The Role of Streptococcus spp. in Bovine Mastitis
Abstract
1. Introduction
2. Classification
3. Reservoirs, Occurrence and Frequency of Streptococcal Mastitis
4. Epidemiologic Factors Influencing Susceptibility for Streptococcal Mastitis
5. Pathogenesis and Virulence Factors
6. Diagnosis of Streptococcal Mastitis
7. Conventional and Alternative Therapy Strategies
8. Antimicrobial Resistances (AMRs) in Bovine Streptococcus spp.
9. Control and Prevention
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bradley, A.J. Bovine Mastitis: An Evolving Disease. Vet. J. 2002, 164, 116–128. [Google Scholar] [CrossRef]
- Leigh, J. Streptococcus uberis: A permanent barrier to the control of bovine mastitis? Vet. J. 1999, 157, 225–238. [Google Scholar] [CrossRef]
- Hogeveen, H.; Huijps, K.; Lam, T.J. Economic aspects of mastitis: New developments. N. Z. Vet. J. 2011, 59, 16–23. [Google Scholar] [CrossRef]
- Ruegg, P.L. A 100-Year Review: Mastitis detection, management, and prevention. J. Dairy Sci. 2017, 100, 10381–10397. [Google Scholar] [CrossRef]
- Cheng, W.N.; Han, S.G. Bovine mastitis: Risk factors, therapeutic strategies, and alternative treatments—A review. Asian Australas J. Anim. Sci. 2020, 33, 1699–1713. [Google Scholar] [CrossRef] [PubMed]
- Heringstad, B.; Klemetsdal, G.; Ruane, J. Selection for mastitis resistance in dairy cattle: A review with focus on the situation in the Nordic countries. Livest. Prod. Sci. 2000, 64, 95–106. [Google Scholar] [CrossRef]
- Kibebew, K. Bovine mastitis: A review of causes and epidemiological point of view. J. Biol. Agric. Healthc. 2017, 7, 1–14. [Google Scholar]
- Martin, P.; Barkema, H.; Brito, L.; Narayana, S.; Miglior, F. Symposium review: Novel strategies to genetically improve mastitis resistance in dairy cattle. J. Dairy Sci. 2018, 101, 2724–2736. [Google Scholar] [CrossRef]
- Hogan, J.S.; Smith, K.L. Environmental streptococcal mastitis: Facts, fables, and fallacies. In Proceedings of the Fifth International Dairy Housing Conference, Fort Worth, TX, USA, 29–31 January 2003; pp. 9–16. [Google Scholar]
- Jain, B.; Tewari, A.; Bhandari, B.B.; Jhala, M.K. Antibiotic resistance and virulence genes in Streptococcus agalactiae isolated from cases of bovine subclinical mastitis. Vet. Arhiv 2012, 82, 423–432. [Google Scholar]
- Benić, M.; Maćešić, N.; Cvetnić, L.; Habrun, B.; Cvetnić, Ž.; Turk, R.; Đuričić, D.; Lojkić, M.; Dobranić, V.; Valpotić, H. Bovine mastitis: A persistent and evolving problem requiring novel approaches for its control-a review. Vet. Arhiv 2018, 88, 535–557. [Google Scholar] [CrossRef]
- Botrel, M.-A.; Haenni, M.; Morignat, E.; Sulpice, P.; Madec, J.-Y.; Calavas, D. Distribution and Antimicrobial Resistance of Clinical and Subclinical Mastitis Pathogens in Dairy Cows in Rhône-Alpes, France. Foodborne Pathog. Dis. 2009, 7, 479–487. [Google Scholar] [CrossRef]
- Gomes, F.; Henriques, M. Control of Bovine Mastitis: Old and Recent Therapeutic Approaches. Curr. Microbiol. 2016, 72, 377–382. [Google Scholar] [CrossRef]
- Kaczorek, E.; Małaczewska, J.; Wójcik, R.; Rękawek, W.; Siwicki, A.K. Phenotypic and genotypic antimicrobial susceptibility pattern of Streptococcus spp. isolated from cases of clinical mastitis in dairy cattle in Poland. J. Dairy Sci. 2017, 100, 6442–6453. [Google Scholar] [CrossRef]
- Oliver, S.P.; Pighetti, G.M. Mastitis Pathogens|Environmental Pathogens. Encycl. Dairy Sci. 2002, 1728–1734. [Google Scholar] [CrossRef]
- Wente, N.; Krömker, V. Streptococcus dysgalactiae—Contagious or Environmental? Animals 2020, 10, 2185. [Google Scholar] [CrossRef]
- Raabe, V.N.; Shane, A.L. Group B streptococcus (Streptococcus agalactiae). Gram Posit. Pathog. 2019, 228–238. [Google Scholar] [CrossRef]
