Isolation and Characterization of Euglena gracilis-Associated Bacteria, Enterobacter sp. CA3 and Emticicia sp. CN5, Capable of Promoting the Growth and Paramylon Production of E. gracilis under Mixotrophic Cultivation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microalgae and Culture Conditions
2.2. Sewage Effluent Sample and Collection of Indigenous Bacterial Communities from the Effluent
2.3. Culturing E. gracilis with a Bacterial Community Derived from Sewage Effluent and Isolating Bacteria Associated with E. gracilis
2.4. Screening of MGPB Capable of Enhancing Both E. gracilis Growth and Paramylon Production
2.5. Identification and Characterization of the CA3 and CN5 Strains
2.6. Growth of CA3 and CN5 Utilizing Glucose
2.7. Co-Culturing E. gracilis with CA3 or CN5 under Acidic and Neutral pH with or without Glucose
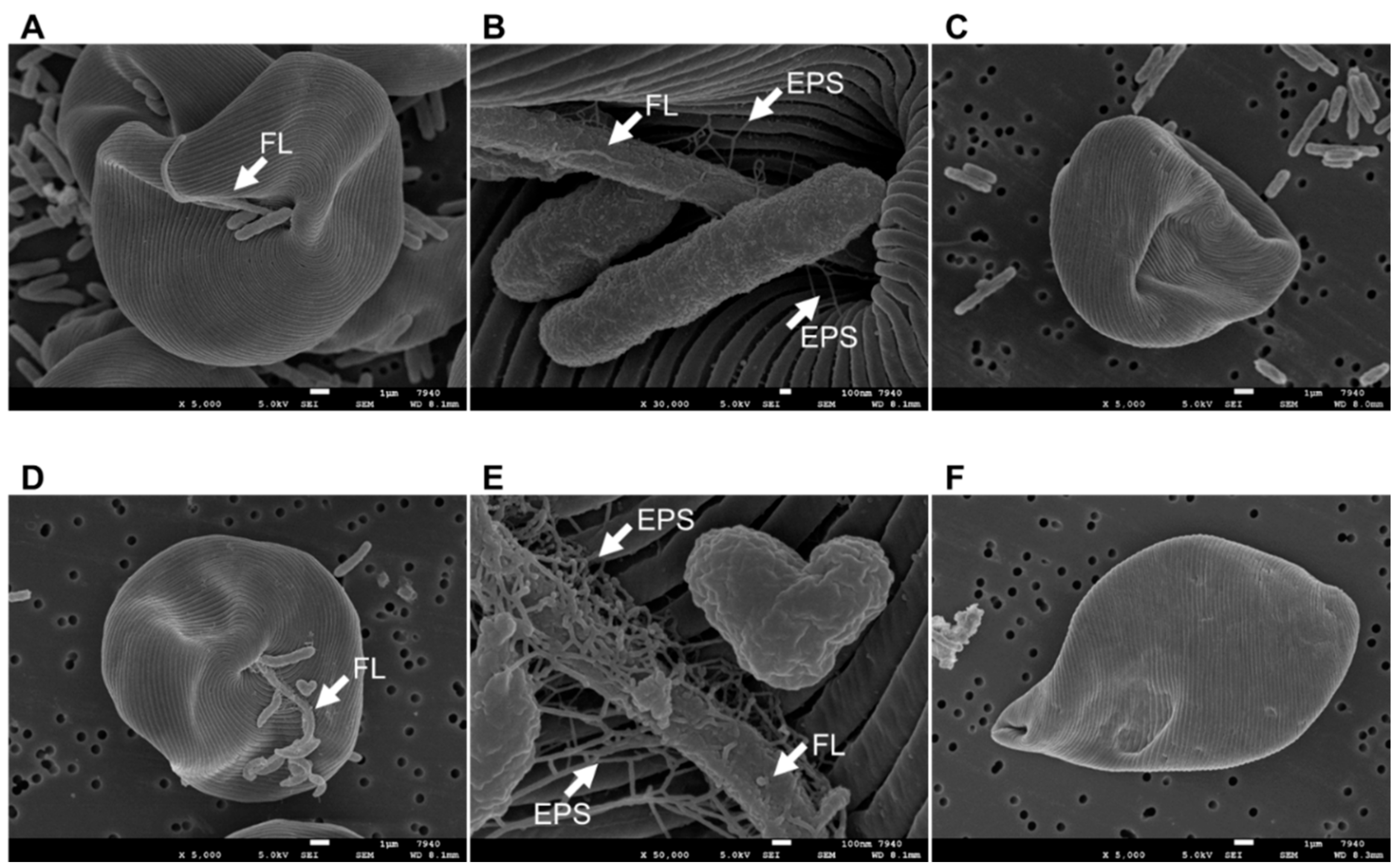
2.8. Scanning Electron Microscopy (SEM) of E. gracilis Cell Surfaces
2.9. Analytical Procedures
2.10. Statistical Analysis
3. Results
3.1. Isolation and Identification of Bacteria Promoting the Growth and Paramylon Production of E. gracilis
3.2. Growth of CA3 and CN5 Utilizing Glucose as a Sole Carbon Source
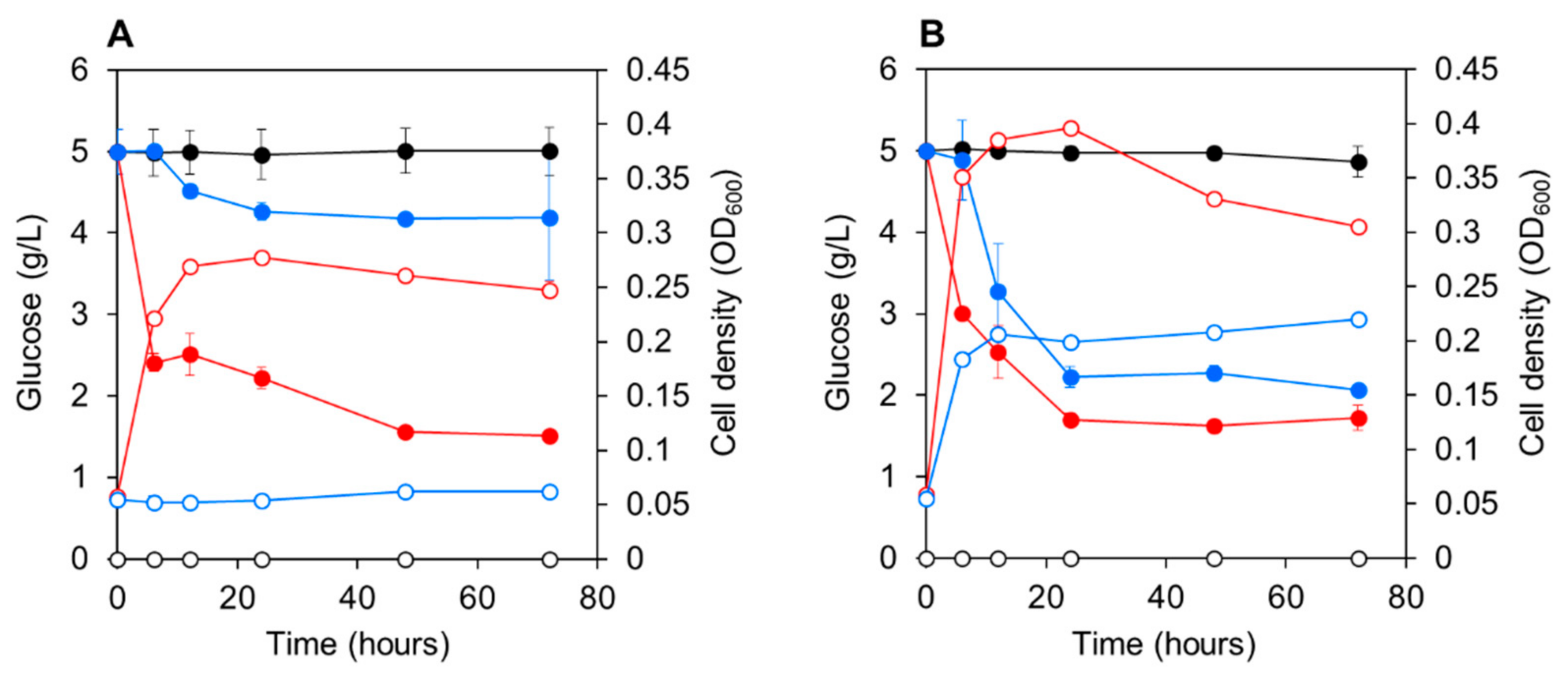
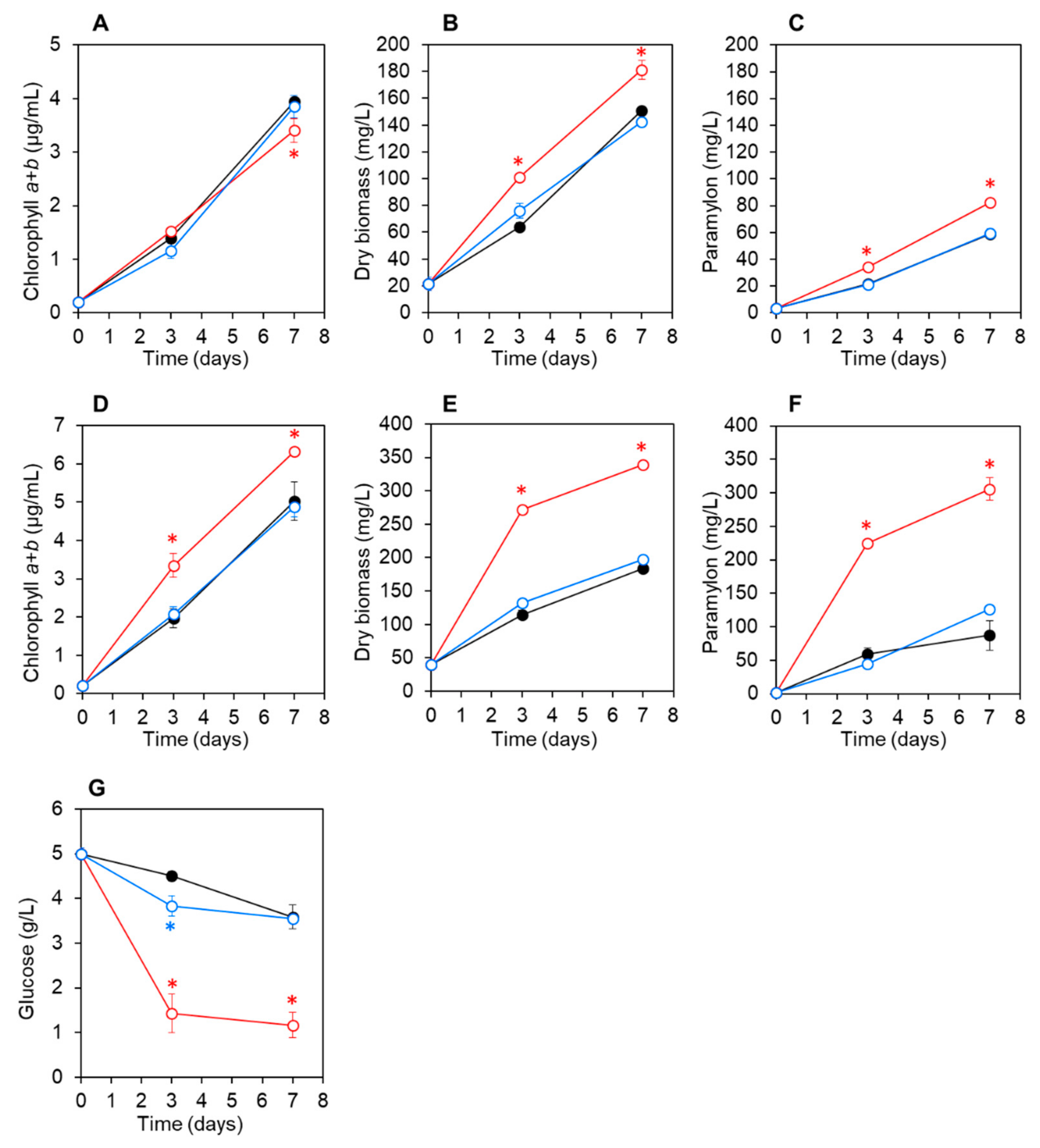
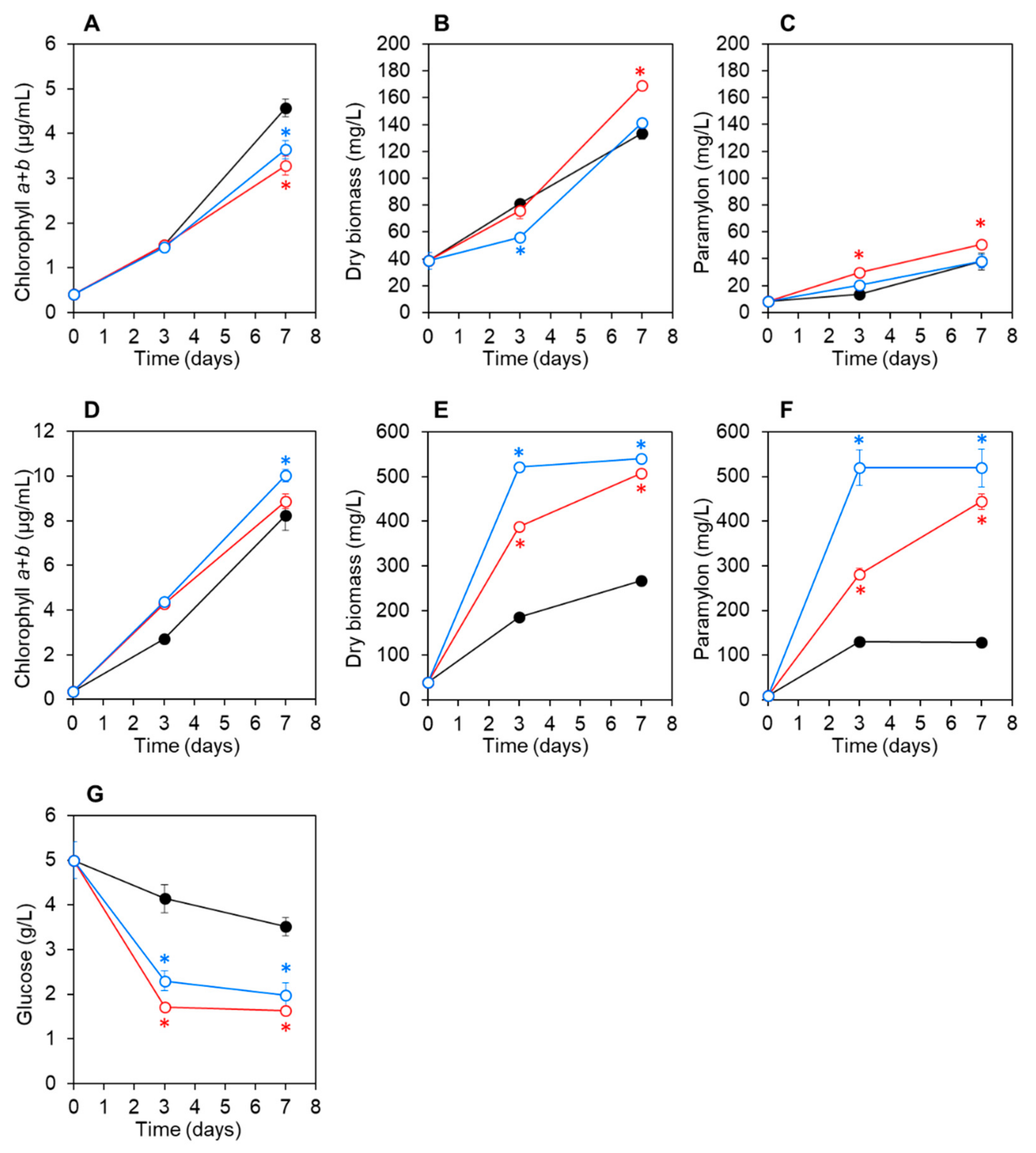
3.3. Co-Culturing E. gracilis with Strain CA3 or CN5 under Photoautotrophic or Mixotrophic Conditions at pH 4.5 or 7.5
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Barsanti, L.; Gualtieri, P. Is exploitation of microalgae economically and energetically sustainable? Algal. Res. 2018, 31, 107–115. [Google Scholar] [CrossRef]
- Gissibl, A.; Sun, A.; Care, A.; Nevalainen, H.; Sunna, A. Bioproducts from Euglena gracilis: Synthesis and applications. Front. Bioeng. Biotechnol. 2019, 7, 108. [Google Scholar] [CrossRef]
- Yamane, Y.I.; Utsunomiya, T.; Watanabe, M.; Sasaki, K. Biomass production in mixotrophic culture of Euglena gracilis under acidic condition and its growth energetics. Biotechnol. Lett. 2001, 23, 1223–1228. [Google Scholar] [CrossRef]
