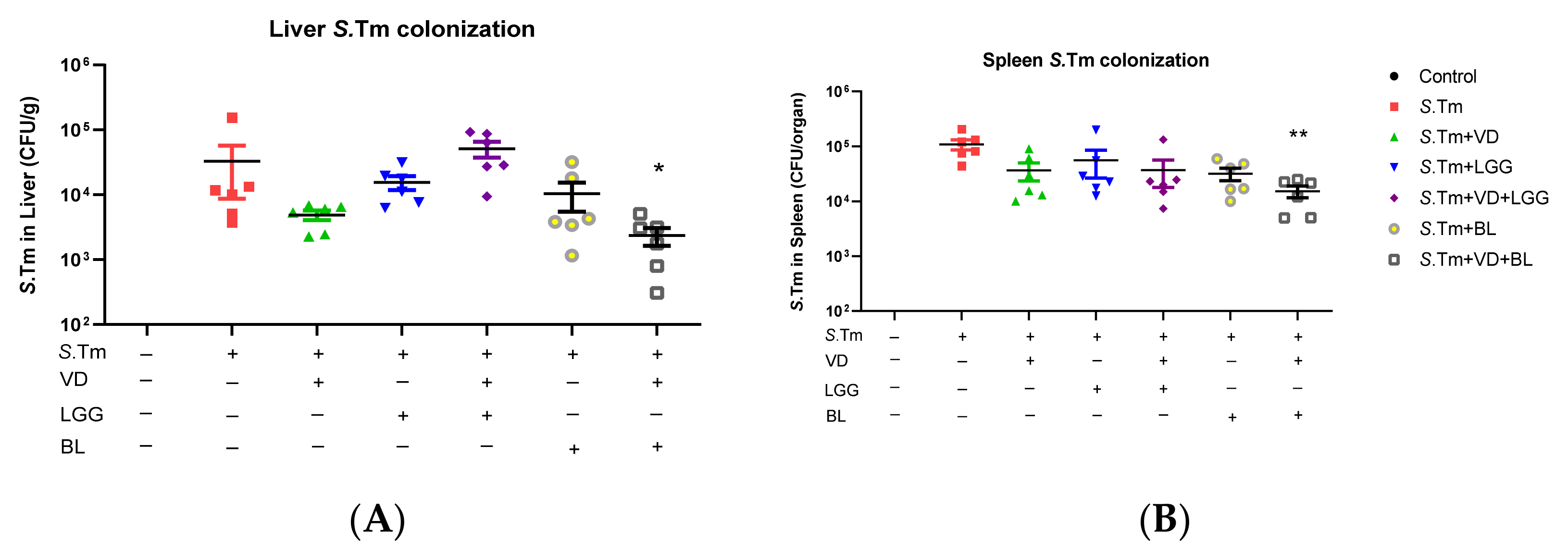
2.4. Analysis of S.Tm Loads in the Spleens and Livers
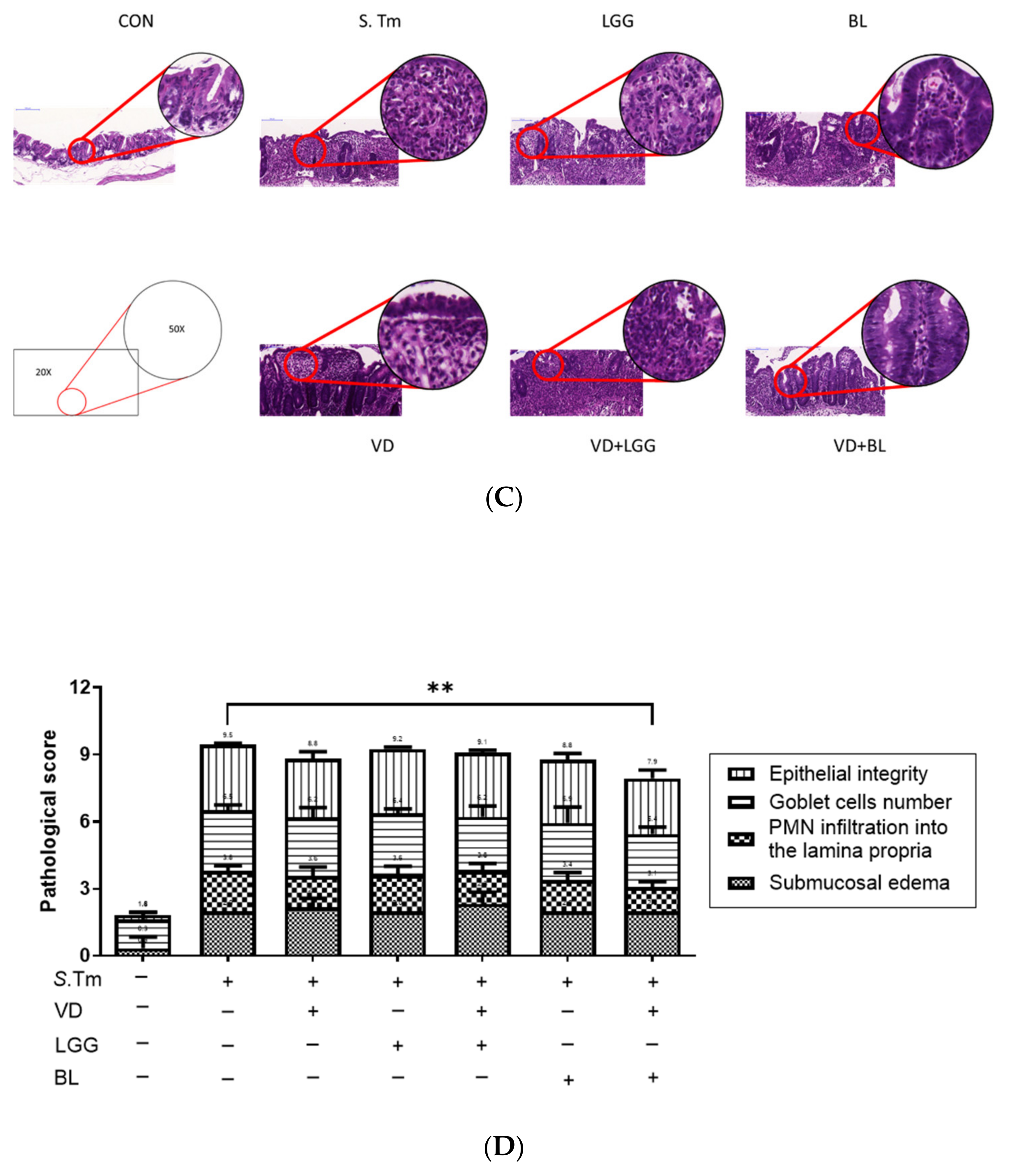
2.5. Histological Colitis Scoring
2.6. Quantitative Real-Time PCR Analysis of Cecum RNA
2.7. RNA Interference (RNAi) in Cultured Cells
2.8. Statistical Analysis
3. Results
3.1. In Vivo Study
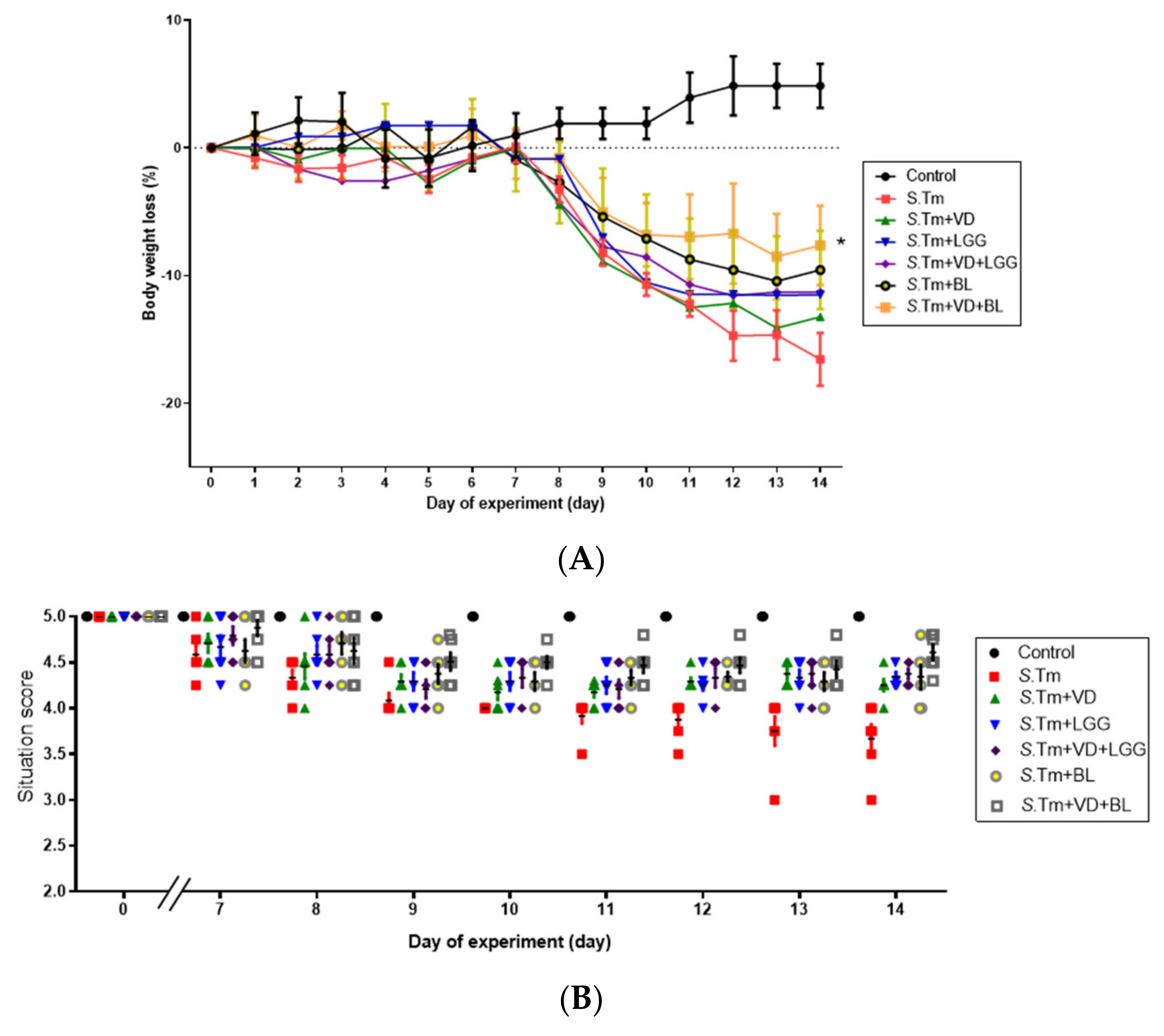
3.1.1. Combination of Bifidobacterium Longum and Active 1,25D3 Attenuates the Severity of Salmonella Colitis in Mice
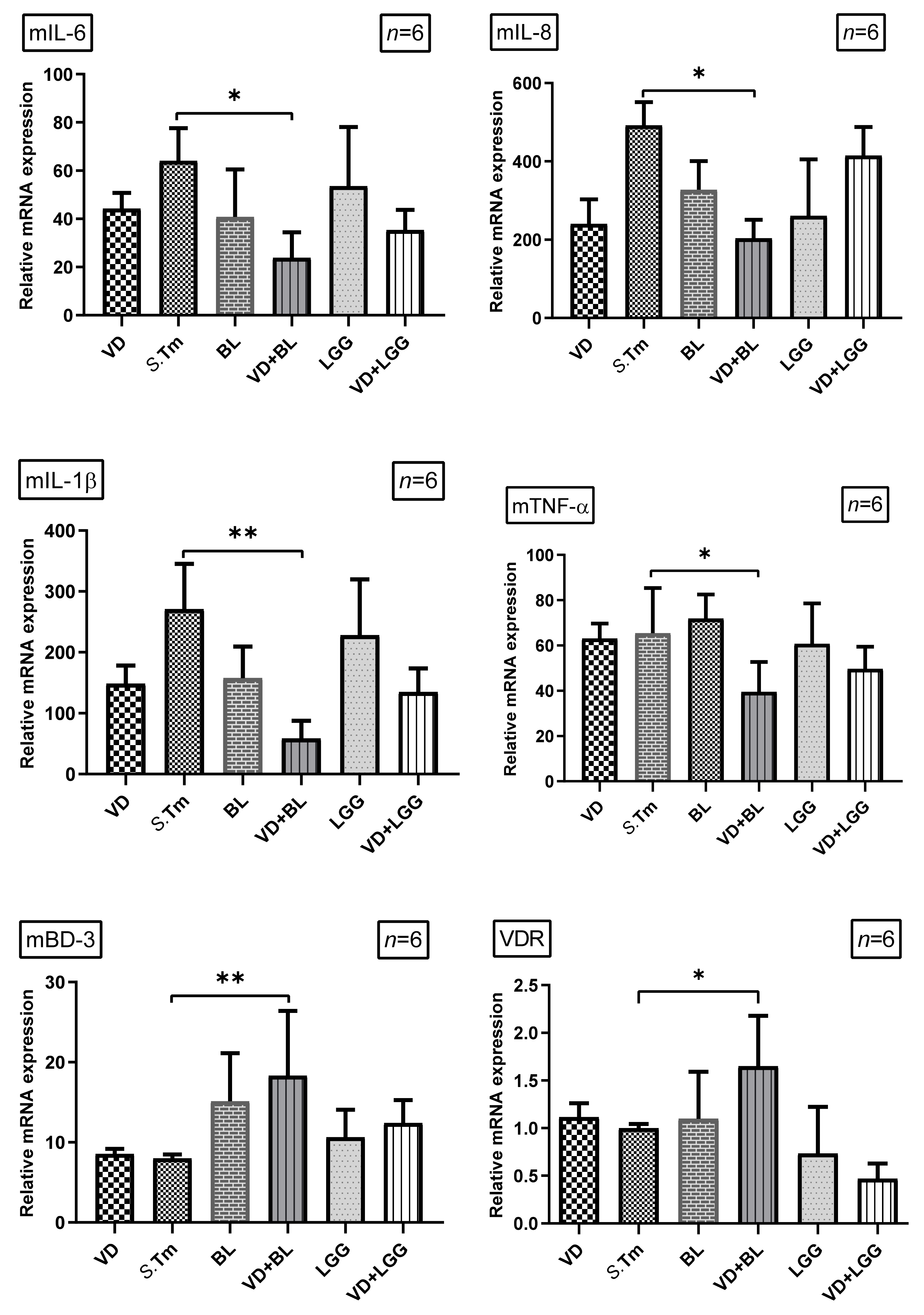
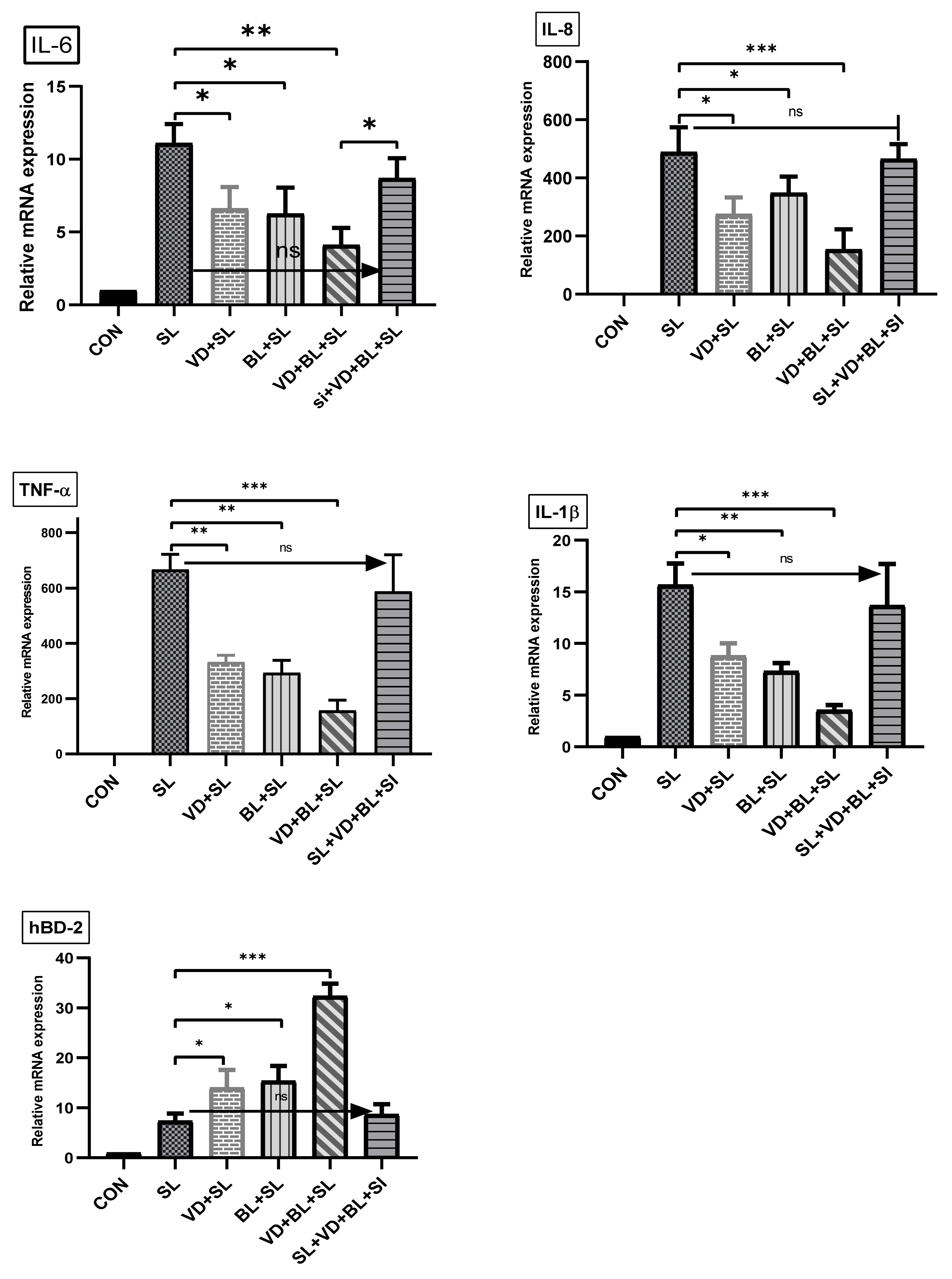
3.1.2. Combination of 1,25D3 and Bifidobacterium longum Exerts Cecal Anti-Inflammatory Responses and Antimicrobial Peptide in Salmonella Colitis Mice
3.1.3. Combination of 1,25D3 and Bifidobacterium Longum Exerted Reduction of Bacterial Translocation in Salmonella-Infected Mice
3.2. In Vitro Study
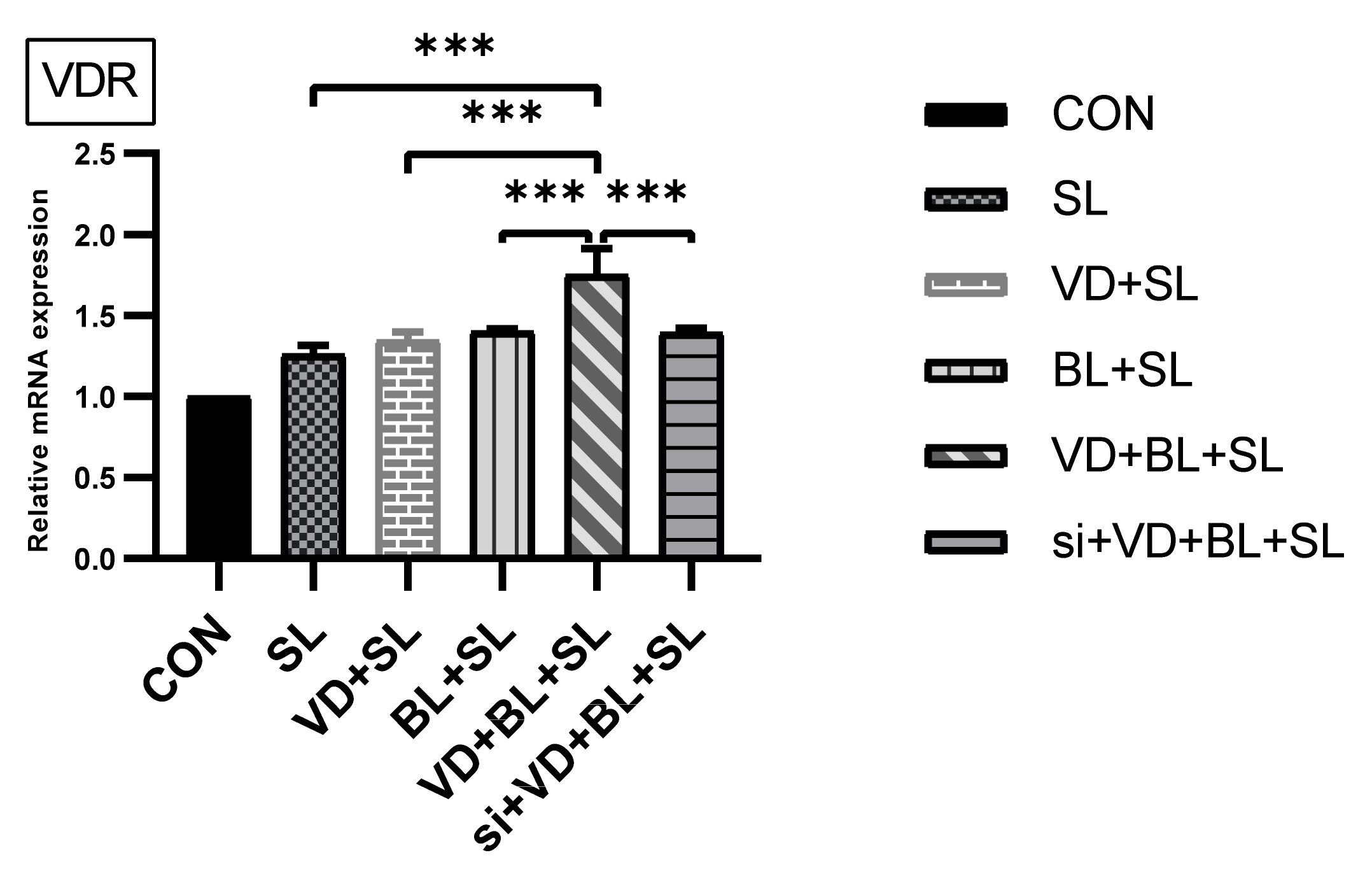
3.2.1. Combination of Bifidobacterium Longum and 1,25D3 Exerted Synergistic Effect on Vitamin D Receptor mRNA Expression in Salmonella-Infected SW480 Cells
3.2.2. The Synergistic Effect of Bifidobacterium Longum on Vitamin D-Induced mRNA Expression Is Dependent on VDR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pang, T.; Bhutta, Z.A.; Finlay, B.B.; Altwegg, M. Typhoid fever and other salmonellosis: A continuing challenge. Trends Microbiol. 1995, 3, 253–255. [Google Scholar] [CrossRef]
- Glynn, M.K.; Bopp, C.; Dewitt, W.; Mokhtar, M.; Angulo, F.J. Emergence of multidrug-resistant Salmonella enterica serotype typhimurium DT104 infections in the United States. N. Engl. J. Med. 1998, 338, 1333–1338. [Google Scholar] [CrossRef] [Green Version]