- Evans, J.J.; Bohnsack, J.F.; Klesius, P.H.; Whiting, A.A.; Garcia, J.C.; Shoemaker, C.A.; Takahashi, S. Phylogenetic relationships among Streptococcus agalactiae isolated from piscine, dolphin, bovine and human sources: A dolphin and piscine lineage associated with a fish epidemic in Kuwait is also associated with human neonatal infections in Japan. J. Med. Microbiol. 2008, 57, 1369–1376. [Google Scholar] [CrossRef]
- Lyhs, U.; Kulkas, L.; Katholm, J.; Waller, K.P.; Saha, K.; Tomusk, R.J.; Zadoks, R.N. Streptococcus agalactiae serotype IV in humans and cattle, northern Europe. Emerg. Infect. Dis. 2016, 22, 2097. [Google Scholar] [CrossRef]
- Pereira, U.; Mian, G.; Oliveira, I.; Benchetrit, L.; Costa, G.; Figueiredo, H. Genotyping of Streptococcus agalactiae strains isolated from fish, human and cattle and their virulence potential in Nile tilapia. Vet. Microbiol. 2010, 140, 186–192. [Google Scholar] [CrossRef]
- Phuektes, P.; Mansell, P.D.; Dyson, R.S.; Hooper, N.D.; Dick, J.S.; Browning, G.F. Molecular epidemiology of Streptococcus uberis isolates from dairy cows with mastitis. J. Clin. Microbiol. 2001, 39, 1460–1466. [Google Scholar] [CrossRef]
- Cvetnić, L.; Samardžija, M.; Habrun, B.; Kompes, G.; Benić, M. Microbiological monitoring of mastitis pathogens in the control of udder health in dairy cows. Slov. Vet. Res. 2016, 53, 131–140. [Google Scholar]
- Hillerton, J.E.; Berry, E.A. The management and treatment of environmental streptococcal mastitis. Vet. Clin. Food Anim. Pract. 2003, 19, 157–169. [Google Scholar] [CrossRef]
- Krömker, V.; Reinecke, F.; Paduch, J.-H.; Grabowski, N. Bovine Streptococcus uberis intramammary infections and mastitis. Clin. Microbiol. Open Access 2014. [Google Scholar] [CrossRef]
- Pitkälä, A.; Koort, J.; Björkroth, J. Identification and antimicrobial resistance of Streptococcus uberis and Streptococcus parauberis isolated from bovine milk samples. J. Dairy Sci. 2008, 91, 4075–4081. [Google Scholar] [CrossRef]
- Calvinho, L.F.; Almeida, R.A.; Oliver, S.P. Potential virulence factors of Streptococcus dysgalactiae associated with bovine mastitis. Vet. Microbiol. 1998, 61, 93–110. [Google Scholar] [CrossRef]
- Alnakip, M.E.A.; Rhouma, N.R.; Abd-Elfatah, E.N.; Quintela-Baluja, M.; Böhme, K.; Fernández-No, I.; Bayoumi, M.A.; Abdelhafez, M.M.; Taboada-Rodríguez, A.; Calo-Mata, P.; et al. Discrimination of major and minor streptococci incriminated in bovine mastitis by MALDI-TOF MS fingerprinting and 16S rRNA gene sequencing. Res. Vet. Sci. 2020, 132, 426–438. [Google Scholar] [CrossRef]
- Chaffer, M.; Friedman, S.; Saran, A.; Younis, A. An outbreak of Streptococcus canis mastitis in a dairy herd in Israel. N. Z. Vet. J. 2005, 53, 261–264. [Google Scholar] [CrossRef]
- Chen, P.; Qiu, Y.; Liu, G.; Li, X.; Cheng, J.; Liu, K.; Qu, W.; Zhu, C.; Kastelic, J.P.; Han, B.; et al. Characterization of Streptococcus lutetiensis isolated from clinical mastitis of dairy cows. J. Dairy Sci. 2021, 104, 702–714. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.A.; Akineden, O.; Usleber, E. Identification of Streptococcus canis isolated from milk of dairy cows with subclinical mastitis. J. Clin. Microbiol. 2005, 43, 1234–1238. [Google Scholar] [CrossRef] [PubMed]
- Król, J.; Twardoń, J.; Mrowiec, J.; Podkowik, M.; Dejneka, G.; Dębski, B.; Nowicki, T.; Zalewski, W. Streptococcus canis is able to establish a persistent udder infection in a dairy herd. J. Dairy Sci. 2015, 98, 7090–7096. [Google Scholar] [CrossRef] [PubMed]
- Tikofsky, L.; Zadoks, R. Cross-infection between cats and cows: Origin and control of Streptococcus canis mastitis in a dairy herd. J. Dairy Sci. 2005, 88, 2707–2713. [Google Scholar] [CrossRef]