- Ogbonna, J.C.; Ichige, E.; Tanaka, H. Regulating the ratio of photoautotrophic to heterotrophic metabolic activities in photoheterotrophic culture of Euglena gracilis and its application to α-tocopherol production. Biotechnol. Lett. 2002, 24, 953–958. [Google Scholar] [CrossRef]
- Wang, Y.; Seppänen-Laakso, T.; Rischer, H.; Wiebe, M.G. Euglena gracilis growth and cell composition under different temperature, light and trophic conditions. PLoS ONE 2018, 13, e0195329. [Google Scholar] [CrossRef] [Green Version]
- Hasan, M.T.; Sun, A.; Khatiwada, B.; McQuade, L.; Mirzaei, M.; Te’o, J.; Hobba, G.; Sunna, A.; Nevalainen, H. Comparative proteomics investigation of central carbon metabolism in Euglena gracilis grown under predominantly phototrophic, mixotrophic and heterotrophic cultivations. Algal. Res. 2019, 43, 101638. [Google Scholar] [CrossRef]
- Takeyama, H.; Kanamaru, A.; Yoshino, Y.; Kakuta, H.; Kawamura, Y.; Matsunaga, T. Production of antioxidant vitamins, β-carotene, vitamin C, and vitamin E, by two-step culture of Euglena gracilis Z. Biotechnol. Bioeng. 1997, 53, 185–190. [Google Scholar] [CrossRef]
- Barsanti, L.; Bastianini, A.; Passarelli, V.; Tredici, M.R.; Gualtieri, P. Fatty acid content in wild type and WZSL mutant of Euglena gracilis. J. Appl. Phycol. 2000, 12, 515–520. [Google Scholar] [CrossRef]
- Kim, S.; Lee, D.; Lim, D.; Lim, S.; Park, S.; Kang, C.; Yu, J.; Lee, T. Paramylon production from heterotrophic cultivation of Euglena gracilis in two different industrial byproducts: Corn steep liquor and brewer’s spent grain. Algal. Res. 2020, 47, 101826. [Google Scholar] [CrossRef]
- Russo, R.; Barsanti, L.; Evangelista, V.; Frassanito, A.M.; Longo, V.; Pucci, L.; Penno, G.; Gualtieri, P. Euglena gracilis paramylon activates human lymphocytes by upregulating pro-inflammatory factors. Food Sci. Nutr. 2016, 5, 205–214. [Google Scholar] [CrossRef]
