Probiotic Endophytes for More Sustainable Banana Production
Abstract
:1. Introduction
2. Plant Microbiomes: The Origin of Plant Probiotic Bacteria
3. Synthetic Communities as Probiotic Bioinoculants
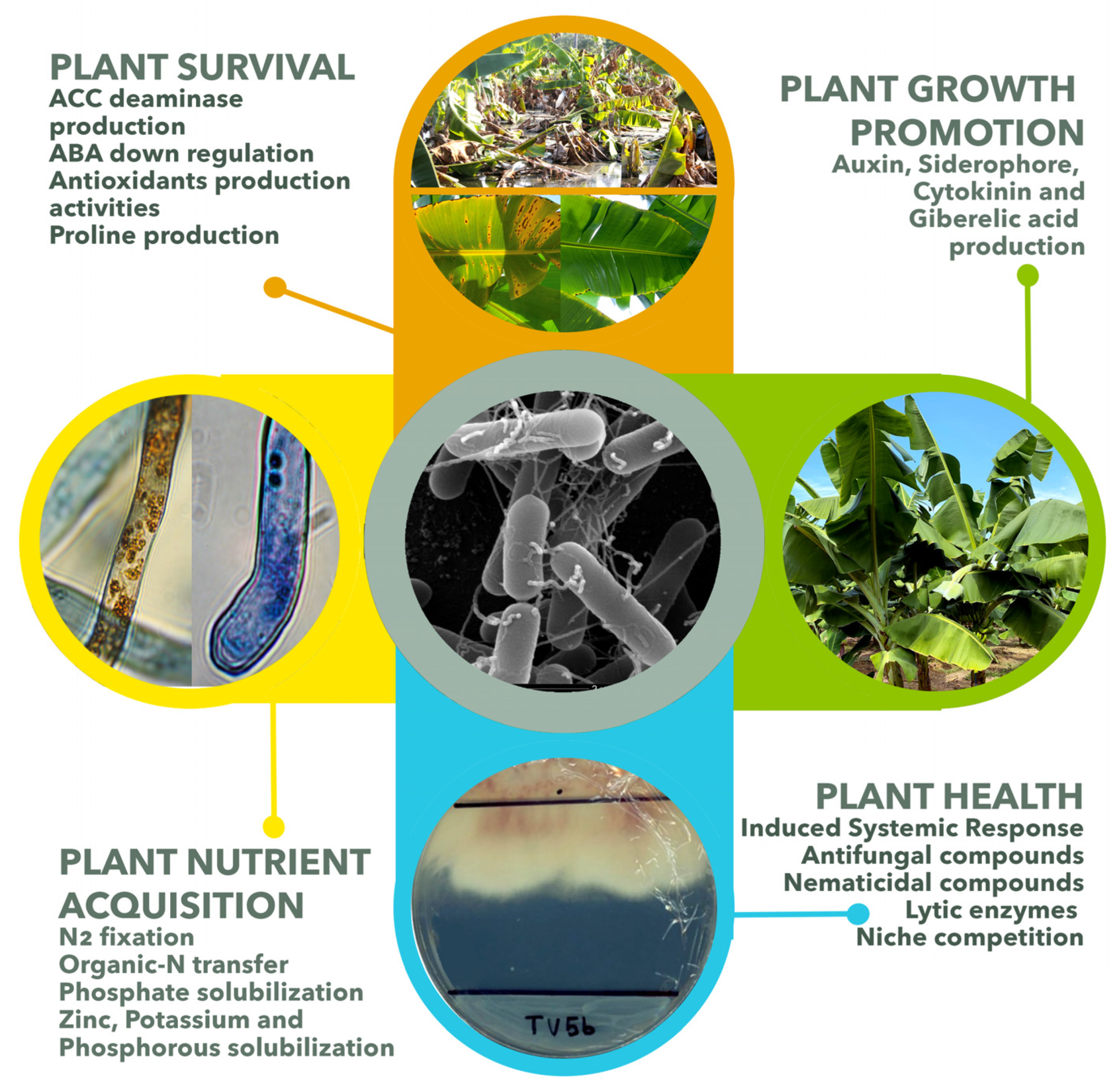
4. The Banana Endophytic Microbiome or Endophytome: History, Diversity, Functionality, and the Cry for Help Phenomena
4.1. Pioneer Studies of Banana Endophyte
4.2. The Banana Bacterial Endophytome
4.3. Fusarium Wilt Disease Shifts Endophytic Communities in Banana Plants
4.4. Banana Endophyte Probiotics for Black Sigatoka
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations, Teams on International Investment and Tropical fruits Trade and Market Division. Banana Market Review: Preliminary Results. 2019. Available online: http://www.fao.org/faostat/en/?#data/QC (accessed on 28 June 2021).