- Erskine, R.J.; Wagner, S.; DeGraves, F.J. Mastitis therapy and pharmacology. Vet. Clin. N. Am. Food Anim. Pract. 2003, 19, 109–138. [Google Scholar] [CrossRef]
- Kalińska, A.; Gołębiewski, M.; Wójcik, A. Mastitis pathogens in dairy cattle—A review. World Sci. News 2017, 89, 22–31. [Google Scholar]
- Amin, B.; Deneke, Y.; Abdela, N. Bovine Mastitis: Prevalence, Risk Factors and Isolation of Streptoccocus Species from Small Holders Dairy Farms in and Around Haramaya Town, Eastern Ethiopia. J. Med. Res. 2017, 17, 27–38. [Google Scholar]
- Dego, O.K. Current status of antimicrobial resistance and prospect for new vaccines against major bacterial bovine mastitis pathogens. In Animal Reproduction in Veterinary Medicine; Aral, F., Ed.; IntechOpen: London, UK, 2020; p. 78921. [Google Scholar] [CrossRef]
- Oikonomou, G.; Machado, V.S.; Santisteban, C.; Schukken, Y.H.; Bicalho, R.C. Microbial diversity of bovine mastitic milk as described by pyrosequencing of metagenomic 16s rDNA. PLoS ONE 2012, 7, e47671. [Google Scholar]
- Derakhshani, H.; Fehr, K.B.; Sepehri, S.; Francoz, D.; De Buck, J.; Barkema, H.W.; Plaizier, J.C.; Khafipour, E. Invited review: Microbiota of the bovine udder: Contributing factors and potential implications for udder health and mastitis susceptibility. J. Dairy Sci. 2018, 101, 10605–10625. [Google Scholar] [CrossRef]
- Falentin, H.; Rault, L.; Nicolas, A.; Bouchard, D.S.; Lassalas, J.; Lamberton, P.; Aubry, J.-M.; Marnet, P.-G.; Le Loir, Y.; Even, S. Bovine Teat Microbiome Analysis Revealed Reduced Alpha Diversity and Significant Changes in Taxonomic Profiles in Quarters with a History of Mastitis. Front. Microbiol. 2016, 7, 480. [Google Scholar] [CrossRef]
- Cole, J.N.; Pence, M.A.; Köckritz-Blickwede, M.V.; Hollands, A.; Gallo, R.L.; Walker, M.J.; Nizet, V.; Norrby-Teglund, A.; Low, D.E. M Protein and Hyaluronic Acid Capsule Are Essential for In Vivo Selection of covRS Mutations Characteristic of Invasive Serotype M1T1 Group A Streptococcus. Mbio 2010, 1, e00191-10. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, L. Understanding the regulation of Group B Streptococcal virulence factors. Future Microbiol. 2009, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Kell, D.B.; Heyden, E.L.; Pretorius, E. The biology of lactoferrin, an iron-binding protein that can help defend against viruses and bacteria. Front. Immunol. 2020, 11, 1221. [Google Scholar] [CrossRef] [PubMed]
- O’Halloran, F.; Beecher, C.; Chaurin, V.; Sweeney, T.; Giblin, L. Lactoferrin affects the adherence and invasion of Streptococcus dysgalactiae ssp. dysgalactiae in mammary epithelial cells. J. Dairy Sci. 2016, 99, 4619–4628. [Google Scholar] [CrossRef] [PubMed]
- Almeida, R.A.; Dego, O.K.; Headrick, S.I.; Lewis, M.J.; Oliver, S.P. Role of Streptococcus uberis adhesion molecule in the pathogenesis of Streptococcus uberis mastitis. Vet. Microbiol. 2015, 179, 332–335. [Google Scholar] [CrossRef] [PubMed]
- Egan, S.A.; Ward, P.N.; Watson, M.; Field, T.R.; Leigh, J.A. Vru (Sub0144) controls expression of proven and putative virulence determinants and alters the ability of Streptococcus uberis to cause disease in dairy cattle. Microbiology 2012, 158, 1581–1592. [Google Scholar] [CrossRef]
- Patel, D.; Almeida, R.A.; Dunlap, J.R.; Oliver, S.P. Bovine lactoferrin serves as a molecular bridge for internalization of Streptococcus uberis into bovine mammary epithelial cells. Vet. Microbiol. 2009, 137, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-M.; Almeida, R.; Luther, D.; Oliver, S. Binding of bovine lactoferrin to Streptococcus dysgalactiae subsp. dysgalactiae isolated from cows with mastitis. FEMS Microbiol. Lett. 2002, 208, 35–39. [Google Scholar] [CrossRef][Green Version]