- Rodrigue, D.C.; Tauxe, R.V.; Rowe, B. International increase in Salmonella enteritidis: A new pandemic? Epidemiol. Infect. 1990, 105, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Threlfall, E.J.; Fisher, I.S.; Berghold, C.; Gerner-Smidt, P.; Tschäpe, H.; Cormican, M.; Luzzi, I.; Schnieder, F.; Wannet, W.; Machado, J.; et al. Antimicrobial drug resistance in isolates of Salmonella enterica from cases of salmonellosis in humans in Europe in 2000: Results of international multi-centre surveillance. Euro. Surveill. 2003, 8, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Su, L.H.; Wu, T.L.; Chia, J.H.; Chu, C.; Kuo, A.J.; Chiu, C.H. Increasing ceftriaxone resistance in Salmonella isolates from a university hospital in Taiwan. J. Antimicrob. Chemother. 2005, 55, 846–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauderdale, T.L.; Aarestrup, F.M.; Chen, P.C.; Lai, J.F.; Wang, H.Y.; Shiau, Y.R.; Huang, I.W.; Hung, C.L. Multidrug resistance among different serotypes of clinical Salmonella isolates in Taiwan. Diagn. Microbiol. Infect. Dis. 2006, 55, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Chiappini, E.; Galli, L.; Pecile, P.; Martino, M.D. Results of a 5-year prospective surveillance study of antibiotic resistance among Salmonella enterica isolates and ceftriaxone therapy among children hospitalized for acute diarrhea. Clin. Ther. 2002, 24, 1585–1594. [Google Scholar] [CrossRef]
- Helms, M.; Vastrup, P.; Gerner-Smidt, P.; Mølbak, K. Excess mortality associated with antimicrobial drug-resistant Salmonella typhimurium. Emerg. Infect. Dis. 2002, 8, 490–495. [Google Scholar] [CrossRef]
- Liu, P.T.; Stenger, S.; Li, H.; Wenzel, L.; Tan, B.H.; Krutzik, S.R.; Ochoa, M.T.; Schauber, J.; Wu, K.; Meinken, C.; et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 2006, 311, 1770–1773. [Google Scholar] [CrossRef]
- White, J.H. Vitamin D signaling, infectious diseases, and regulation of innate immunity. Infect. Immun. 2008, 76, 3837–3843. [Google Scholar] [CrossRef] [Green Version]