- Scott, G.J. A review of root, tuber and banana crops in developing countries: Past, present and future. Int. J. Food Sci. Technol. 2021, 56, 1093–1114. [Google Scholar] [CrossRef]
- Panigrahi, N.; Thompson, A.; Zubelzu, S.; Knox, J. Identifying opportunities to improve management of water stress in banana production. Sci. Hortic. 2020, 276, 109735. [Google Scholar] [CrossRef]
- Meya, A.; Ndakidemi, P.; Mtei, K.; Swennen, R.; Merckx, R. Optimizing soil fertility management strategies to enhance banana production in volcanic soils of the Northern Highlands, Tanzania. Agronomy 2020, 10, 289. [Google Scholar] [CrossRef] [Green Version]
- Aguilar-Barragan, A.; García-Torres, A.; Odriozola-Casas, O.; Macedo-Raygoza, G.; Ogura, T.; Manzo-Sánchez, G.; James, A.; Islas-Flores, I.; Beltran-García, M. Chemical management in fungicide sensivity of Mycosphaerella fijiensis collected from banana fields in México. Braz. J. Microbiol. 2014, 45, 359–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dita, M.; Barquero, M.; Heck, D.; Mizubuti, E.S.G.; Staver, C.P. Fusarium wilt of banana: Current knowledge on epidemiology and research needs toward sustainable disease management. Front. Plant Sci. 2018, 9, 1468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thangavelu, R.; Saraswathi, M.S.; Uma, S.; Loganathan, M.; Backiyarani, S.; Durai, P.; Raj, E.E.; Marimuthu, N.; Kannan, G.; Swennen, R. Identification of sources resistant to a virulent Fusarium wilt strain (VCG 0124) infecting Cavendish bananas. Sci. Rep. 2021, 11, 3183. [Google Scholar] [CrossRef]
- Raphael, L.; Recous, S.; Ozier-Lafontaine, H.; Sierra, J. Fate of a 15N-labeled urea pulse in heavily fertilized banana crops. Agronomy 2020, 10, 666. [Google Scholar] [CrossRef]
- Aryal, D.R.; Geissen, V.; Ponce-Mendoza, A.; Ramos-Reyes, R.; Becker, M. Water quality under intensive banana production and extensive pastureland in tropical Mexico. J. Soil. Sci. Plant Nutr. 2012, 175, 553–559. [Google Scholar] [CrossRef]
- White, J.F.; Chang, X.; Kingsley, K.L.; Zhang, Q.; Chiaranunt, P.; Micci, A.; Velazquez, F.; Elmore, M.; Crane, S.; Li, S.; et al. Endophytic bacteria in grass crop growth promotion and biostimulation. Grass Res. 2021, 1, 1–9. [Google Scholar] [CrossRef]
- Souza, R.; Ambrosini, A.; Passaglia, L.M. Plant growth-promoting bacteria as inoculants in agricultural soils. Genet. Mol. Biol. 2015, 38, 401–419. [Google Scholar] [CrossRef]
- Ambrosini, A.; de Souza, R.; Passaglia, L.M.P. Ecological role of bacterial inoculants and their potential impact on soil microbial diversity. Plant Soil. 2016, 400, 193–207. [Google Scholar] [CrossRef]
- White, J.F.; Kingsley, K.L.; Zhang, Q.; Verma, R.; Obi, N.; Dvinskikh, S.; Elmore, M.T.; Verma, S.K.; Gond, S.K.; Kowalski, K.P. Endophytic microbes and their potential applications in crop management. Pest. Manag. Sci. 2019, 10, 2558–2565. [Google Scholar] [CrossRef] [PubMed]
- Vandana, U.K.; Rajkumari, J.; Singha, L.P.; Satish, L.; Alavilli, H.; Sudheer, P.D.V.N.; Chauhan, S.; Ratnala, R.; Satturu, V.; Mazumder, P.B.; et al. The endophytic microbiome as a hotspot of synergistic interactions, with prospects of plant growth promotion. Biology 2021, 10, 101. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Sekhar, A.C. Live cell imaging reveals extensive intracellular cytoplasmic colonization of banana by normally non-cultivable endophytic bacteria. AoB Plants 2014, 6, plu002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, P.; Franco, C.M.M. Intracellular bacteria in Plants: Elucidation of abundant and diverse cytoplasmic bacteria in healthy plant cells using in vitro cell and callus cultures. Microorganisms 2021, 28, 269. [Google Scholar] [CrossRef] [PubMed]
- Beltran-Garcia, M.J.; White, J.F., Jr.; Prado, F.M.; Prieto, K.R.; Yamaguchi, L.F.; Torres, M.S.; Kato, M.J.; Medeiros, M.H.G.; Di Mascio, P. Nitrogen acquisition in Agave tequilana from degradation of endophytic bacteria. Sci. Rep. 2014, 4, 1–7. [Google Scholar] [CrossRef]
- Macedo-Raygoza, G.M.; Valdez, B.S.; Prado, F.M.; Prieto, K.R.; Yamaguchi, L.F.; Kato, M.J.; Canto-Canche, B.B.; Carrillo-Beltran, M.; Di Mascio, P.; White, J.F.; et al. Enterobacter cloacae, an endophyte that establishes a nutrient-transfer symbiosis with banana plants and protects against the black Sigatoka pathogen. Front. Microbiol. 2019, 10, 804. [Google Scholar] [CrossRef] [Green Version]
- Dini-Andreote, F. Endophytes: The second layer of plant defense. Trends Plant Sci. 2020, 25, 319–322. [Google Scholar] [CrossRef]