- Barsanti, L.; Gualtieri, P. Paramylon, a potent immunomodulator from WZSL mutant of Euglena gracilis. Molecules 2019, 24, 3114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koizumi, N.; Sakagami, H.; Utsumi, A.; Fujinaga, S.; Takeda, M.; Asano, K.; Sugawara, I.; Ichikawa, S.; Kondo, H.; Mori, S.; et al. Anti-HIV (human immunodeficiency virus) activity of sulfated paramylon. Antiviral Res. 1993, 21, 1–14. [Google Scholar] [CrossRef]
- Aoe, S.; Yamanaka, C.; Koketsu, K.; Nishioka, M.; Onaka, N.; Nishida, N.; Takahashi, M. Effects of paramylon extracted from Euglena gracilis EOD-1 on parameters related to metabolic syndrome in diet-induced obese mice. Nutrients 2019, 11, 1674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, T.; Shimada, R.; Matsuyama, A.; Yuasa, M.; Sawamura, H.; Yoshida, E.; Suzuki, K. Antitumor activity of the β-glucan paramylon from Euglena against preneoplastic colonic aberrant crypt foci in mice. Food Funct. 2013, 4, 1685–1690. [Google Scholar] [CrossRef]
- Nakashima, A.; Suzuki, K.; Asayama, Y.; Konno, M.; Saito, K.; Yamazaki, N.; Takimoto, H. Oral administration of Euglena gracilis Z and its carbohydrate storage substance provides survival protection against influenza virus infection in mice. Biochem. Biophys. Res. Commun. 2017, 494, 379–383. [Google Scholar] [CrossRef]
- Villa, J.A.; Ray, E.E.; Barney, B.M. Azotobacter vinelandii siderophore can provide nitrogen to support the culture of the green algae Neochloris oleoabundans and Scenedesmus sp. BA032. FEMS Microbiol. Lett. 2014, 351, 70–77. [Google Scholar] [CrossRef] [Green Version]
- Croft, M.T.; Lawrence, A.D.; Raux-Deery, E.; Warren, M.J.; Smith, A.G. Algae acquire vitamin B12 through a symbiotic relationship with bacteria. Nature 2005, 438, 90–93. [Google Scholar] [CrossRef]
- Amin, S.A.; Hmelo, L.R.; Tol, H.M.; Durham, B.P.; Carlson, L.T.; Heal, K.R.; Morales, R.L.; Berthiaume, C.T.; Parker, M.S.; Djunaedi, B.; et al. Interaction and signalling between a cosmopolitan phytoplankton and associated bacteria. Nature 2015, 522, 98–101. [Google Scholar] [CrossRef]