- Gubatan, J.; Moss, A.C. Vitamin D in inflammatory bowel disease: More than just a supplement. Curr. Opin. Gastroenterol. 2018, 34, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Kellermann, L.; Jensen, K.B.; Bergenheim, F.; Gubatan, J.; Chou, N.D.; Moss, A.; Nielsen, O.H. Mucosal vitamin D signaling in inflammatory bowel disease. Autoimmun. Rev. 2020, 19, 102672. [Google Scholar] [CrossRef] [PubMed]
- Gubatan, J.; Mehigan, G.A.; Villegas, F.; Mitsuhashi, S.; Longhi, M.S.; Malvar, G.; Csizmadia, E.; Robson, S.; Moss, A.C. Cathelicidin Mediates a Protective Role of Vitamin D in Ulcerative Colitis and Human Colonic Epithelial Cells. Inflamm. Bowel Dis. 2020, 26, 885–897. [Google Scholar] [CrossRef]
- Wang, T.T.; Dabbas, B.; Laperriere, D.; Bitton, A.J.; Soualhine, H.; Tavera-Mendoza, L.E.; Dionne, S.; Servant, M.J.; Bitton, A.; Seidman, E.G.; et al. Direct and indirect induction by 1,25-dihydroxyvitamin D3 of the NOD2/CARD15-defensin beta2 innate immune pathway defective in Crohn disease. J. Biol. Chem. 2010, 285, 2227–2231. [Google Scholar] [CrossRef] [Green Version]
- Jeng, L.; Yamshchikov, A.V.; Judd, S.E.; Blumberg, H.M.; Martin, G.S.; Ziegler, T.R.; Tangpricha, V. Alterations in vitamin D status and anti-microbial peptide levels in patients in the intensive care unit with sepsis. J. Transl. Med. 2009, 7, 28. [Google Scholar] [CrossRef] [Green Version]
- Hertting, O.; Holm, A.; Luthje, P.; Brauner, H.; Dyrdak, R.; Jonasson, A.F.; Wiklund, P.; Chromek, M.; Brauner, A. Vitamin D induction of the human antimicrobial Peptide cathelicidin in the urinary bladder. PLoS ONE 2010, 5, e15580. [Google Scholar] [CrossRef] [Green Version]
- Bergman, P.; Norlin, A.C.; Hansen, S.; Rekha, R.S.; Agerberth, B.; Björkhem-Bergman, L.; Ekström, L.; Lindh, J.D.; Andersson, J. Vitamin D3 supplementation in patients with frequent respiratory tract infections: A randomised and double-blind intervention study. BMJ Open 2012, 2. [Google Scholar] [CrossRef] [Green Version]