- Bradshaw, M.J.; Pane, A.M. Field inoculations of nitrogen fixing endophytes on turfgrass. Physiol. Mol. Plant Pathol. 2020, 112, 101557. [Google Scholar] [CrossRef]
- Gupta, S.; White, J.; Kulkarni, M. An outlook on current and future directions in Endophyte research. Editorial Note-Endophyte Special Issue (South African Journal of Botany). S Afr. J. Bot. 2020, 134, 1–2. [Google Scholar] [CrossRef]
- Babalola, O.O.; Fadiji, A.E.; Enagbonma, B.J.; Alori, E.T.; Ayilara, M.S.; Ayangbenro, A.S. The nexus between plant and plant microbiome: Tevelation of the networking strategies. Front. Microbiol. 2020, 11, 548037. [Google Scholar] [CrossRef]
- Ray, P.; Lakshmanan, V.; Labbé, J.L.; Craven, K.D. Microbe to Microbiome: A paradigm shift in the application of microorganisms for sustainable agriculture. Front. Microbiol. 2020, 11, 622926. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Guo, S.; Jousset, A.; Zhao, Q.; Wu, H.; Rong, L.; Kowalchuk, G.; Shen, Q. Bio-fertilizer application induces soil suppressiveness against Fusarium wilt disease by reshaping the soil microbiome. Soil. Biol. Biochem. 2017, 114, 238–247. [Google Scholar] [CrossRef]
- Liu, H.; Carvalhais, L.C.; Crawford, M.; Singh, E.; Dennis, P.G.; Pieterse, C.M.J.; Schenk, P.M. Inner plant values: Diversity, colonization and benefits from endophytic bacteria. Front. Microbiol. 2017, 8, 2552. [Google Scholar] [CrossRef]
- White, J.F.; Kingsley, K.L.; Verma, S.K.; Kowalski, K.P. Rhizophagy cycle: An oxidative process in plants for nutrient extraction from symbiotic microbes. Microorganisms 2018, 6, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardoim, P.R.; van Overbeek, L.S.; Berg, G.; Pirttilä, A.M.; Compant, S.; Campisano, A.; Döring, M.; Sessitsch, A. The hidden world within plants: Ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293–320. [Google Scholar] [CrossRef] [Green Version]
- Santoyo, G.; Moreno-Hagelsiebb, G.; Orozco-Mosqueda, M.C.; Glick, B.R. Plant growth-promoting bacterial endophytes. Microbiol. Res. 2016, 183, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Vorholt, J.A. Microbial life in the phyllosphere. Nat. Rev. Microbiol. 2012, 10, 828–840. [Google Scholar] [CrossRef]
- Thomas, P.; Swarna, G.K.; Patil, P.; Rawal, R. Ubiquitous presence of normally non-culturable endophytic bacteria in field shoot-tips of banana and their gradual activation to quiescent cultivable form in tissue cultures. Plant Cell Tiss. Organ Cult. 2008, 93, 39–54. [Google Scholar] [CrossRef]
- Thomas, P.; Swarna, G.K.; Roy, P.K.; Patil, P. Identification of culturable and originally non-culturable endophytic bacteria isolated from shoot tip cultures of banana cv. Grand Naine. Plant Cell Tiss. Organ Cult. 2008, 93, 55–63. [Google Scholar] [CrossRef]
- Kaushal, M.; Mahuku, G.; Swennen, R. Metagenomic insights of the root colonizing microbiome associated with symptomatic and non-symptomatic bananas in Fusarium wilt infected fields. Plants 2020, 9, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabanás, G.-L.C.; Fernández-González, A.J.; Cardoni, M.; Valverde-Corredor, A.; López-Cepero, J.; Fernández-López, M.; Mercado-Blanco, J. The Banana root endophytome: Differences between mother plants and suckers and evaluation of selected bacteria to control Fusarium oxysporum f.sp. cubense. J. Fungi 2021, 7, 194. [Google Scholar] [CrossRef]
- Anguita-Maeso, M.; Olivares-García, C.; Haro, C.; Imperial, J.; Navas-Cortés, J.A.; Landa, B.B. Culture-dependent and culture-independent characterization of the olive xylem microbiota: Effect of sap extraction methods. Front. Plant Sci. 2020, 10, 700–711. [Google Scholar] [CrossRef] [Green Version]
- Aghdam, S.A.; Brown, A.M.V. Deep learning approaches for natural product discovery from plant endophytic microbiomes. Environ. Microbiome 2021, 16, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Zhai, Y.; Deng, Q.; Tan, H.; Cao, L. Illumina-Based sequencing analysis directed selection for actinobacterial probiotic candidates for banana plants. Probiotics Antimicrob. Proteins 2018, 10, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Bücking, H.; Gonzalez Hernandez, J.L.; Subramanian, S. Single-Cell RNA sequencing of plant-associated bacterial communities. Front. Microbiol. 2019, 10, 2452. [Google Scholar] [CrossRef]
- Liu, C.; Dong, Y.; Hou, L.; Deng, N.; Jiao, R. Acidobacteria community responses to nitrogen dose and form in chinese fir plantations in southern China. Curr. Microbiol. 2017, 74, 396–403. [Google Scholar] [CrossRef]
- Fadiji, A.E.; Ayangbenro, A.S.; Babalola, O.O. Metagenomic profiling of the community structure, diversity, and nutrient pathways of bacterial endophytes in maize plant. Antonie Van Leeuwenhoek 2020, 113, 1559–1571. [Google Scholar] [CrossRef] [PubMed]