- Tong, J.; Sun, M.; Zhang, H.; Yang, D.; Zhang, Y.; Xiong, B.; Jiang, L. Proteomic analysis of bovine mammary epithelial cells after in vitro incubation with S. agalactiae: Potential biomarkers. Vet. Res. 2020, 51, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mamo, W.; Fröman, G.; Sundås, A.; Wadström, T. Binding of fibronectin, fibrinogen and type II collagen to streptococci isolated from bovine mastitis. Microb. Pathog. 1987, 2, 417–424. [Google Scholar] [CrossRef]
- Filippsen, L.F. Bovine S protein (vitronectin) increases phagocytosis of Streptococcus dysgalactiae. Rev. Microbiol. 1999, 30, 15–18. [Google Scholar] [CrossRef]
- Varhimo, E.; Varmanen, P.; Fallarero, A.; Skogman, M.; Pyörälä, S.; Iivanainen, A.; Sukura, A.; Vuorela, P.; Savijoki, K. Alpha-and β-casein components of host milk induce biofilm formation in the mastitis bacterium Streptococcus uberis. Vet. Microbiol. 2011, 149, 381–389. [Google Scholar] [CrossRef]
- Reinoso, E.B. Bovine mastitis caused by streptococcus uberis. Virulence factors and biofilm. J. Microb. Biochem. Technol. 2017. [Google Scholar] [CrossRef]
- Shukla, R.; Pandey, V.; Vadnere, G.P.; Lodhi, S. Role of flavonoids in management of inflammatory disorders. In Bioactive Food as Dietary Interventions for Arthritis and Related Inflammatory Disease; Elsevier: Amsterdam, The Netherlands, 2019; pp. 293–322. [Google Scholar]
- Alves-Barroco, C.; Roma-Rodrigues, C.; Balasubramanian, N.; Guimarães, M.A.; Ferreira-Carvalho, B.T.; Muthukumaran, J.; Nunes, D.; Fortunato, E.; Martins, R.; Santos-Silva, T.; et al. Biofilm development and computational screening for new putative inhibitors of a homolog of the regulatory protein BrpA in Streptococcus dysgalactiae subsp. dysgalactiae. Int. J. Med. Microbiol. 2019, 309, 169–181. [Google Scholar] [CrossRef]
- Emaneini, M.; Khoramian, B.; Jabalameli, F.; Abani, S.; Dabiri, H.; Beigverdi, R. Comparison of virulence factors and capsular types of Streptococcus agalactiae isolated from human and bovine infections. Microb. Pathog. 2016, 91, 1–4. [Google Scholar] [CrossRef]
- Mudzana, R.; Mavenyengwa, R.T.; Gudza-Mugabe, M. Analysis of virulence factors and antibiotic resistance genes in group B streptococcus from clinical samples. BMC Infect. Dis. 2021, 21, 125. [Google Scholar] [CrossRef] [PubMed]
- Loures, R.A.; de Pádua Pereira, U.; de Carvalho-Castro, G.d.A.; Mian, G.F.; da Costa Custódio, D.A.; da Silva, J.R.; da Costa, G.M. Genetic diversity and virulence genes in Streptococcus uberis strains isolated from bovine mastitis. Semin. Ciências Agrárias 2017, 38, 2595–2605. [Google Scholar] [CrossRef]
- Lämmler, C.; Frede, C. Binding of immunoglobulin G and albumin to Streptococcus dysgalactiae. Zent. Bakteriol. 1989, 271, 321–329. [Google Scholar] [CrossRef]
- Jonsson, H.; Frykberg, L.; Rantamäki, L.; Guss, B. MAG, a novel plasma protein receptor from Streptococcus dysgalactiae. Gene 1994, 143, 85–89. [Google Scholar] [CrossRef]
- Valentin-Weigand, P.; Traore, M.Y.; Blobel, H.; Chhatwal, G.S. Role of α2-macroglobulin in phagocytosis of group A and C streptococci. FEMS Microbiol. Lett. 1990, 70, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Bobadilla, F.J.; Novosak, M.G.; Cortese, I.J.; Delgado, O.D.; Laczeski, M.E. Prevalence, serotypes and virulence genes of Streptococcus agalactiae isolated from pregnant women with 35–37 weeks of gestation. BMC Infect. Dis. 2021, 21, 73. [Google Scholar] [CrossRef] [PubMed]
- Pang, M.; Sun, L.; He, T.; Bao, H.; Zhang, L.; Zhou, Y.; Zhang, H.; Wei, R.; Liu, Y.; Wang, R. Molecular and virulence characterization of highly prevalent Streptococcus agalactiae circulated in bovine dairy herds. Vet. Res. 2017, 48, 1–12. [Google Scholar] [CrossRef] [PubMed]