- Camargo, C.A., Jr.; Ganmaa, D.; Frazier, A.L.; Kirchberg, F.F.; Stuart, J.J.; Kleinman, K.; Sumberzul, N.; Rich-Edwards, J.W. Randomized trial of vitamin D supplementation and risk of acute respiratory infection in Mongolia. Pediatrics 2012, 130, e561–e567. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Zhang, H.; Wu, H.; Li, H.; Liu, L.; Guo, J.; Li, C.; Shih, D.Q.; Zhang, X. Protective role of 1,25(OH)2 vitamin D3 in the mucosal injury and epithelial barrier disruption in DSS-induced acute colitis in mice. BMC Gastroenterol. 2012, 12, 57. [Google Scholar] [CrossRef] [Green Version]
- Nicholson, I.; Dalzell, A.M.; El-Matary, W. Vitamin D as a therapy for colitis: A systematic review. J. Crohns. Colitis. 2012, 6, 405–411. [Google Scholar] [CrossRef] [Green Version]
- Huang, F.C.; Huang, S.C. Active vitamin D3 attenuates the severity of Salmonella colitis in mice by orchestrating innate immunity. Immun. Inflamm. Dis. 2021, 9, 481–491. [Google Scholar] [CrossRef]
- Huang, F.C. The differential effects of 1,25-dihydroxyvitamin D3 on Salmonella-induced interleukin-8 and human beta-defensin-2 in intestinal epithelial cells. Clin. Exp. Immunol. 2016. [CrossRef] [Green Version]
- Huang, F.C. Vitamin D differentially regulates Salmonella-induced intestine epithelial autophagy and interleukin-1beta expression. World J. Gastroenterol. 2016, 22, 10353–10363. [Google Scholar] [CrossRef]
- Coconnier, M.H.; Lievin, V.; Bernet-Camard, M.F.; Hudault, S.; Servin, A.L. Antibacterial effect of the adhering human Lactobacillus acidophilus strain LB. Antimicrob. Agents Chemother. 1997, 41, 1046–1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, S.J.; Okoko, B.; Martinez, E.; Gregorio, G.; Dans, L.F. Probiotics for treating infectious diarrhoea. Cochrane Database Syst. Rev. 2004, CD003048. [Google Scholar] [CrossRef]
- Huang, F.C.; Huang, S.C. The different effects of probiotics treatment on Salmonella-induced interleukin-8 response in intestinal epithelia cells via PI3K/Akt and NOD2 expression. Benef. Microbes 2016, 7, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.T.; Huang, F.C. Probiotics exert reciprocal effects on autophagy and interleukin-1beta expression in Salmonella-infected intestinal epithelial cells via autophagy-related 16L1 protein. Benef. Microbes 2019, 10, 913–922. [Google Scholar] [CrossRef]