- Reva, O.N.; Swanevelder, D.Z.H.; Mwita, L.A.; Mwakilili, A.D.; Muzondiwa, D.; Joubert, M.; Chan, W.Y.; Lutz, S.; Ahrens, C.H.; Avdeeva, L.V.; et al. Genetic, epigenetic and phenotypic diversity of four Bacillus velezensis strains used for plant protection or as probiotics. Front. Microbiol. 2019, 10, 2610. [Google Scholar] [CrossRef] [Green Version]
- Mastan, A.; Rane, D.; Dastager, S.G.; Vivek-Babu, C.S. Plant probiotic bacterial endophyte, Alcaligenes faecalis, modulates plant growth and forskolin biosynthesis in Coleus forskohlii. Probiotics Antimicrob. Proteins 2020, 12, 481–493. [Google Scholar] [CrossRef]
- Jayakumar, A.; Padmakumar, P.; Nair, I.; Krishnankutty, R. Drought tolerant bacterial endophytes with potential plant probiotic effects from Ananas comosus. Biologia 2020, 75, 1769–1778. [Google Scholar] [CrossRef]
- Cueva-Yesquén, L.G.; Goulart, M.C.; Attili de Angelis, D.; Nopper Alves, M.; Fantinatti-Garboggini, F. Multiple plant growth-promotion traits in Endophytic Bacteria retrieved in the vegetative stage from passion flower. Front. Plant Sci. 2021, 11, 2282. [Google Scholar] [CrossRef]
- Saha, C.; Mukherjee, G.; Agarwal-Banka, P.; Seal, A. A consortium of non-rhizobial endophytic microbes from Typha angustifolia functions as probiotic in rice and improves nitrogen metabolism. Plant Biol. 2016, 18, 938–946. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Rodríguez, J.; De la Mora-Amutio, M.; Plascencia-Correa, L.A.; Audelo-Regalado, E.; Guardado, F.R.; Hernández-Sánchez, E.; Peña-Ramírez, Y.J.; Escalante, A.; Beltrán-García, M.J.; Ogura, T. Cultivable endophytic bacteria from leaf bases of Agave tequilana and their role as plant growth promoters. Braz. J. Microbiol. 2015, 45, 1333–1339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Rodriguez, A.; Macedo-Raygoza, G.; Huerta, A.; Reyes-Sepulveda, I.; Lozano-Lopez, J.; García-Ochoa, E.; Fierro-Kong, L.; Medeiros, M.; Di Mascio, P.; White, J.; et al. Agave seed endophytes: Ecology and impacts on root architecture, nutrient acquisition, and cold stress tolerance. Seed Endophytes. Springer 2019, 5, 139–170. [Google Scholar]
- Kazerooni, E.A.; Maharachchikumbura, S.S.N.; Adhikari, A.; Al-Sadi, A.M.; Kang, S.M.; Kim, L.R.; Lee, I.J. Rhizospheric Bacillus amyloliquefaciens protects Capsicum annuum cv. Geumsugangsan from multiple abiotic stresses via multifarious plant growth-promoting attributes. Front. Plant Sci. 2021, 12, 12. [Google Scholar] [CrossRef]
- Orozco-Mosqueda, M.D.C.; Glick, B.R.; Santoyo, G. ACC deaminase in plant growth-promoting bacteria (PGPB): An efficient mechanism to counter salt stress in crops. Microbiol. Res. 2020, 235, 126439. [Google Scholar] [CrossRef]
- Orozco-Mosqueda, M.C.; Flores, A.; Rojas-Sánchez, B.; Urtis-Flores, C.A.; Morales-Cedeño, L.R.; Valencia-Marin, M.F.; Chávez-Avila, S.; Rojas-Solis, D.; Santoyo, G. Plant growth-promoting bacteria as bioinoculants: Attributes and challenges for sustainable crop improvement. Agronomy 2021, 11, 50. [Google Scholar] [CrossRef]
- Adhikari, P.; Pandey, A. Bioprospecting plant growth promoting endophytic bacteria isolated from Himalayan yew (Taxus wallichiana Zucc.). Microbiol. Res. 2020, 239, 126536. [Google Scholar] [CrossRef]
- Chen, L.; Wu, Y.D.; Chong, X.Y.; Xin, Q.H.; Wang, D.X.; Bian, K. Seed-borne endophytic Bacillus velezensis LHSB1 mediate the biocontrol of peanut stem rot caused by Sclerotium rolfsii. J. Appl. Microbiol. 2020, 128, 803–813. [Google Scholar] [CrossRef]
- Zheng, T.; Liu, L.; Nie, Q.; Hsiang, T.; Sun, Z.; Zhou, Y. Isolation, identification and biocontrol mechanisms of endophytic bacterium D61-A from Fraxinus hupehensis against Rhizoctonia solani. Biological. Control. 2021, 158, 104621. [Google Scholar] [CrossRef]
- López, S.M.Y.; Pastorino, G.N.; Balatti, P.A. Volatile organic compounds profile synthesized and released by endophytes of tomato (Solanum lycopersici L.) and their antagonistic role. Arch. Microbiol. 2021, 203, 1383–1397. [Google Scholar] [CrossRef]
- Merino-Martin, L.; Stokes, A.; Gweon, H.; Moragues, L.; Staunton, S.; Plassard, C.; Oliver, A.; Le Bissonnais, Y.; Griffiths, R. Interacting effects of land use type, microbes and plant traits on soil aggregate stability. Soil Biol. Biochem. 2021, 154, 108072. [Google Scholar] [CrossRef]
- Du, J.; Li, Y.; Yin, Z.; Wang, H.; Zhang, X.; Ding, X. High-throughput customization of plant microbiomes for sustainable agriculture. Front. Plant Sci. 2020, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, P.; Rothballer, M.; Paul Chowdhury, S.; Nussbaumer, T.; Gutjahr, C.; Falter-Braun, P. Systems biology of plant-microbiome interactions. Mol. Plant 2019, 12, 804–821. [Google Scholar] [CrossRef] [Green Version]