- International Dairy Federation. Guidelines for the Use and Interpretation of Bovine Milk Somatic Cell Count; International Dairy Federation (IDF/FIL): Schaerbeek, Belgium, 2013; pp. 2–8. [Google Scholar]
- Blagitz, M.G.; Souza, F.N.; Batista, C.F.; Azevedo, L.F.; Benites, N.R.; Melville, P.A.; Diniz, S.A.; Silva, M.X.; Haddad, J.P.; Heinnemann, M.B.; et al. The neutrophil function and lymphocyte profile of milk from bovine mammary glands infected with Streptococcus dysgalactiae. J. Dairy Res. 2015, 82, 460–469. [Google Scholar] [CrossRef]
- Beecher, C.; Daly, M.; Ross, R.P.; Flynn, J.; McCarthy, T.V.; Giblin, L. Characterization of the bovine innate immune response in milk somatic cells following intramammary infection with Streptococcus dysgalactiae subspecies dysgalactiae. J. Dairy Sci. 2012, 95, 5720–5729. [Google Scholar] [CrossRef]
- Florindo, C.; Barroco, C.A.; Silvestre, I.; Damião, V.; Gomes, J.P.; Spellerberg, B.; Santos-Sanches, I.; Borrego, M.J. Capsular Type, Sequence Type and Microbial Resistance Factors Impact on DNase Activity of Streptococcus agalactiae Strains from Human and Bovine Origin. Eur. J. Microbiol. Immunol. 2018, 8, 149–154. [Google Scholar] [CrossRef]
- Smeesters, P.R.; McMillan, D.J.; Sriprakash, K.S. The streptococcal M protein: A highly versatile molecule. Trends Microbiol. 2010, 18, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Vasi, J.; Frykberg, L.; Carlsson, L.E.; Lindberg, M.; Guss, B. M-like proteins of Streptococcus dysgalactiae. Infect. Immun. 2000, 68, 294–302. [Google Scholar] [CrossRef]
- Duarte, R.S.; Bellei, B.C.; Miranda, O.P.; Brito, M.A.V.P.; Teixeira, L.M. Distribution of antimicrobial resistance and virulence-related genes among Brazilian group B streptococci recovered from bovine and human sources. Antimicrob. Agents Chemother. 2005, 49, 97–103. [Google Scholar] [CrossRef]
- Ashraf, A.; Imran, M. Diagnosis of bovine mastitis: From laboratory to farm. Trop. Anim. Health Prod. 2018, 50, 1193–1202. [Google Scholar] [CrossRef]
- Duarte, C.M.; Freitas, P.P.; Bexiga, R. Technological advances in bovine mastitis diagnosis:an overview. J. Vet. Diagn. Investig. 2015, 27, 665–672. [Google Scholar] [CrossRef]
- Keefe, G. Update on control of Staphylococcus aureus and Streptococcus agalactiae for management of mastitis. Vet. Clin. Food Anim. Pract. 2012, 28, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Damm, M.; Holm, C.; Blaabjerg, M.; Bro, M.N.; Schwarz, D. Differential somatic cell count—A novel method for routine mastitis screening in the frame of Dairy Herd Improvement testing programs. J. Dairy Sci. 2017, 100, 4926–4940. [Google Scholar] [CrossRef] [PubMed]
- Wethal, K.; Svendsen, M.; Heringstad, B. A genetic study of new udder health indicator traits with data from automatic milking systems. J. Dairy Sci. 2020, 103, 7188–7198. [Google Scholar] [CrossRef] [PubMed]
- Viguier, C.; Arora, S.; Gilmartin, N.; Welbeck, K.; O’Kennedy, R. Mastitis detection: Current trends and future perspectives. Trends Biotechnol. 2009, 27, 486–493. [Google Scholar] [CrossRef]
- Mansor, R.; Mullen, W.; Albalat, A.; Zerefos, P.; Mischak, H.; Barrett, D.C.; Biggs, A.; Eckersall, P.D. A peptidomic approach to biomarker discovery for bovine mastitis. J. Proteom. 2013, 85, 89–98. [Google Scholar] [CrossRef]
- Tenhagen, B.-A.; Köster, G.; Wallmann, J.; Heuwieser, W. Prevalence of mastitis pathogens and their resistance against antimicrobial agents in dairy cows in Brandenburg, Germany. J. Dairy Sci. 2006, 89, 2542–2551. [Google Scholar] [CrossRef]
