Behavior of Listeria monocytogenes and Other Microorganisms in Sliced Riojano Chorizo (Spanish Dry-Cured Sausage) during Storage under Modified Atmospheres
Abstract
:1. Introduction
2. Materials and Methods
2.1. Riojano Chorizo Samples
2.2. Experimental Design
2.3. L. monocytogenes Strains and Inoculum Preparation
2.4. Chorizo Contamination
2.5. Packaging, Storage Conditions and Sampling
2.6. Physicochemical Analyses
2.7. Microbiological Analyses
2.8. Statistical Analysis
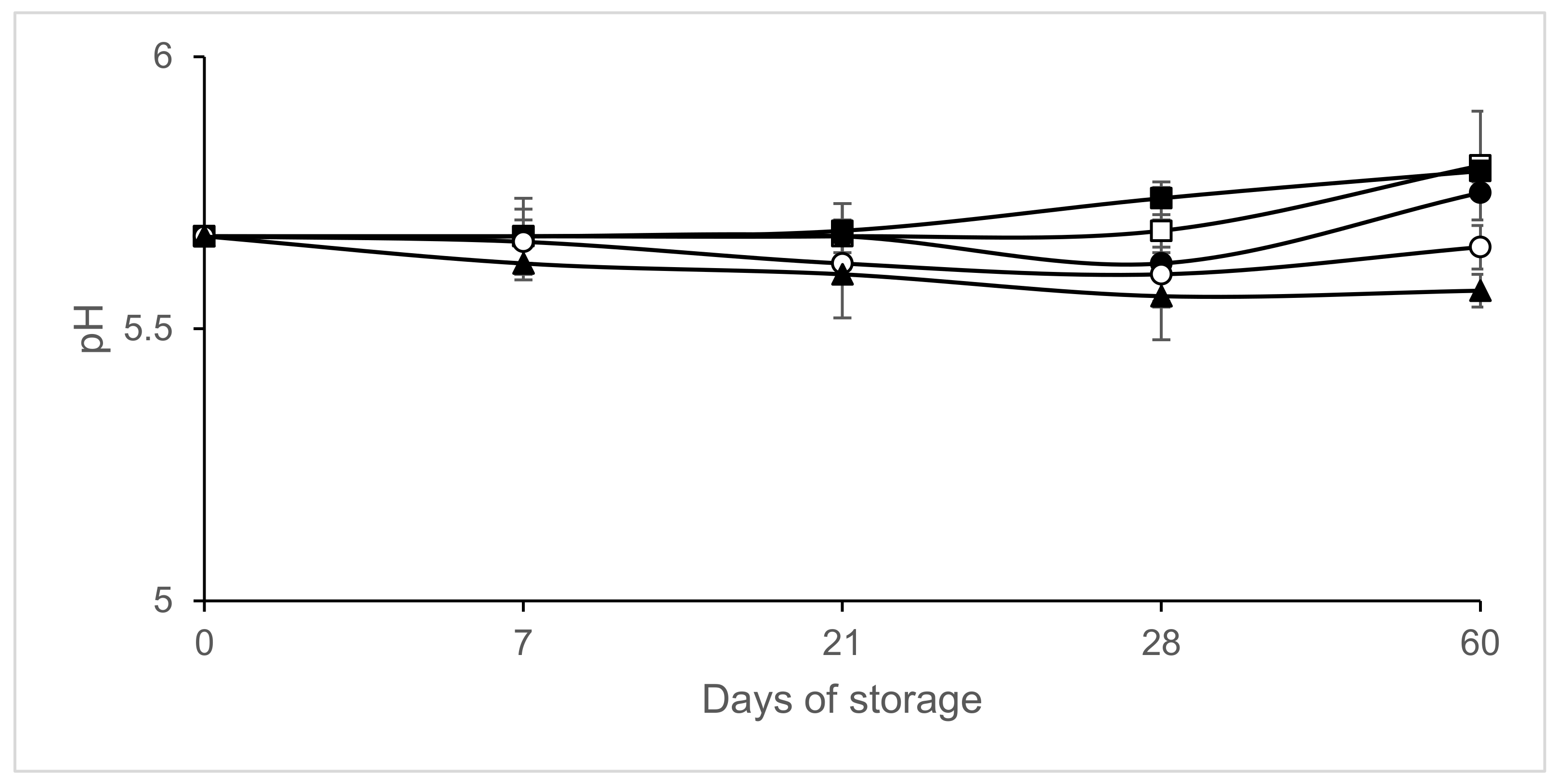
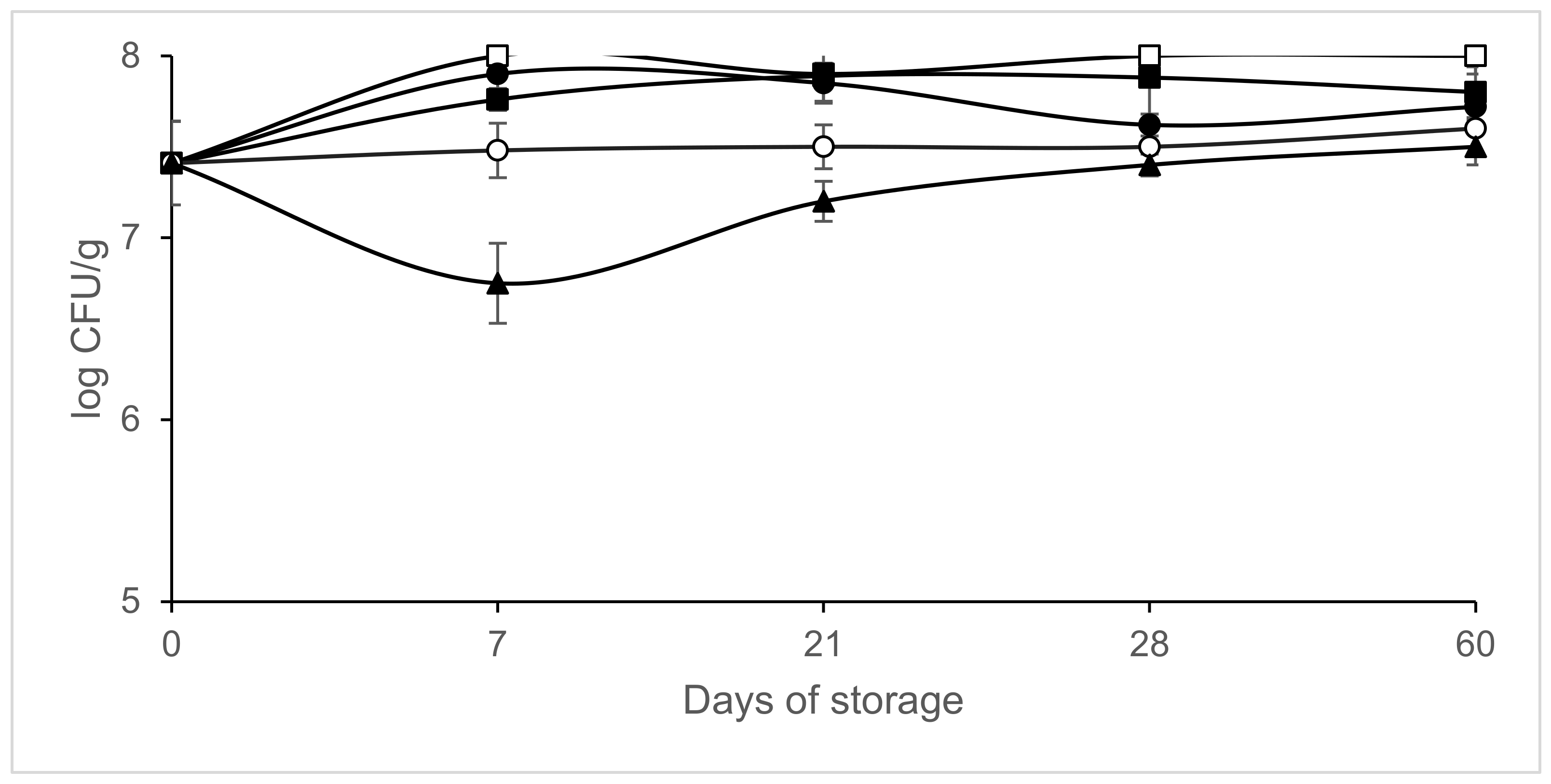
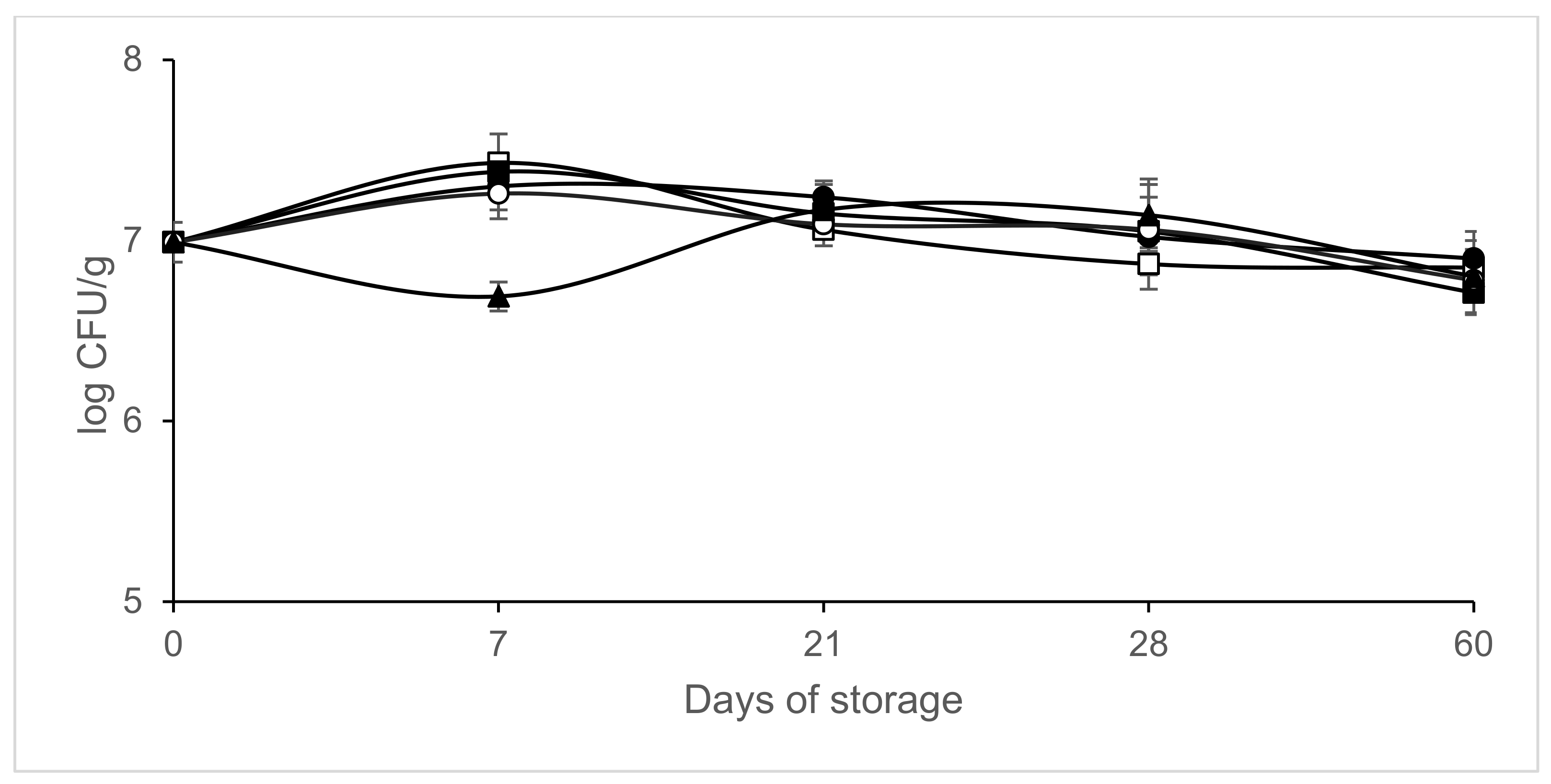
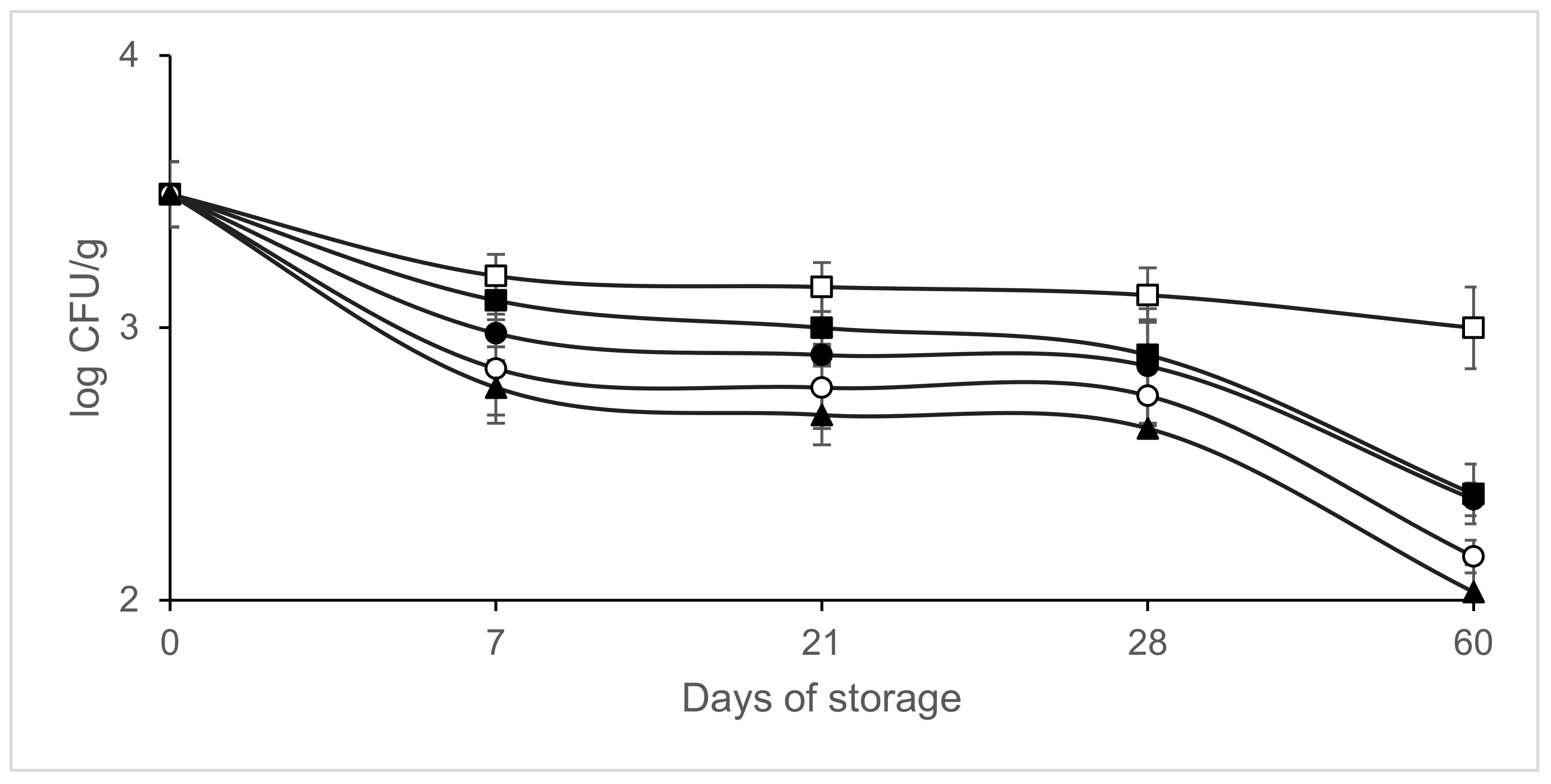