- De Souza, R.S.C.; Armanhi, J.S.L.; Arruda, P. From microbiome to traits: Designing synthetic microbial communities for improved crop resiliency. Front. Plant Sci. 2020, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Saad, M.; Eida, A.A.; Hirt, H. Tailoring plant-associated microbial inoculants in agricultura—A roadmap for successful application. J. Exp. Bot. 2020, 71, 3878–3901. [Google Scholar] [CrossRef] [Green Version]
- Ke, J.; Wang, B.; Yoshikuni, Y. Microbiome engineering: Synthetic biology of plant-associated microbiomes in sustainable agriculture. Trends Biotechnol. 2021, 39, 244–261. [Google Scholar] [CrossRef]
- Marín, O.; González, B.; Poupin, M.J. From microbial dynamics to functionality in the rhizosphere: A systematic review of the opportunities with synthetic microbial communities. Front. Plant Sci. 2021, 12, 12. [Google Scholar] [CrossRef]
- Ma, K.W.; Niu, Y.; Jia, Y.; Ordon, J.; Copeland, C.; Emonet, A.; Geldner, N.; Guan, R.; Stolze, S.C.; Nakagami, H.; et al. Coordination of microbe-host homeostasis by crosstalk with plant innate immunity. Nat. Plants 2021, 7, 814–825. [Google Scholar] [CrossRef]
- Yin, J.; Yu, Y.; Zhang, Z.; Chen, L.; Ruan, L. Enrichment of potentially beneficial bacteria from the consistent microbial community confers canker resistance on tomato. Microbiol. Res. 2020, 234, 126446. [Google Scholar] [CrossRef] [PubMed]
- Carrión, V.J.; Perez-Jaramillo, J.; Cordovez, V.; Tracanna, V.; de Hollander, M.; Ruiz-Buck, D.; Mendes, L.W.; van Ijcken, W.F.J.; Gomez-Exposito, R.; Elsayed, S.S.; et al. Pathogen-induced activation of disease-suppressive functions in the endophytic root microbiome. Science 2019, 366, 606–612. [Google Scholar] [CrossRef]
- Niu, B.; Paulson, J.; Zheng, X.; Kolter, R. Simplified and representative bacterial community of maize roots. Proc. Natl. Acad. Sci. USA 2017, 114, E2450–E2459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finkel, O.M.; Salas-González, I.; Castrillo, G.; Conway, J.M.; Law, T.F.; Teixeira, P.J.P.L.; Wilson, E.D.; Fitzpatrick, C.R.; Jones, C.D.; Dangl, J.L. A single bacterial genus maintains root growth in a complex microbiome. Nature 2020, 587, 103–108. [Google Scholar] [CrossRef]
- Wu, L.; Yang, B.; Li, M.; Chen, J.; Xiao, Z.; Wu, H.; Tong, Q.; Luo, X.; Lin, W. Modification of rhizosphere bacterial community structure and functional potentials to control Pseudostellaria heterophylla replant disease. Plant Dis. 2020, 104, 25–34. [Google Scholar] [CrossRef]
- Durán, P.; Thiergart, T.; Garrido-Oter, R.; Agler, M.; Kemenm, E.; Schulze-Lefert, P.; Hacquard, S. Microbial interkingdom interactions in roots promote Arabidopsis survival. Cell 2018, 175, 973–983. [Google Scholar] [CrossRef]
- Knoth, J.; Kim, S.H.; Ettl, G.; Doty, S. Biological nitrogen fixation and biomass accumulation within poplar clones as a result of inoculations with diazotrophic endophyte consortia. New Phytol. 2014, 201, 599–609. [Google Scholar] [CrossRef]
- Finkel, O.M.; Salas-González, I.; Castrillo, G.; Spaepen, S.; Law, T.F.; Teixeira, P.J.P.L.; Jones, C.D.; Dangl, J.L. The effects of soil phosphorus content on plant microbiota are driven by the plant phosphate starvation response. PLoS Biol. 2019, 17, e3000534. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Liu, Y.X.; Zhang, N.; Hu, B.; Jin, T.; Xu, H.; Qin, Y.; Yan, P.; Zhang, X.; Guo, X.; et al. NRT1.1B is associated with root microbiota composition and nitrogen use in field-grown rice. Nat. Biotechnol. 2019, 37, 676–684. [Google Scholar] [CrossRef]
- Brown, K.B.; Hyde, K.; Guest, D. Preliminary studies on endophytic fungal communities of Musa acuminata species complex in Hong Kong and Australia. Fungal Divers. 1998, 1, 27–51. [Google Scholar]
- Pereira, J.; Vieira, M.C.; Azevedo, J. Endophytic fungi from Musa acuminata and their reintroduction into axenic plants. World J. Microbiol. Biotechnol. 1999, 15, 37–40. [Google Scholar] [CrossRef]
- Sikora, R.A.; Schuster, R.F.; Griesbach, M. Improved plant health through biological enhancement of banana planting material with mutualistic endophytes. Acta Hortic. 2000, 540, 409–413. [Google Scholar] [CrossRef]
- Martinez, L.; Caballero-Mellado, J.; Orozco, J.; Martínez-Romero, E. Diazotrophic bacteria associated with banana (Musa spp.). Plant Soil. 2003, 257, 35–47. [Google Scholar] [CrossRef]