- Bonsaglia, E.C.R.; Gomes, M.S.; Canisso, I.F.; Zhou, Z.; Lima, S.F.; Rall, V.L.M.; Oikonomou, G.; Bicalho, R.C.; Lima, F.S. Milk microbiome and bacterial load following dry cow therapy without antibiotics in dairy cows with healthy mammary gland. Sci. Rep. 2017, 7, 8067. [Google Scholar] [CrossRef]
- Gill, J.J.; Pacan, J.C.; Carson, M.E.; Leslie, K.E.; Griffiths, M.W.; Sabour, P.M. Efficacy and pharmacokinetics of bacteriophage therapy in treatment of subclinical Staphylococcus aureus mastitis in lactating dairy cattle. Antimicrob. Agents Chemother. 2006, 50, 2912–2918. [Google Scholar] [CrossRef]
- O’flaherty, S.; Coffey, A.; Meaney, W.; Fitzgerald, G.; Ross, R. Inhibition of bacteriophage K proliferation on Staphylococcus aureus in raw bovine milk. Lett. Appl. Microbiol. 2005, 41, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Radzikowski, D.; Kalińska, A.; Ostaszewska, U.; Gołębiewski, M. Alternative solutions to antibiotics in mastitis treatment for dairy cows-a review. Anim. Sci. Pap. Rep. 2020, 38, 117–133. [Google Scholar]
- Yang, X.; Ouyang, W.; Sun, J.; Li, X. Post-antibiotic effect of Amoxicillin nanoparticles against main pathogenic bacteria of Bovine mastitis in vitro. J. Northwest A F Univ. Nat. Sci. Ed. 2009, 37, 1–6. [Google Scholar]
- Serna-Cock, L.; Enríquez-Valencia, C.E.; Jiménez-Obando, E.M.; Campos-Gaona, R. Effects of fermentation substrates and conservation methods on the viability and antimicrobial activity of Weissella confusa and its metabolites. Electron. J. Biotechnol. 2012, 15, 6. [Google Scholar] [CrossRef]
- Wu, J.; Hu, S.; Cao, L. Therapeutic effect of nisin Z on subclinical mastitis in lactating cows. Antimicrob. Agents Chemother. 2007, 51, 3131–3135. [Google Scholar] [CrossRef] [PubMed]
- Abd El Hafez, S.M.; Ismael, A.B.; Mahmoud, M.B.; Elaraby, A.K.A. Development of new strategy for non-antibiotic therapy: Bovine lactoferrin has a potent antimicrobial and immunomodulatory effects. Adv. Infect. Dis. 2013, 3, 185–192. [Google Scholar] [CrossRef]
- Kutila, T.; Pyörälä, S.; Kaartinen, L.; Isomäki, R.; Vahtola, K.; Myllykoski, L.; Saloniemi, H. Lactoferrin and Citrate Concentrations at Drying-off and During Early Mammary Involution of Dairy Cows. J. Vet. Med. Ser. A 2003, 50, 350–353. [Google Scholar] [CrossRef] [PubMed]
- Malinowski, E.; Niewitecki, W.; Nadolny, M.; Lassa, H.; Smulski, S. Effect of lysozyme dimer injections on results of intramammary treatment of acute mastitis in cows. Med. Weter. 2006, 62, 1395–1399. [Google Scholar]
- Ananda Baskaran, S.; Kazmer, G.W.; Hinckley, L.; Andrew, S.M.; Venkitanarayanan, K. Antibacterial effect of plant-derived antimicrobials on major bacterial mastitis pathogens in vitro. J. Dairy Sci. 2009, 92, 1423–1429. [Google Scholar] [CrossRef] [PubMed]
- De Barros, M.; Perciano, P.G.; Dos Santos, M.H.; De Oliveira, L.L.; Costa, É.D.M.; Moreira, M.A.S. Antibacterial Activity of 7-Epiclusianone and Its Novel Copper Metal Complex on Streptococcus spp. Isolated from Bovine Mastitis and Their Cytotoxicity in MAC-T Cells. Molecules 2017, 22, 823. [Google Scholar] [CrossRef]
- Fonseca, A.P.; Estrela, F.T.; Moraes, T.S.; Carneiro, L.J.; Bastos, J.K.; Santos, R.A.d.; Ambrósio, S.R.; Martins, C.H.G.; Veneziani, R.C.S. In Vitro Antimicrobial Activity of Plant-Derived Diterpenes against Bovine Mastitis Bacteria. Molecules 2013, 18, 7865–7872. [Google Scholar] [CrossRef]
- Gopinath, S.M.; Suneetha, T.B.; Mruganka, V.D.; Ananda, S. Evaluation of antibacterial activity of Tabernaemontana divaricata (L.) leaves against the causative organisms of bovine mastitis. Int. J. Res. Phytochem. Pharmacol. 2011, 1, 211–213. [Google Scholar]