- Dao, G.; Wang, S.; Wang, X.; Chen, Z.; Wu, Y.; Wu, G.; Lu, Y.; Liu, S.; Hu, H. Enhanced Scenedesmus sp. growth in response to gibberellin secretion by symbiotic bacteria. Sci. Total Environ. 2020, 740, 140099. [Google Scholar] [CrossRef]
- Amin, S.A.; Green, D.H.; Hart, M.C.; Küpper, F.C.; Sunda, W.G.; Carrano, C.J. Photolysis of iron-siderophore chelates promotes bacterial-algal mutualism. Proc. Natl. Acad. Sci. USA 2009, 106, 17071–17076. [Google Scholar] [CrossRef] [Green Version]
- Amavizca, E.; Bashan, Y.; Ryu, C.-M.; Farag, M.A.; Bebout, B.M.; de-Bashan, L.E. Enhanced performance of the microalga Chlorella sorokiniana remotely induced by the plant growth-promoting bacteria Azospirillum brasilense and Bacillus pumilus. Sci. Rep. 2017, 7, 41310. [Google Scholar] [CrossRef]
- Kim, B.H.; Ramanan, R.; Cho, D.H.; Oh, H.M.; Kim, H.S. Role of Rhizobium, a plant growth promoting bacterium, in enhancing algal biomass through mutualistic interaction. Biomass. Bioenergy 2014, 69, 95–105. [Google Scholar] [CrossRef]
- Rivas, M.O.; Vargas, P.; Riquelme, C.E. Interactions of Botryococcus braunii cultures with bacterial biofilms. Microb. Ecol. 2010, 60, 628–635. [Google Scholar] [CrossRef]
- Tanabe, Y.; Okazaki, Y.; Yoshida, M.; Matsuura, H.; Kai, A.; Shiratori, T.; Ishida, K.; Nakano, S.; Watanabe, M.M. A novel alphaproteobacterial ectosymbiont promotes the growth of the hydrocarbon-rich green alga Botryococcus braunii. Sci. Rep. 2015, 5, 10467. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Park, B.S.; Wang, P.; Patidar, S.K.; Kim, J.H.; Kim, S.H.; Han, M.S. Phycospheric native bacteria Pelagibaca bermudensis and Stappia sp. ameliorate biomass productivity of Tetraselmis striata (KCTC1432BP) in co-cultivation system through mutualistic interaction. Front. Plant Sci. 2017, 8, 289. [Google Scholar] [CrossRef] [Green Version]