- Barthel, M.; Hapfelmeier, S.; Quintanilla-Martinez, L.; Kremer, M.; Rohde, M.; Hogardt, M.; Pfeffer, K.; Rüssmann, H.; Hardt, W.D. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect. Immun. 2003, 71, 2839–2858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kammanadiminti, S.J.; Chadee, K. Suppression of NF-kappaB activation by Entamoeba histolytica in intestinal epithelial cells is mediated by heat shock protein 27. J. Biol. Chem. 2006, 281, 26112–26120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, F.C. Plasma membrane cholesterol plays a critical role in the Salmonella-induced anti-inflammatory response in intestinal epithelial cells. Cell Immunol. 2011, 271, 480–487. [Google Scholar] [CrossRef]
- Huang, F.C. Regulation of Salmonella flagellin-induced interleukin-8 in intestinal epithelial cells by muramyl dipeptide. Cell Immunol. 2012, 278, 1–9. [Google Scholar] [CrossRef]
- Khailova, L.; Frank, D.N.; Dominguez, J.A.; Wischmeyer, P.E. Probiotic administration reduces mortality and improves intestinal epithelial homeostasis in experimental sepsis. Anesthesiology 2013, 119, 166–177. [Google Scholar] [CrossRef] [Green Version]
- Jones, M.L.; Martoni, C.J.; Prakash, S. Oral supplementation with probiotic L. reuteri NCIMB 30242 increases mean circulating 25-hydroxyvitamin D: A post hoc analysis of a randomized controlled trial. J. Clin. Endocrinol. Metab. 2013, 98, 2944–2951. [Google Scholar] [CrossRef] [Green Version]
- Abboud, M.; Rizk, R.; AlAnouti, F.; Papandreou, D.; Haidar, S.; Mahboub, N. The Health Effects of Vitamin D and Probiotic Co-Supplementation: A Systematic Review of Randomized Controlled Trials. Nutrients 2020, 13, 111. [Google Scholar] [CrossRef]
