Bacillus licheniformis and Bacillus subtilis, Probiotics That Induce the Formation of Macrophage Extracellular Traps
Abstract
1. Introduction
2. Materials and Methods
2.1. J774A.1 Macrophage Culture
2.2. Cultivation of Bacillus licheniformis and Bacillus subtilis
2.3. Obtaining Intracellular CFU and Cell Viability of Infected Macrophages
2.4. Analysis of Cellular Alterations and Observation of the Formation of Extracellular Traps
2.5. Determination of ROS
2.6. Quantification of Extracellular DNA
2.7. Inhibitory Activity of Bacillus-Induced METs against Staphylococcus aureus
2.8. Statistical Analyses
3. Results
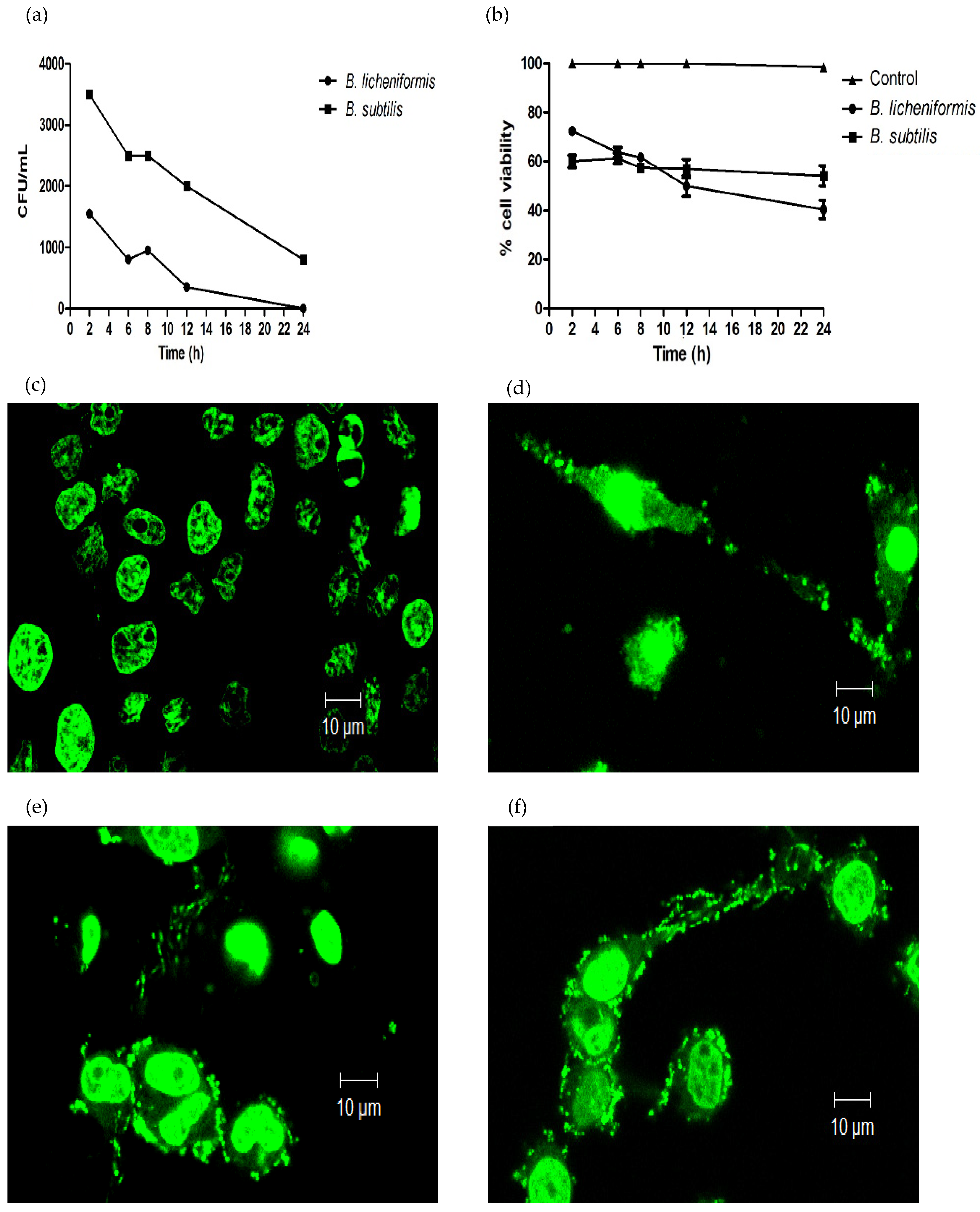
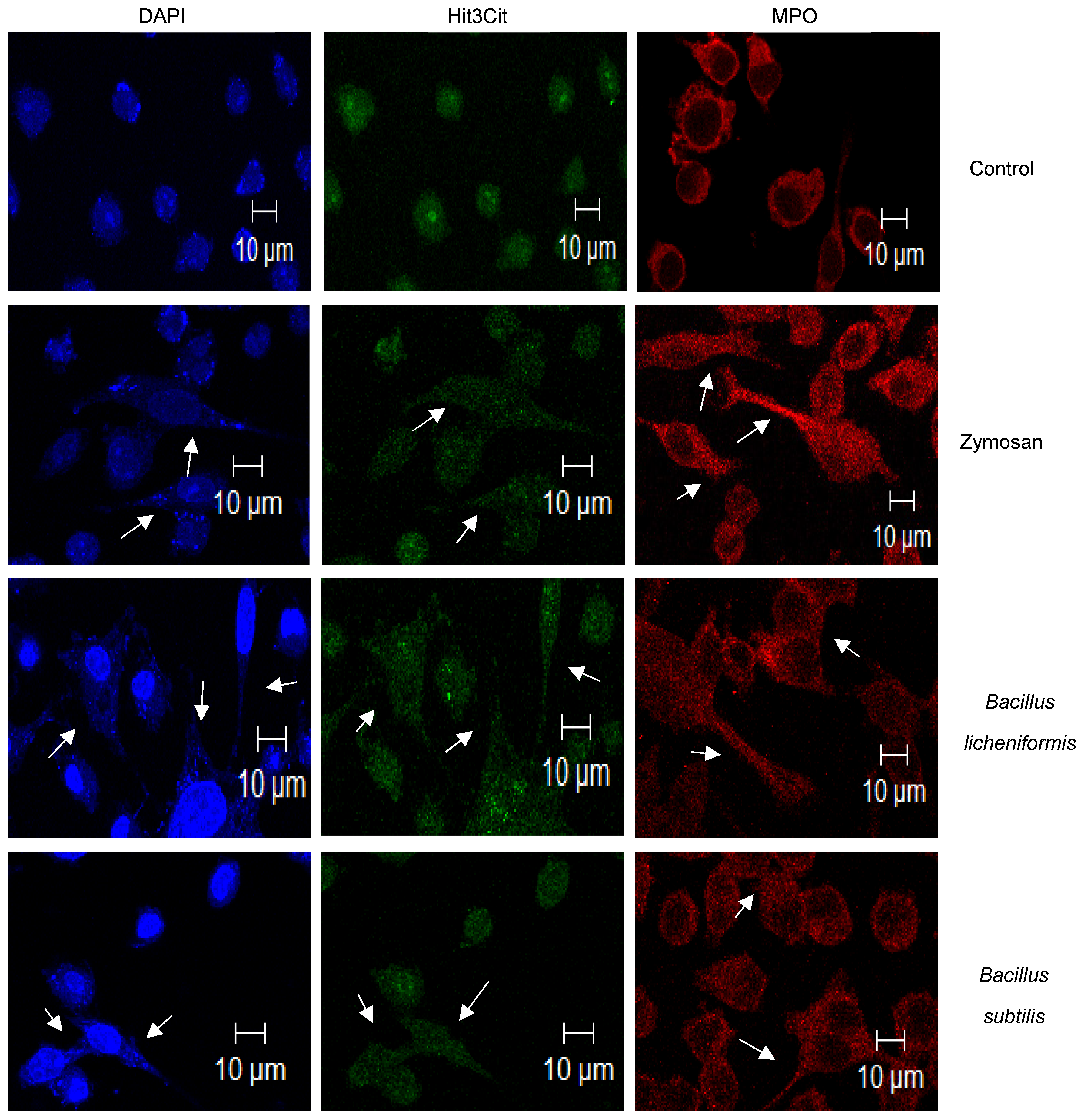
3.1. B. licheniformis and B. subtilis Are Eliminated by Macrophages, and They Induce the Formation of METs
3.2. ROS-Production during Infection and Induction of METs
3.3. METs Induced by B. licheniformis Inhibit the Growth of Staphylococcus aureus
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO; OMS. Probióticos en los Alimentos: Propiedades Saludables y Nutricionales y Directrices para la Evaluación; FAO: Rome, Italy, 2006; ISSN 1014-2916. ISBN 9253055138. [Google Scholar]
- Goldenberg, J.; Yap, C.; Lytvyn, L.; Lo, C.; Beardsley, J.; Mertz, D.; Johnston, B. Probiotics for the prevention of Clostridium difficile-associated diarrea in adults and children. Coch. Data Syst. Rev. 2017, 12, CD006095. [Google Scholar] [CrossRef]
- Williams, N. Probiotics. Am. J. Health Syst. Pharm. 2010, 67, 449–458. [Google Scholar] [CrossRef]
- Sorokulova, I. Modern status and perspectives of Bacillus bacteria as probiotics. J. Probio. Health 2013, 1. [Google Scholar] [CrossRef]
- Stenfors Arnesen, L.; Fagerlund, A.; Granum, P. From soil to gut: Bacillus cereus and its food poisoning toxins. FEMS Micro. Rev. 2008, 32, 579–606. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ali, S.; Bier, E.; Nizet, V. Innate immune interactions between Bacillus anthracis and host neutrophils. Front. Cell. Infect. Microbiol. 2018, 8, 2. [Google Scholar] [CrossRef]
- Rhayat, L.; Maresca, M.; Nicoletti, C.; Perrier, J.; Brinch, K.S.; Christian, S.; Devillard, E.; Eckhardt, E. Effect of Bacillus subtilis strains on intestinal barrier function and inflammatory response. Front. Immunol. 2019, 10, 564. [Google Scholar] [CrossRef]