- Dao, G.H.; Wu, G.X.; Wang, X.X.; Zhang, T.Y.; Zhan, X.M.; Hu, H.Y. Enhanced microalgae growth through stimulated secretion of indole acetic acid by symbiotic bacteria. Algal. Res. 2018, 33, 345–351. [Google Scholar] [CrossRef]
- Toyama, T.; Hanaoka, T.; Yamada, K.; Suzuki, K.; Tanaka, Y.; Morikawa, M.; Mori, K. Enhanced production of biomass and lipids by Euglena gracilis via co-culturing with a microalga growth-promoting bacterium, Emticicia sp. EG3. Biotechnol. Biofuels 2019, 12, 205. [Google Scholar] [CrossRef]
- Kim, J.Y.; Oh, J.J.; Jeon, M.S.; Kim, G.H.; Choi, Y.E. Improvement of Euglena gracilis paramylon production through a cocultivation strategy with the indole-3-acetic acid-producing bacterium Vibrio natriegens. Appl. Environ. Microbiol. 2019, 85, e01548-19. [Google Scholar] [CrossRef] [Green Version]
- Jeon, M.S.; Oh, J.J.; Kim, J.Y.; Han, S.I.; Sim, S.J.; Choi, Y.E. Enhancement of growth and paramylon production of Euglena gracilis by co-cultivation with Pseudoalteromonas sp. MEBiC 03485. Bioresour. Technol. 2019, 288, 121513. [Google Scholar] [CrossRef]
- Jeon, M.S.; Han, S.I.; Kim, J.Y.; Choi, Y.E. Co-cultivation of Euglena gracilis and Pseudoalteromonas sp. MEBiC 03607 for paramylon production. J. Appl. Phycol. 2020, 32, 3679–3686. [Google Scholar] [CrossRef]
- Toyama, T.; Kasuya, M.; Hanaoka, T.; Kobayashi, N.; Tanaka, Y.; Inoue, D.; Sei, K.; Morikawa, M.; Mori, K. Growth promotion of three microalgae, Chlamydomonas reinhardtii, Chlorella vulgaris and Euglena gracilis, by in situ indigenous bacteria in wastewater effluent. Biotechnol. Biofuels 2018, 11, 176. [Google Scholar] [CrossRef] [Green Version]
- Rahman, A.; Sitepu, I.R.; Tang, S.Y.; Hashidoko, Y. Salkowski’s reagent test as a primary screening index for functionalities of rhizobacteria isolated from wild dipterocarp saplings growing naturally on medium-strongly acidic tropical peat soil. Biosci. Biotechnol. Biochem. 2010, 74, 2202–2208. [Google Scholar] [CrossRef] [Green Version]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Hipkins, M.F.; Baker, N.F. Photosynthesis: Energy Transduction a Practical Approach; IRL Press: Oxford, UK, 1986. [Google Scholar]