- Wang, H.; Gong, J.; Wang, W.; Long, Y.; Fu, X.; Fu, Y.; Qian, W.; Hou, X. Are there any different effects of Bifidobacterium, Lactobacillus and Streptococcus on intestinal sensation, barrier function and intestinal immunity in PI-IBS mouse model? PLoS ONE 2014, 9, e90153. [Google Scholar] [CrossRef] [Green Version]
- O‘Mahony, L.; McCarthy, J.; Kelly, P.; Hurley, G.; Luo, F.; Chen, K.; O’Sullivan, G.C.; Kiely, B.; Collins, J.K.; Shanahan, F.; et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: Symptom responses and relationship to cytokine profiles. Gastroenterology 2005, 128, 541–551. [Google Scholar] [CrossRef]
- O‘Sullivan, M.A.; O‘Morain, C.A. Bacterial supplementation in the irritable bowel syndrome. A randomised double-blind placebo-controlled crossover study. Dig. Liver Dis. 2000, 32, 294–301. [Google Scholar] [CrossRef]
- Singh, N.; Arioli, S.; Wang, A.; Villa, C.R.; Jahani, R.; Song, Y.S.; Mora, D.; Guglielmetti, S.; Comelli, E.M. Impact of Bifidobacterium bifidum MIMBb75 on mouse intestinal microorganisms. FEMS Microbiol. Ecol. 2013, 85, 369–375. [Google Scholar] [CrossRef] [Green Version]
- Yun, B.; Song, M.; Park, D.J.; Oh, S. Beneficial Effect of Bifidobacterium longum ATCC 15707 on Survival Rate of Clostridium difficile Infection in Mice. Korean J. Food Sci. Anim. Resour. 2017, 37, 368–375. [Google Scholar] [CrossRef] [Green Version]
- Celiberto, L.S.; Bedani, R.; Dejani, N.N.; de Medeiros, A.I.; Zuanon, J.A.S.; Spolidorio, L.C.; Adorno, M.A.T.; Varesche, M.B.A.; Galvão, F.C.; Valentini, S.R.; et al. Effect of a probiotic beverage consumption (Enterococcus faecium CRL 183 and Bifidobacterium longum ATCC 15707) in rats with chemically induced colitis. PLoS ONE 2017, 12, e0175935. [Google Scholar] [CrossRef] [Green Version]
- Tannock, G.W.; Munro, K.; Harmsen, H.J.; Welling, G.W.; Smart, J.; Gopal, P.K. Analysis of the fecal microflora of human subjects consuming a probiotic product containing Lactobacillus rhamnosus DR20. Appl Environ. Microbiol. 2000, 66, 2578–2588. [Google Scholar] [CrossRef] [Green Version]