- Lim, H.; Oh, S.; Yu, S.; Kim, M. Isolation and Characterization of Probiotic Bacillus subtilis MKHJ 1-1 Possessing L-Asparaginase Activity. Appl. Sci. 2021, 11, 4466. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, Y. Bacillus licheniformis-fermented products improve growth performance and the fecal microbiota community in broilers. Poult. Sci. 2020, 99, 1432–1443. [Google Scholar] [CrossRef]
- Earl, A.; Losick, R.; Kolter, R. Ecology and genomics of Bacillus subtilis. Tren. Microbiol. 2008, 16, 269–275. [Google Scholar] [CrossRef]
- Kovács, Á. Bacillus subtilis. Trends Microbiol. 2019, 27, 724–725. [Google Scholar] [CrossRef]
- Inatsu, Y.; Nakamura, N.; Yuriko, Y.; Fushimi, T.; Watanasiritum, L.; Kawamoto, S. Characterization of Bacillus subtilis strains in Thua nao, a traditional fermented soybean food in northern Thailand. Lett. Appl. Microbiol. 2006, 43, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Sankararaman, S.; Velayuthan, S. Bacillus cereus. Ped. Rev. 2013, 34, 196–197. [Google Scholar] [CrossRef][Green Version]
- Sorokulova, I.; Pinchuk, I.; Denayrolles, M.; Osipova, I.; Huang, J.; Cutting, S.; Urdaci, M. The safety of two bacillus probiotic strains for human use. Dig. Dis. Sci. 2008, 53, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Agerholm, J.; Jensen, H.; Jensen, N. Experimental infection in mice with Bacillus licheniformis. J. Vet. Med. 1995, 42, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Jelacic, T.; Ribot, W.; Chua, J.; Boyer, A.; Woolfitt, A.; Barr, J.; Friedlander, A. Human innate immune cells respond differentially to Poly-ɣ-Glutamic acid polymers from Bacillus anthracis and non-pathogenic Bacillus species. J. Immunol. 2020, 204, 1263–1273. [Google Scholar] [CrossRef] [PubMed]
- Lefevre, M.; Racedo, S.M.; Ripert, G.; Housez, B.; Cazaubiel, M.; Maudet, C.; Jüsten, P.; Marteau, P.; Urdaci, M.C. Probiotic strain Bacillus subtilis CU1 stimulates immune system of elderly during common infectious disease period: A randomized, double-blind placebo-controlled study. Immun. Ageing 2015, 12. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.; Weintrauch, Y.; Zychlinsky, A. Neutrophil Extracellular Traps Kill Bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Muñoz-Caro, T.; Silva, L.; Ritter, C.; Taubert, A.; Hermosilla, C. Besnoitia besnoiti tachyzoites induce monocyte extracellular trap formation. Parasitol. Res. 2014, 113, 4189–4197. [Google Scholar] [CrossRef]