- Laloučková, K.; Malá, L.; Slaničková, P.; Skřivanová, E. In vitro antimicrobial effect of palm oils rich in medium-chain fatty acids against mastitis-causing Gram-positive bacteria. Czech J. Anim. Sci. 2019, 64, 325–331. [Google Scholar] [CrossRef]
- Montironi, I.D.; Cariddi, L.N.; Reinoso, E.B. Evaluation of the antimicrobial efficacy of Minthostachys verticillata essential oil and limonene against Streptococcus uberis strains isolated from bovine mastitis. Rev. Argent. Microbiol. 2016, 48, 210–216. [Google Scholar] [CrossRef]
- Krömker, V.; Leimbach, S. Mastitis treatment—Reduction in antibiotic usage in dairy cows. Reprod. Domest. Animals 2017, 52, 21–29. [Google Scholar] [CrossRef]
- Barlow, J. Mastitis therapy and antimicrobial susceptibility: A multispecies review with a focus on antibiotic treatment of mastitis in dairy cattle. J. Mammary Gland Biol. Neoplasia 2011, 16, 383–407. [Google Scholar] [CrossRef]
- Kateete, D.P.; Kabugo, U.; Baluku, H.; Nyakarahuka, L.; Kyobe, S.; Okee, M.; Najjuka, C.F.; Joloba, M.L. Prevalence and antimicrobial susceptibility patterns of bacteria from milkmen and cows with clinical mastitis in and around Kampala, Uganda. PLoS ONE 2013, 8, e63413. [Google Scholar] [CrossRef]
- Makovec, J.A.; Ruegg, D.P.L. Antimicrobial resistance of bacteria isolated from dairy cow milk samples submitted for bacterial culture: 8905 samples (1994–2001). J. Am. Vet. Med. Assoc. 2003, 222, 1582–1589. [Google Scholar] [CrossRef]
- Persson, Y.; Nyman, A.-K.J.; Grönlund-Andersson, U. Etiology and antimicrobial susceptibility of udder pathogens from cases of subclinical mastitis in dairy cows in Sweden. Acta Vet. Scand. 2011, 53, 1–8. [Google Scholar] [CrossRef]
- Rato, M.G.; Bexiga, R.; Florindo, C.; Cavaco, L.M.; Vilela, C.L.; Santos-Sanches, I. Antimicrobial resistance and molecular epidemiology of streptococci from bovine mastitis. Vet. Microbiol. 2013, 161, 286–294. [Google Scholar] [CrossRef]
- Rossitto, P.; Ruiz, L.; Kikuchi, Y.; Glenn, K.; Luiz, K.; Watts, J.; Cullor, J.S. Antibiotic susceptibility patterns for environmental streptococci isolated from bovine mastitis in central California dairies. J. Dairy Sci. 2002, 85, 132–138. [Google Scholar] [CrossRef]
- Thomas, V.; de Jong, A.; Moyaert, H.; Simjee, S.; El Garch, F.; Morrissey, I.; Marion, H.; Vallé, M. Antimicrobial susceptibility monitoring of mastitis pathogens isolated from acute cases of clinical mastitis in dairy cows across Europe: VetPath results. Int. J. Antimicrob. Agents 2015, 46, 13–20. [Google Scholar] [CrossRef]
- Boireau, C.; Cazeau, G.; Jarrige, N.; Calavas, D.; Madec, J.-Y.; Leblond, A.; Haenni, M.; Gay, É. Antimicrobial resistance in bacteria isolated from mastitis in dairy cattle in France, 2006–2016. J. Dairy Sci. 2018, 101, 9451–9462. [Google Scholar] [CrossRef] [PubMed]
- Minst, K.; Märtlbauer, E.; Miller, T.; Meyer, C. Streptococcus species isolated from mastitis milk samples in Germany and their resistance to antimicrobial agents. J. Dairy Sci. 2012, 95, 6957–6962. [Google Scholar] [CrossRef]
- Burović, J. Isolation of bovine clinical mastitis bacterial pathogens and their antimicrobial susceptibility in the Zenica region in 2017. Vet. Stanica 2020, 51, 47–52. [Google Scholar] [CrossRef]
- Haenni, M.; Lupo, A.; Madec, J.-Y. Antimicrobial Resistance in Streptococcus spp. ASM J. 2018, 6, 2. [Google Scholar] [CrossRef]
- Pitkälä, A.; Haveri, M.; Pyörälä, S.; Myllys, V.; Honkanen-Buzalski, T. Bovine mastitis in Finland 2001—prevalence, distribution of bacteria, and antimicrobial resistance. J. Dairy Sci. 2004, 87, 2433–2441. [Google Scholar] [CrossRef]