- Ghadimi, D.; de Vrese, M.; Heller, K.J.; Schrezenmeir, J. Lactic acid bacteria enhance autophagic ability of mononuclear phagocytes by increasing Th1 autophagy-promoting cytokine (IFN-gamma) and nitric oxide (NO) levels and reducing Th2 autophagy-restraining cytokines (IL-4 and IL-13) in response to Mycobacterium tuberculosis antigen. Int. Immunopharmacol. 2010, 10, 694–706. [Google Scholar] [CrossRef] [PubMed]
- Schlee, M.; Harder, J.; Koten, B.; Wehkamp, J.; Fellermann, K. Probiotic lactobacilli and VSL#3 induce enterocyte beta-defensin 2. Clin. Exp. Immunol. 2008, 151, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Schlee, M.; Wehkamp, J.; Altenhoefer, A.; Stange, E.F.; Fellermann, K. Induction of human beta-defensin 2 by the probiotic Escherichia coli Nissle 1917 is mediated through flagellin. Infect. Immun. 2007, 75, 2399–2407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salzman, N.H.; Hung, K.; Haribhai, D.; Chu, H.; Karlsson-Sjöberg, J.; Amir, E.; Teggatz, P.; Barman, M.; Hayward, M.; Eastwood, D.; et al. Enteric defensins are essential regulators of intestinal microbial ecology. Nat. Immunol. 2010, 11, 76–83. [Google Scholar] [CrossRef]
- Gong, J.; Xu, J.; Zhu, W.; Gao, X.; Li, N.; Li, J. Epithelial-specific blockade of MyD88-dependent pathway causes spontaneous small intestinal inflammation. Clin. Immunol. 2010, 136, 245–256. [Google Scholar] [CrossRef]
- Kong, J.; Zhang, Z.; Musch, M.W.; Ning, G.; Sun, J.; Hart, J.; Bissonnette, M.; Li, Y.C. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G208–G216. [Google Scholar] [CrossRef] [Green Version]
- Froicu, M.; Cantorna, M.T. Vitamin D and the vitamin D receptor are critical for control of the innate immune response to colonic injury. BMC Immunol. 2007, 8, 5. [Google Scholar] [CrossRef] [Green Version]