- Cao, L.; Qiu, Z.; You, J.; Tan, H.; Zhou, S. Isolation and characterization of endophytic Streptomycete antagonists of Fusarium wilt pathogen from surface-sterilized banana roots. FEMS Microbiol. Lett. 2006, 247, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Soly, T.A. Endophytic bacteria associated with growing shoot tips of banana (Musa sp.) cv. Grand Naine and the affinity of endophytes to the host. Microb. Ecol. 2009, 58, 952–964. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Reddy, K. Microscopic elucidation of abundant endophytic bacteria colonizing the cell wall-plasma membrane peri-space in the shoot-tip tissue of banana. AoB Plants 2013, 5, 5. [Google Scholar] [CrossRef] [Green Version]
- Thomas, P.; Sekhar, A.C. Effects due to rhizospheric soil application of an antagonistic bacterial endophyte on native bacterial community and its survival in soil: A case study with Pseudomonas aeruginosa from banana. Front. Microbiol. 2016, 7, 7. [Google Scholar] [CrossRef] [Green Version]
- Arango-Isaza, R.E.; Diaz-Trujillo, C.; Dhillon, B.; Aerts, A.; Carlier, J.; Crane, C.F.; de Jong, T.; de Vries, I.; Dietrich, R.; Farmer, A.D.; et al. Combating a global threat to a clonal crop: Banana Black Sigatoka pathogen Pseudocercospora fijiensis (Synonym Mycosphaerella fijiensis) genomes reveal clues for disease control. PLoS Genet. 2016, 12, 12. [Google Scholar] [CrossRef]
- Nakkeeran, S.; Rajamanickam, S.; Saravanan, R.; Vanthana, M.; Soorianathasundaram, K. Bacterial endophytome-mediated resistance in banana for the management of Fusarium wilt. Biotech 2021, 11, 11. [Google Scholar]
- Thomas, P.; Sekhar, A.C. Cultivation Versus Molecular Analysis of Banana (Musa sp.) Shoot-Tip tissue reveals enormous diversity of normally uncultivable endophytic bacteria. Microb. Ecol. 2017, 73, 885–899. [Google Scholar] [CrossRef]
- Rossmann, B.; Müller, H.; Smalla, K.; Mpiira, S.; Tumuhairwe, J.B.; Staver, C.; Berg, G. Banana-associated microbial communities in Uganda are highly diverse but dominated by Enterobacteriaceae. Appl. Environ. Microbiol. 2012, 78, 4933–4941. [Google Scholar] [CrossRef] [Green Version]
- Ngamau, C.N.; Matiru, V.N.; Tani, A.; Murthuri, C.W. Isolation and identification of endophytic bacteria of bananas (Musa spp.) in Kenya and their potential as biofertilizers for sustainable banana production. Afr. J. Microbiol. Res. 2012, 6, 6414–6422. [Google Scholar]
- Souza, S.; Xavier, A.; Costa, M.; Cardoso, A.; Pereira, M.; Nietsche, S. Endophytic bacterial diversity in banana ‘Prata Anã’ (Musa spp.) roots. Genet. Mol. Biol. 2013, 36, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Andrade, L.F.; de Souza, G.L.; Nietsche, S.; Xavier, A.A.; Costa, M.R.; Cardoso, A.M.; Pereira, M.C.; Pereira, D.F. Analysis of the abilities of endophytic bacteria associated with banana tree roots to promote plant growth. J. Microbiol. 2014, 52, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.; Nietsche, S.; Xavier, A.; Souza, S.; Costa, M.; Duarte, A. Characterization and activity of endophytic bacteria from ‘Prata Anã’ banana crop (Musa sp., AAB). Rev. Ceres 2018, 65, 381–387. [Google Scholar] [CrossRef] [Green Version]
- Marcano, I.E.; Díaz-Alcántara, C.A.; Urbano, B.; González-Andrés, F. Assessment of bacterial populations associated with banana tree roots and development of successful plant probiotics for banana crop. Soil Biol. Biochem. 2016, 99, 1–20. [Google Scholar] [CrossRef]
- Karthik, M.; Periyasamy, P.; Ramasamy, K.; Murugaiyan, S. Endophytic bacteria associated with banana cultivars and their inoculation effect on plant growth. J. Hortic. Sci. Biotechnol. 2017, 92, 1–9. [Google Scholar] [CrossRef]
- Beltran-Garcia, M.J.; Martínez-Rodríguez, A.; Olmos-Arriaga, I.; Valdes-Salas, B.; Di Mascio, P.; White, J. Nitrogen fertilization and stress factors drive shifts in microbial diversity in soils and plants. Symbiosis 2021, 1, 1–12. [Google Scholar]
- Fan, K.; Delgado-Baquerizo, M.; Guo, X.; Wang, D.; Wu, Y.; Zhu, M.; Yu, W.; Yao, H.; Zhu, Y.; Chu, H. Suppressed N fixation and diazotrophs after four decades of fertilization. Microbiome 2019, 7, 7. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, A.; Tan, H.; Cao, L.; Zhang, R. Engineering banana endosphere microbiome to improve Fusarium wilt resistance in banana. Microbiome 2019, 7, 7. [Google Scholar] [CrossRef]