- Zadoks, R.; Allore, H.; Barkema, H.; Sampimon, O.; Wellenberg, G.; Gröhn, Y.; Schukken, Y. Cow-and quarter-level risk factors for Streptococcus uberis and Staphylococcus aureus mastitis. J. Dairy Sci. 2001, 84, 2649–2663. [Google Scholar] [CrossRef]
- Leigh, J.; Finch, J.; Field, T.; Real, N.; Winter, A.; Walton, A.; Hodgkinson, S. Vaccination with the plasminogen activator from Streptococcus uberis induces an inhibitory response and protects against experimental infection in the dairy cow. Vaccine 1999, 17, 851–857. [Google Scholar] [CrossRef]
- Finch, J.M.; Hill, A.W.; Field, T.R.; Leigh, J.A. Local vaccination with killed Streptococcus uberis protects the bovine mammary gland against experimental intramammary challenge with the homologous strain. Infect. Immun. 1994, 62, 3599–3603. [Google Scholar] [CrossRef]
- Collado, R.; Montbrau, C.; Sitjà, M.; Prenafeta, A. Study of the efficacy of a Streptococcus uberis mastitis vaccine against an experimental intramammary infection with a heterologous strain in dairy cows. J. Dairy Sci. 2018, 101, 10290–10302. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, M.C.; Perez-Casal, J.; Song, X.-M.; Shelford, J.; Willson, P.J.; Potter, A.A. Immunisation of dairy cattle with recombinant Streptococcus uberis GapC or a chimeric CAMP antigen confers protection against heterologous bacterial challenge. Vaccine 2002, 20, 2278–2286. [Google Scholar] [CrossRef]
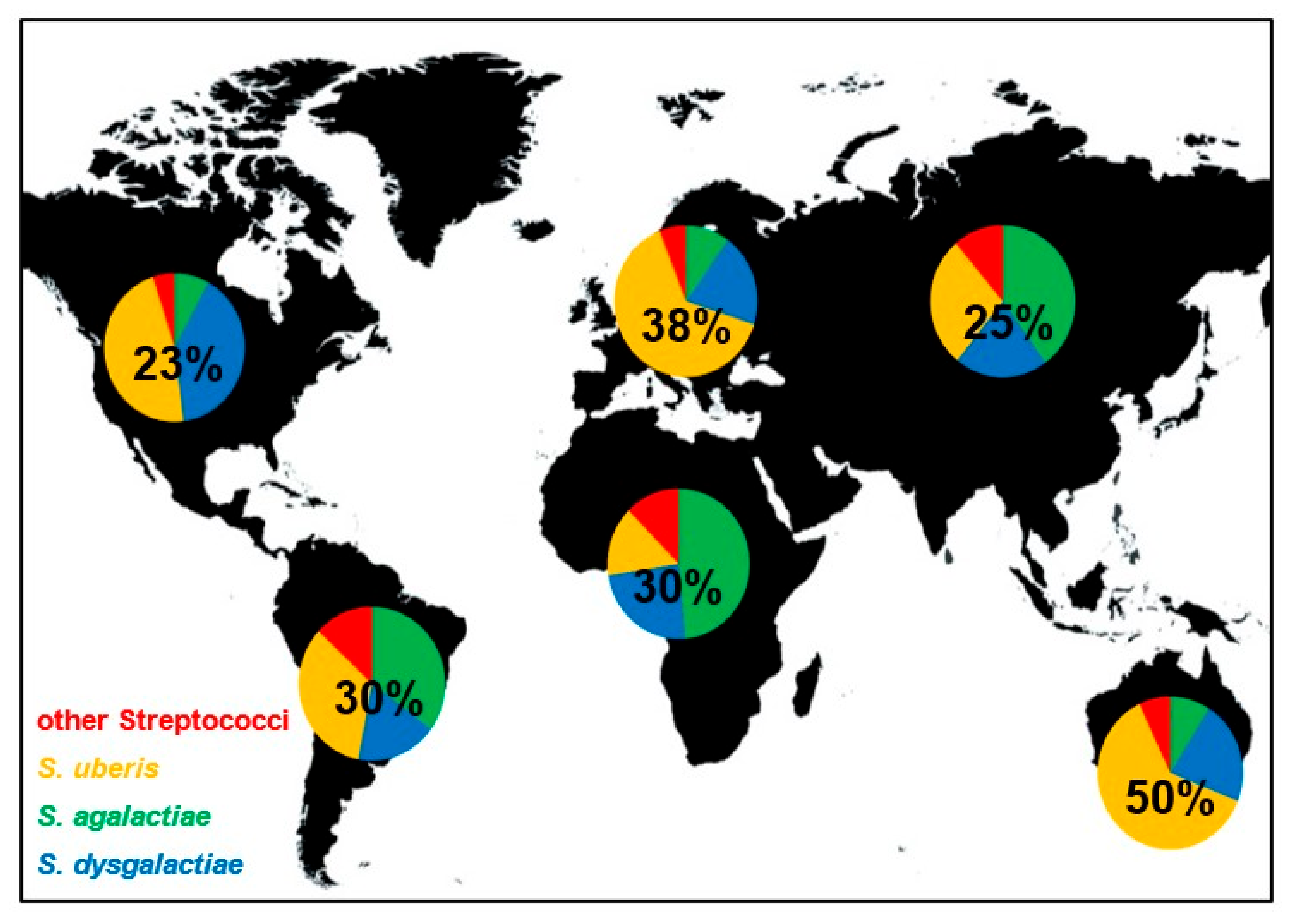

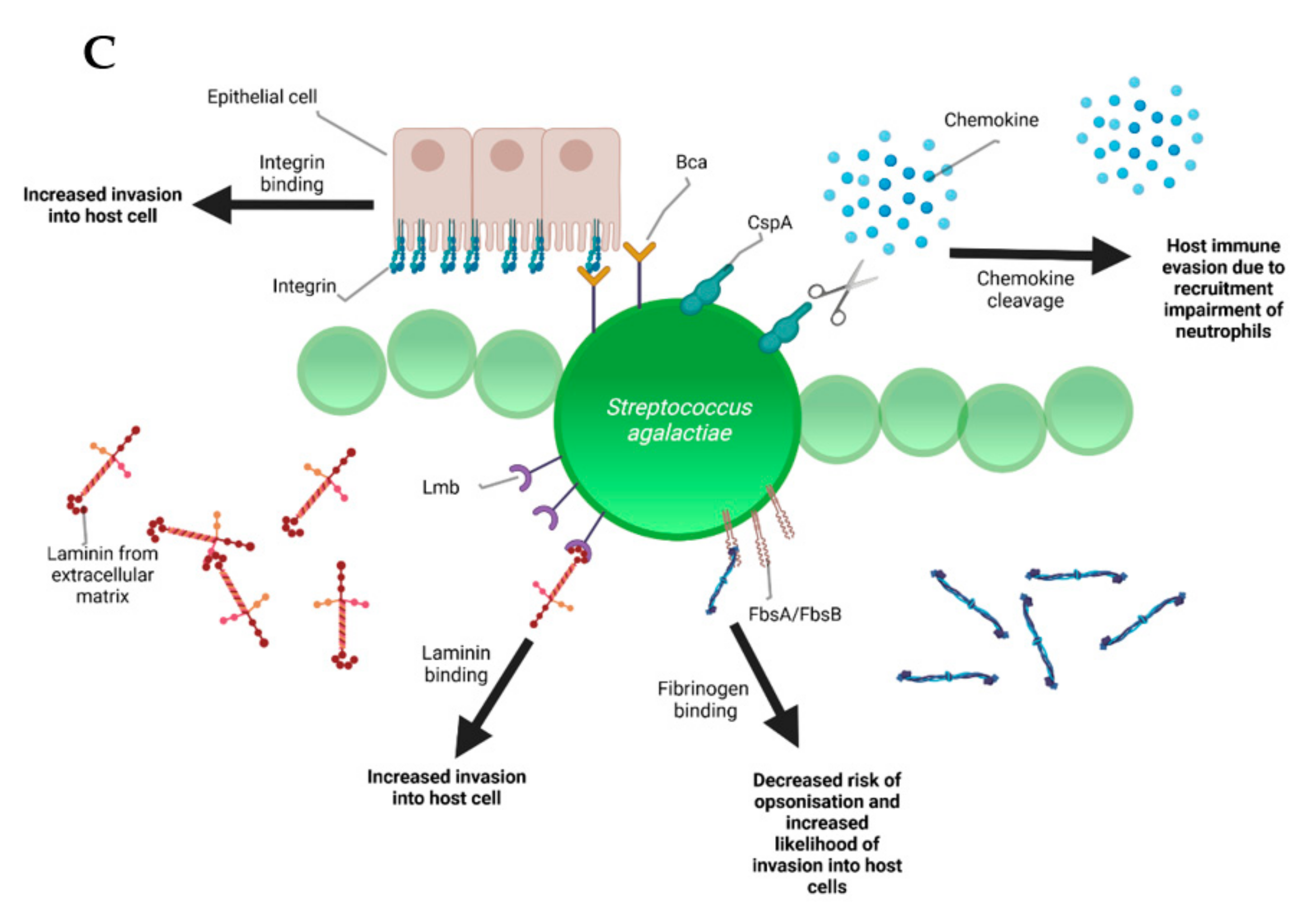
| Streptococcus Species | Lancefield Classification | Transmission | Phenotype (+ Positive − Negative) | Virulence Factors | Antibiotic Resistance (Top 3) | Risk Factors | Host Specificity |
|---|---|---|---|---|---|---|---|
| Streptococcus agalactiae | B | contagious | beta-hemolytic + CAMP − aesculin | CspA Bca FbsA/FbsB Lmb | 50.5% sulfatrimethoprim 46.2% tetracycline 15.4% erythromycin | milking | zoonotic |
| Streptococcuscanis | G | contagious | beta-hemolytic − CAMP + aesculin | SCM SLS | 33% penicillin 0% cephalothin 0% oxacillin | pathogen transfer from cats and dogs | zoonotic |
| Streptococcus dysgalactiae ssp. dysgalactiae | C | intermediate | non-hemolytic − CAMP − aesculin | MAG DemA Superantigen | 38.5% tetracycline 4.8% erythromycin 4.4% streptomycin | summer (transfer by flies) | cattle- specific |
| Streptococcus equinus (previpously S. bovis type II/2) | D | environmental | variable hemolytic − CAMP + aesculin | zoonotic | |||
| Streptococcus lutetiensis (previous S. bovis type II/1) | D | contagious | alpha-hemolysis − CAMP + aesculin | Hly scpB Bca Superantigen | 63% enrofloxacin 49% ceftiofur 43% tetracycline | zoonotic | |
| Streptococcus uberis (Streptococcus parauberis) | diverse (mostly E) | environmental | alpha-hemolytic variable CAMP + aesculin | Sua/Vru HasC PauA/SkC | 32.9% enrofloxacin 20.0% erythromycin 19.1% lincomycin | straw as bedding material | cattle- specific |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kabelitz, T.; Aubry, E.; van Vorst, K.; Amon, T.; Fulde, M. The Role of Streptococcus spp. in Bovine Mastitis. Microorganisms 2021, 9, 1497. https://doi.org/10.3390/microorganisms9071497
Kabelitz T, Aubry E, van Vorst K, Amon T, Fulde M. The Role of Streptococcus spp. in Bovine Mastitis. Microorganisms. 2021; 9(7):1497. https://doi.org/10.3390/microorganisms9071497
Chicago/Turabian StyleKabelitz, Tina, Etienne Aubry, Kira van Vorst, Thomas Amon, and Marcus Fulde. 2021. "The Role of Streptococcus spp. in Bovine Mastitis" Microorganisms 9, no. 7: 1497. https://doi.org/10.3390/microorganisms9071497
APA StyleKabelitz, T., Aubry, E., van Vorst, K., Amon, T., & Fulde, M. (2021). The Role of Streptococcus spp. in Bovine Mastitis. Microorganisms, 9(7), 1497. https://doi.org/10.3390/microorganisms9071497