- Davin-Regli, A.; Lavigne, J.-P.; Pagès, J.M. Enterobacter spp.: Update on taxonomy, clinical aspects, and emerging antimicrobial resistance. Clin. Microbiol. Rev. 2019, 32, e00002-19. [Google Scholar] [CrossRef] [PubMed]
- Mazalan, N.Z.S.; Oyeleye, A.; Rahman, R.N.Z.R.A.; Aris, A.Z.; Salleh, A.B.; Normi, Y.M. Isolation and characterization of an acid and metal tolerant Enterobacter cloacae NZS strain from former mining lake in Selangor, Malaysia. Beni-Suef Univ. J. Basic Appl. Sci. 2020, 9, 27. [Google Scholar] [CrossRef]
- Sanket, A.S.; Ghosh, S.; Sahoo, R.; Nayak, S.; Das, A.P. Molecular identification of acidophilic manganese (Mn)-solubilizing bacteria from mining effluents and their application in mineral beneficiation. Geomicrobiol. J. 2017, 34, 71–80. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, I.H.; Lee, W.J.; Lee, J.H. Characterization of thiosulfate-oxidizing Enterobacter hormaechei JH isolated from barnyard manure. Korean J. Chem. Eng. 2008, 25, 1131–1135. [Google Scholar] [CrossRef]
- Joung, Y.; Seo, M.A.; Kang, H.; Kim, H.; Ahn, T.S.; Cho, J.C.; Joh, K. Emticicia aquatica sp. nov., a species of the family Cytophagaceae isolated from fresh water. Int. J. Syst. Evol. Microbiol. 2015, 65, 4358–4362. [Google Scholar] [CrossRef]
- Nam, G.G.; Joung, Y.; Song, J.; Lim, Y.; Cho, J.C. Emticiciafontis sp. nov., isolated from a freshwater pond. Int. J. Syst. Evol. Microbiol. 2016, 66, 5161–5166. [Google Scholar] [CrossRef]
- Ten, L.N.; Li, W.; Ha, A.; Kim, M.K.; Rooney, A.P.; Jung, H.Y. Emticicia agri sp. nov., a novel member of the family Cytophagaceae. Int. J. Syst. Evol. Microbiol. 2019, 69, 3492–3499. [Google Scholar] [CrossRef]