- Wagg, C.; Schlaeppi, K.; Banerjee, S.; Kuramae, E.; Van der Heijden, M. Fungal-bacterial diversity and microbiome complexity predict ecosystem functioning. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Banerjee, S.; Schlaeppi, K.; van der Heijden, M.G.A. Keystone taxa as drivers of microbiome structure and functioning. Nat. Rev. Microbiol. 2018, 16, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Walder, F.; Büchi, L.; Meyer, M.; Held, A.Y.; Gattinger, A.; Keller, T.; Charles, R.; van der Heijden, M.G.A. Agricultural intensification reduces microbial network complexity and the abundance of keystone taxa in roots. ISME J. 2019, 13, 1722–1736. [Google Scholar] [CrossRef] [Green Version]
- Yin, C.; Casa Vargas, J.M.; Schlatter, D.C.; Hagerty, C.H.; Hulbert, S.H.; Paulitz, T.C. Rhizosphere community selection reveals bacteria associated with reduced root disease. Microbiome 2021, 9, 86. [Google Scholar] [CrossRef]
- Rolfe, S.A.; Griffiths, J.; Ton, J. Crying out for help with root exudates: Adaptive mechanisms by which stressed plants assemble health-promoting soil microbiomes. Curr. Opin. Microbiol. 2019, 49, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lambers, H. Root-released organic anions in response to low phosphorus availability: Recent progress, challenges and future perspectives. Plant Soil. 2020, 447, 135–156. [Google Scholar] [CrossRef]
- Rudrappa, T.; Czymmek, K.J.; Paré, P.W.; Bais, H.P. Root-secreted malic acid recruits beneficial soil bacteria. Plant Physiol. 2008, 148, 1547–1556. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Li, J.; Carvalhais, L.C.; Percy, C.D.; Prakash Verma, J.; Schenk, P.M.; Singh, B.K. Evidence for the plant recruitment of beneficial microbes to suppress soil-borne pathogens. New Phytol. 2021, 229, 2873–2885. [Google Scholar] [CrossRef] [PubMed]
- Köberl, M.; Dita, M.; Martinuz, A.; Staver, C.; Berg, G. Members of Gammaproteobacteria as indicator species of healthy banana plants on Fusarium wilt-infested fields in Central America. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Lu, N.; Zongzhuan, S.; Chengyuan, T.; Yannan, O.; Beibei, W.; Yunze, R.; Rong, L.; Qirong, S. The characteristics of culturable bacterial community in soils and tissue parts of banana. Nanjing Nong Ye Da Xue Xue Bao 2019, 42, 1088–1097. [Google Scholar]
- Sang, M.K.; Jeong, J.J.; Kim, J.; Kim, K.D. Growth promotion and root colonisation in pepper plants by phosphate-solubilising Chryseobacterium sp. strain ISE14 that suppresses Phytophthora blight. Ann. Appl. Biol. 2018, 172, 208–223. [Google Scholar] [CrossRef]
- Alsultan, W.; Vadamalai, G.; Ahmad, K.; Halimi, M.; Al-Sadi, A.; Rashed, O.; Mohd Jaaffar, A.K.; Nasehi, A. Isolation, identification and characterization of endophytic bacteria antagonistic to Phytophthora palmivora causing black pod of cocoa in Malaysia. Eur. J. Plant Pathol. 2019, 155, 1077–1091. [Google Scholar] [CrossRef]
- Jasrotia, S.; Salgotra, R.K.; Sharma, M. Efficacy of bioinoculants to control of bacterial and fungal diseases of rice (Oryza sativa L.) in northwestern Himalaya. Braz. J. Microbiol. 2021, 52, 687–704. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Su, G.; Li, M.; Wang, B.; Lin, R.; Yang, Y.; Wei, T.; Zhou, B.; Gao, Z. Distribution of bacterial endophytes in the non-lesion tissues of potato and their response to potato common scab. Front. Microbiol. 2021, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- De Lima, D.R.M.; Dos Santos, I.B.; Oliveira, J.T.C.; da Costa, D.P.; de Queiroz, J.V.J.; Romagnoli, E.M.; Andreote, F.D.; Freire, F.J.; Kuklinsky-Sobral, J. Genetic diversity of N-fixing and plant growth-promoting bacterial community in different sugarcane genotypes, association habitat and phenological phase of the crop. Arch. Microbiol. 2021, 203, 1089–1105. [Google Scholar] [CrossRef]
- Korshunova, T.Y.; Bakaeva, M.D.; Kuzina, E.V.; Rafikova, G.F.; Chetverikov, S.P.; Chetverikova, D.V.; Loginov, O.N. Role of bacteria of the genus Pseudomonas in the sustainable development of agricultural systems and environmental protection (Review). J. Appl. Microbiol. Biochem. 2021, 57, 281–296. [Google Scholar] [CrossRef]
- Gang, S.; Sharma, S.; Saraf, M.; Buck, M.; Schumacher, J. Bacterial indole-3-acetic acid influences soil nitrogen acquisition in barley and chickpea. Plants 2021, 10, 780. [Google Scholar] [CrossRef]
- Köberl, M.; Dita, M.; Nimusiima, J.; Tumuhairwe, J.; Kubiriba, J.; Staver, C.; Berg, G. The banana microbiome: Stability and potential health indicators. Acta Hortic. 2018, 1196, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Oljira, A.M.; Hussain, T.; Waghmode, T.R.; Zhao, H.; Sun, H.; Liu, X.; Wang, X.; Liu, B. Trichoderma enhances net photosynthesis, water use efficiency, and growth of wheat (Triticum aestivum L.) under salt stress. Microorganisms 2020, 8, 1565. [Google Scholar] [CrossRef]