- Stoiber, W.; Obermayer, A.; Steinbacher, P.; Krautgartner, W. The role of reactive oxygen species (ROS) in the formation of extracellular traps (ETs) in humans. Biomolecules 2015, 5, 702–723. [Google Scholar] [CrossRef]
- Granger, V.; Faille, D.; Marani, V.; Noël, B.; Gallais, Y.; Szely, N.; Flament, H.; Pallardy, M.; Chollet-Martin, S.; Chaisemartin, L. Human blood monocytes are able to form extracellular traps. J. Leuko. Biol. 2017, 102, 775–781. [Google Scholar] [CrossRef]
- Je, S.; Quan, H.; Yoon, Y.; Na, Y.; Kim, B.; Seok, S. Mycobacterium massiliense induces macrophage extracellular traps with facilitating bacterial growth. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Aulik, N.; Hellebrand, K.; Czuprynski, C. Mannheimia haemolytica and its leukotoxin cause macrophage extracellular trap formation by bovine macrophages. Infect. Immun. 2012, 80, 1923–1933. [Google Scholar] [CrossRef]
- García-Pérez, B.; Villagómez-Palatto, D.; Castañeda-Sánchez, J.; Coral-Vázquez, R.; Ramírez-Sánchez, I.; Ordoñez-Razo, R.; Luna-Herrera, J. Innate response of human endothelial cells infected with mycobacteria. Immunobiology 2011, 216, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Campillo-Navarro, M.; Leyva-Paredes, K.; Donis-Maturano, L.; González-Jiménez, M.; Paredes-Vivas, Y.; Cerbulo-Vázquez, A.; Serafín-López, J.; García-Pérez, B.; Urlich, S.; Flores-Romo, L.; et al. Listeria monocytogenes induces mast cell extracelular traps. Immunobiology 2017, 222, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Halder, L.; Abdelfatah, M.; Jo, E.; Jacobsen, I.; Westermann, M.; Beyersdorf, N.; Lorkowski, S.; Zipfel, P.; Skerka, C. Factor H binds to extracellular DNA traps released from human blood monocytes in response to Candida albicans. Front. Immunol. 2017, 7, 671. [Google Scholar] [CrossRef]
- Wang, Y.; Wysocka, J.; Sayegh, J.; Lee, Y.; Perlín, J.; Leonelli, L.; Sonbuchner, L.; McDonald, C.; Cook, R.; Dou, Y.; et al. Human PAD4 regulates histone arginine methylation levels via demethylimination. Science 2004, 306, 279–283. [Google Scholar] [CrossRef]