- Masojídek, J.; Torzillo, G.; Koblížek, M. Photosynthesis in microalgae. In Handbook of Microalgal Culture: Applied Phycology and Biotechnology, 2nd ed.; John Wiley & Sons: West Sussex, UK, 2013; pp. 21–36. [Google Scholar]
- Zhu, J.; Wakisaka, M. Growth promotion of Euglena gracilis by ferulic acid from rice bran. AMB Express 2018, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Noble, A.; Kisiala, A.; Galer, A.; Clysdale, D.; Emery, R.J.N. Euglena gracilis (Euglenophyceae) produces abscisic acid and cytokinins and responds to their exogenous application singly and in combination with other growth regulators. Eur. J. Phycol. 2014, 49, 244–254. [Google Scholar] [CrossRef]
| Strain and pH Condition | IAA-Producing Activity | EPS-Producing Activity | ||||
|---|---|---|---|---|---|---|
| IAA Concentration with l-Tryptophan (μmol L−1) 1 | IAA Concentration without l-Tryptophan | Free EPS | Cell-Bound EPS | |||
| Sugar (mg L−1) 1 | Protein (mg L−1) 1 | Sugar (mg L−1) 1 | Protein (mg L−1) 1 | |||
| CA3 at pH 4.5 | 5.48 ± 0.24 | Negative | 26.7 ± 0.03 | 16.5 ± 0.85 | 1.22 ± 0.00 | 1.07 ± 0.21 |
| CA3 at pH 7.5 | 8.11 ± 0.23 | Negative | 35.1 ± 0.08 | 17.1 ± 0.11 | 2.01 ± 0.00 | 2.49 ± 0.24 |
| CN5 at pH 4.5 | NT 2 | NT 2 | NT 2 | NT 2 | NT 2 | NT 2 |
| CN5 at pH 7.5 | 2.51 ± 0.07 | Negative | 31.7 ± 0.02 | 17.1 ± 0.06 | 2.14 ± 0.00 | 2.54 ± 0.32 |
| Culture Condition | Co-Culture Condition | Final Biomass and Paramylon Concentration after 7 d | ||
|---|---|---|---|---|
| Biomass (mg L−1) 1 | Paramylon (mg L−1) 1 | |||
| pH 4.5 | Autotrophic | Co-culture with CA3 | 181.3 ± 7.1 (1.2-fold) 2 | 82.5 ± 3.5 (1.4-fold) |
| Co-culture with CN5 | 142.7 ± 1.3 (0.95-fold) | 59.4 ± 2.8 (1.0-fold) | ||
| Control | 150.7 ± 0.7 | 58.9 ± 2.3 | ||
| Mixotrophic with glucose | Co-culture with CA3 | 338.7 ± 4.2 (1.8-fold) | 305.7 ± 16.8 (3.5-fold) | |
| Co-culture with CN5 | 197.3 ± 4.8 (1.1-fold) | 126.3 ± 1.3 (1.4-fold) | ||
| Control | 184.0 ± 5.3 | 187.1 ± 22.4 | ||
| pH 7.5 | Autotrophic | Co-culture with CA3 | 169.3 ± 0.2 (1.3-fold) | 50.6 ± 1.1 (1.3-fold) |