- Rizaludin, M.S.; Stopnisek, N.; Raaijmakers, J.; Garbeva, P. The chemistry of stress: Understanding the ‘Cry for Help’ of plant roots. Metabolites 2021, 11, 357. [Google Scholar] [CrossRef]
- Chong, P.; Essoh, J.N.; Arango-Isaza, R.E.; Keizer, P.; Stergiopoulos, I.; Seidl, M.F.; Guzman, M.; Sandoval, J.; Verweij, P.E.; Scalliet, G.; et al. A world-wide analysis of reduced sensitivity to DMI fungicides in the banana pathogen Pseudocercospora fijiensis. Pest. Manag. Sci. 2021, 77, 3273–3288. [Google Scholar] [CrossRef]
- Beltrán-García, M.J.; Prado, F.M.; Oliveira, M.S.; Ortiz-Mendoza, D.; Scalfo, A.C.; Pessoa, A., Jr.; Medeiros, M.H.; White, J.F.; Di Mascio, P. Singlet molecular oxygen generation by light-activated DHN-melanin of the fungal pathogen Mycosphaerella fijiensis in black Sigatoka disease of bananas. PLoS ONE 2014, 9, e091616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, P.; Eskerc, P.; Umaña, G. Incorporation of microorganisms to reduce chemical fungicide usage in black Sigatoka control programs in Costa Rica by use of biological fungicides. Crop Prod. 2021, 146, 105657. [Google Scholar] [CrossRef]
- Marcano, I.E.; Díaz-Alcántara, C.A.; Seco, V.; Urbano, B.; González-Andrés, F. Induced Systemic Resistance Could Explain the Reduction in the Incidence of Black Sigatoka (Mycosphaerella fijiensis) in Banana Plants Inoculated with Bacteria Isolated from Banana Tree Roots in the Dominican Republic. In Bacteria Isolated from Banana Tree Roots in the Dominican Republic; González-Andrés, F., James, E., Eds.; Biological Nitrogen Fixation and Beneficial Plant-Microbe Interaction; Springer: Cham, Switzerland, 2016; pp. 155–170. [Google Scholar]
- Beltrán-García, M.J.; Manzo-Sanchez, G.; Guzmán-González, S.; Arias-Castro, C.; Rodríguez-Mendiola, M.; Avila-Miranda, M.; Ogura, T. Oxidative stress response of Mycosphaerella fijiensis, the causal agent of black leaf streak disease in banana plants, to hydrogen peroxide and paraquat. Can. J. Microbiol. 2009, 55, 887–894. [Google Scholar] [CrossRef]
- Beltran-Garcia, M.J.; Martinez-Rodriguez, A.; Macedo-Raygoza, G.M.; Ortiz-Mendoza, D.; Martinez-Molina, C.; Villalobos-Santana, G. Cepas Bacterianas, Mezcla Probiótica, Nutriente y Método Para la Producción Agrícola. Patent Pending Application PCT/Mx2021/000006 and MX/a/2021/002192, 26 February 2021. [Google Scholar]
- Lima, A.S.; Prieto, K.R.; Santos, C.S.; Paula-Valerio, H.; Garcia-Ochoa, E.Y.; Huerta-Robles, A.; Beltran-Garcia, M.J.; Di Mascio, P.; Bertotti, M. In-vivo electrochemical monitoring of H2O2 production induced by root-inoculated endophytic bacteria in Agave tequilana leaves. Biosens. Bioelectron. 2018, 99, 108–114. [Google Scholar] [CrossRef]
- Costa-Júnior, P.S.P.; Cardoso, F.P.; Martins, A.D.; Teixeira-Buttrós, V.H.; Pasqual, M.; Dias, D.R.; Schwan, R.F.; Dória, J. Endophytic bacteria of garlic roots promote growth of micropropagated meristems. Microbiol Res. 2020, 241, 126585. [Google Scholar] [CrossRef] [PubMed]
- Shastry, R.; Welch, M.; Ravishankar, R.; Ghate, S.; Sandeep, K.; Pd, R. The whole-genome sequence analysis of Enterobacter cloacae strain Ghats1: Insights into endophytic lifestyle-associated genomic adaptations. Arch. Microbiol. 2020, 202, 1571–1579. [Google Scholar] [CrossRef]
- Paungfoo-Lonhienne, C.; Rentsch, D.; Robatzek, S.; Webb, R.I.; Sagulenko, E.; Näsholm, T.; Schmidt, S.; Lonhienne, T.G. Turning the table: Plants consume microbes as a source of nutrients. PLoS ONE 2010, 5, e11915. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beltran-Garcia, M.J.; Martinez-Rodriguez, A.; Olmos-Arriaga, I.; Valdez-Salas, B.; Chavez-Castrillon, Y.Y.; Di Mascio, P.; White, J.F. Probiotic Endophytes for More Sustainable Banana Production. Microorganisms 2021, 9, 1805. https://doi.org/10.3390/microorganisms9091805
Beltran-Garcia MJ, Martinez-Rodriguez A, Olmos-Arriaga I, Valdez-Salas B, Chavez-Castrillon YY, Di Mascio P, White JF. Probiotic Endophytes for More Sustainable Banana Production. Microorganisms. 2021; 9(9):1805. https://doi.org/10.3390/microorganisms9091805
Chicago/Turabian StyleBeltran-Garcia, Miguel J., America Martinez-Rodriguez, Ileana Olmos-Arriaga, Benjamin Valdez-Salas, Yur Y. Chavez-Castrillon, Paolo Di Mascio, and James F. White. 2021. "Probiotic Endophytes for More Sustainable Banana Production" Microorganisms 9, no. 9: 1805. https://doi.org/10.3390/microorganisms9091805
APA StyleBeltran-Garcia, M. J., Martinez-Rodriguez, A., Olmos-Arriaga, I., Valdez-Salas, B., Chavez-Castrillon, Y. Y., Di Mascio, P., & White, J. F. (2021). Probiotic Endophytes for More Sustainable Banana Production. Microorganisms, 9(9), 1805. https://doi.org/10.3390/microorganisms9091805