- Cross, H.S.; Nittke, T.; Kallay, E. Colonic vitamin D metabolism: Implications for the pathogenesis of inflammatory bowel disease and colorectal cancer. Mol. Cell Endocrinol. 2011, 347, 70–79. [Google Scholar] [CrossRef]
- Yoon, S.S.; Sun, J. Probiotics, nuclear receptor signaling, and anti-inflammatory pathways. Gastroenterol. Res. Pract. 2011, 2011, 971938. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Liao, A.P.; Xia, Y.; Li, Y.C.; Li, J.D.; Sartor, R.B.; Sun, J. Vitamin D receptor negatively regulates bacterial-stimulated NF-kappaB activity in intestine. Am. J. Pathol. 2010, 177, 686–697. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Yoon, S.; Zhang, Y.G.; Lu, R.; Xia, Y.; Wan, J.; Petrof, E.O.; Claud, E.C.; Chen, D.; Sun, J. Vitamin D receptor pathway is required for probiotic protection in colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G341–G349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, F.-C.; Huang, S.-C. The Cooperation of Bifidobacterium longum and Active Vitamin D3 on Innate Immunity in Salmonella Colitis Mice via Vitamin D Receptor. Microorganisms 2021, 9, 1804. https://doi.org/10.3390/microorganisms9091804
Huang F-C, Huang S-C. The Cooperation of Bifidobacterium longum and Active Vitamin D3 on Innate Immunity in Salmonella Colitis Mice via Vitamin D Receptor. Microorganisms. 2021; 9(9):1804. https://doi.org/10.3390/microorganisms9091804
Chicago/Turabian StyleHuang, Fu-Chen, and Shun-Chen Huang. 2021. "The Cooperation of Bifidobacterium longum and Active Vitamin D3 on Innate Immunity in Salmonella Colitis Mice via Vitamin D Receptor" Microorganisms 9, no. 9: 1804. https://doi.org/10.3390/microorganisms9091804
APA StyleHuang, F.-C., & Huang, S.-C. (2021). The Cooperation of Bifidobacterium longum and Active Vitamin D3 on Innate Immunity in Salmonella Colitis Mice via Vitamin D Receptor. Microorganisms, 9(9), 1804. https://doi.org/10.3390/microorganisms9091804