| Co-culture with CN5 | 141.3 ± 2.3 (1.1-fold) | 37.9 ± 5.0 (1.0-fold) | ||
| Control | 133.3 ± 4.2 | 37.9 ± 6.5 | ||
| Mixotrophic with glucose | Co-culture with CA3 | 508.0 ± 1.1 (1.9-fold) | 443.6 ± 17.7 (3.5-fold) | |
| Co-culture with CN5 | 540.0 ± 2.8 (2.0-fold) | 518.9 ± 41.7 (4.1-fold) | ||
| Control | 266.7 ± 3.1 | 128.0 ± 2.0 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubiyatno; Mori, K.; Inoue, D.; Kim, S.; Yu, J.; Lee, T.; Ike, M.; Toyama, T. Isolation and Characterization of Euglena gracilis-Associated Bacteria, Enterobacter sp. CA3 and Emticicia sp. CN5, Capable of Promoting the Growth and Paramylon Production of E. gracilis under Mixotrophic Cultivation. Microorganisms 2021, 9, 1496. https://doi.org/10.3390/microorganisms9071496
Rubiyatno, Mori K, Inoue D, Kim S, Yu J, Lee T, Ike M, Toyama T. Isolation and Characterization of Euglena gracilis-Associated Bacteria, Enterobacter sp. CA3 and Emticicia sp. CN5, Capable of Promoting the Growth and Paramylon Production of E. gracilis under Mixotrophic Cultivation. Microorganisms. 2021; 9(7):1496. https://doi.org/10.3390/microorganisms9071496
Chicago/Turabian StyleRubiyatno, Kazuhiro Mori, Daisuke Inoue, Sunah Kim, Jaecheul Yu, Taeho Lee, Michihiko Ike, and Tadashi Toyama. 2021. "Isolation and Characterization of Euglena gracilis-Associated Bacteria, Enterobacter sp. CA3 and Emticicia sp. CN5, Capable of Promoting the Growth and Paramylon Production of E. gracilis under Mixotrophic Cultivation" Microorganisms 9, no. 7: 1496. https://doi.org/10.3390/microorganisms9071496
APA StyleRubiyatno, Mori, K., Inoue, D., Kim, S., Yu, J., Lee, T., Ike, M., & Toyama, T. (2021). Isolation and Characterization of Euglena gracilis-Associated Bacteria, Enterobacter sp. CA3 and Emticicia sp. CN5, Capable of Promoting the Growth and Paramylon Production of E. gracilis under Mixotrophic Cultivation. Microorganisms, 9(7), 1496. https://doi.org/10.3390/microorganisms9071496