- Brinkmann, V.; Zychlinsky, A. Neutrophil extracellular traps: Is immunity the second function of chromatin? J. Cell Biol. 2012, 198, 773–783. [Google Scholar] [CrossRef]
- Liu, P.; Wu, X.; Liao, C.; Liu, X.; Du, J.; Shi, H.; Wang, X.; Bai, X.; Peng, P.; Yu, L.; et al. Escherichia coli and Candida albicans induced macrophage extracellular trap-like structures with limited microbicidal activity. PLoS ONE 2014, 9, e90042. [Google Scholar] [CrossRef]
- Papayannopoulos, V.; Metzler, K.; Hakkim, A.; Zychlinsky, A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell Biol. 2010, 191, 677–691. [Google Scholar] [CrossRef]
- Parker, H.; Albrett, A.; Kettle, A.; Winterbourn, C. Myeloperoxidase associated with neutrophil extracellular traps is active and mediates bacterial killing in the presence of hydrogen peroxide. J. Leuk. Biol. 2012, 91, 369–376. [Google Scholar] [CrossRef]
- Abudabos, A.M.; Aljumaah, M.R.; Alkhulaifi, M.M.; Alabdullatif, A.; Suliman, G.M.; ALSulaiman, A.R. Comparative effects of Bacillus subtilis and Bacillus licheniformis on live performance, blood metabolites and intestinal features in broiler inoculated with Salmonella infection during the finisher phase. Microb. Pathog. 2020, 139, 103870. [Google Scholar] [CrossRef] [PubMed]
- Santano, N.; Boll, E.; Capern, L.; Cieplak, T.; Keleszade, E.; Letek, M.; Costabile, A. Comparative evaluation of the antimicrobial and mucus induction properties of selected Bacillus strains against enterotoxigenic Escherichia coli. Antibiotics 2020, 9, 849. [Google Scholar] [CrossRef]
- Dayan, G.; Mohamed, N.; Scully, I.; Cooper, D.; Begier, D.; Eiden, J.; Jansen, K.; Gurtman, A.; Anderson, A. Staphylococcus aureus: The current state of disease, pathophysiology and strategies for prevention. Exp. Rev. Vac. 2016, 15, 1373–1392. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Macías, A.; Pérez-Escajadillo, J.; Calvo, F.; García, B. Enterocolitis por Staphylococcus aureus resistente a meticilina. Enferm. Infecc. Microbiol. Clin. 2003, 21, 600–601. [Google Scholar] [CrossRef]
- Kataoka, K. The intestinal microbiota and its role in human health and disease. J. Med. Investig. 2016, 63, 27–37. [Google Scholar] [CrossRef]
- Kim, S.; Guevarra, R.; Kim, Y.; Kwon, J.; Kim, H.; Cho, J.; Kim, H.; Lee, J. Role of probiotics in human gut microbiome-associated diseases. J. Microbiol. Biotechnol. 2019, 29, 1335–1340. [Google Scholar] [CrossRef]
- Wieërs, G.; Belkhir, L.; Enaud, R.; Leclercq, S.; Philippart de Foy, J.; Dequenne, I.; de Timary, P.; Cani, P. How probiotics affect the microbiota. Front. Cell. Infect. Microbiol. 2020, 9, 454. [Google Scholar] [CrossRef]
- Yan, G.; Elbadawi, M.; Efferth, T. Multiple cell death modalities and their key features. World Acad. Sci. J. 2020, 2, 39–48. [Google Scholar] [CrossRef]
- Wartha, F.; Henriques-Normark, B. ETosis: A novel cell death pathway. Sci. Signal. 2008, 1, pe25. [Google Scholar] [CrossRef] [PubMed]
- Vong, L.; Lorentz, R.; Assa, A.; Glogauer, M.; Sherman, P. Probiotic Lactobacillus rhamnosus inhibits the formation of neutrophil extracellular traps. J. Immunol. 2014, 192, 1870–1877. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Kim, W.; Paik, H. Bacillus strains as human probiotics: Characterization, safety, microbiome and probiotic carrier. Food Sci. Biotechnol. 2019, 28, 1297–1305. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romo-Barrera, C.M.; Castrillón-Rivera, L.E.; Palma-Ramos, A.; Castañeda-Sánchez, J.I.; Luna-Herrera, J. Bacillus licheniformis and Bacillus subtilis, Probiotics That Induce the Formation of Macrophage Extracellular Traps. Microorganisms 2021, 9, 2027. https://doi.org/10.3390/microorganisms9102027
Romo-Barrera CM, Castrillón-Rivera LE, Palma-Ramos A, Castañeda-Sánchez JI, Luna-Herrera J. Bacillus licheniformis and Bacillus subtilis, Probiotics That Induce the Formation of Macrophage Extracellular Traps. Microorganisms. 2021; 9(10):2027. https://doi.org/10.3390/microorganisms9102027
Chicago/Turabian StyleRomo-Barrera, Carol M., Laura E. Castrillón-Rivera, Alejandro Palma-Ramos, Jorge I. Castañeda-Sánchez, and Julieta Luna-Herrera. 2021. "Bacillus licheniformis and Bacillus subtilis, Probiotics That Induce the Formation of Macrophage Extracellular Traps" Microorganisms 9, no. 10: 2027. https://doi.org/10.3390/microorganisms9102027
APA StyleRomo-Barrera, C. M., Castrillón-Rivera, L. E., Palma-Ramos, A., Castañeda-Sánchez, J. I., & Luna-Herrera, J. (2021). Bacillus licheniformis and Bacillus subtilis, Probiotics That Induce the Formation of Macrophage Extracellular Traps. Microorganisms, 9(10), 2027. https://doi.org/10.3390/microorganisms9102027