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ordóñez, J.A.; La Hoz, L. Mediterranean products. In Handbook of Fermented Meat and Poultry; Toldrá, F., Ed.; Blackwell Publishing: Oxford, UK, 2007; pp. 333–348. [Google Scholar]
- González-Fandos, M.E.; Sierra, M.; García-López, M.L.; García-Fernández, C.; Otero, A. The influence of manufacturing and drying conditions on the survival and toxinogenesis of Staphylococcus aureus in two Spanish dry fermented sausages (chorizo and salchichón). Meat Sci. 1999, 52, 411–419. [Google Scholar] [CrossRef]
- European Commision. Commission Regulation (EC). No 249/2010 of 24 March 2010 entering a name in the register of protected designations of origin and protected geographical indications [Riojano Chorizo (PGI)]. Off. J. Eur. Union 2010, L79/3, 1–2. [Google Scholar]
- González-Fandos, E.; Martínez Loza, J.J.; Maya, N.; Vázquez de Castro, M. Prevalence of Salmonella in Riojano chorizo during processing and final product. In Proceedings of the 3rd International Congress HACCP, Córdoba, Spain, 25 April 2008. [Google Scholar]
- Omer, M.K.; Álvarez-Ordoñez, A.; Prieto, M.; Skjerve, E.; Asehun, T.; Alvseike, A. A Systematic Review of Bacterial Foodborne Outbreaks Related to Red Meat and Meat. Foodborne Pathog. Dis. 2018, 15, 598–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lücke, F.K.; Zangerl, P. Food safety challenges associated with traditional foods in German-speaking regions. Food Control 2014, 43, 217–230. [Google Scholar] [CrossRef]
- Lebert, I.; Leroy, S.; Giammarinaro, P.; Lebert, A.; Chacornac, J.P.; Bover-Cid, S.; Vidal-Carou, M.C.; Talon, R. Diversity of microorganisms in the environment and dry fermented sausages of small traditional French processing units. Meat Sci. 2007, 77, 570–579. [Google Scholar] [CrossRef] [PubMed]
- EFSA. The European Union One Health 2019 Zoonoses Report. EFSA J. 2021, 19, 6406. [Google Scholar]
- EFSA. The European Union One Health 2018 Zoonoses Report. EFSA J. 2019, 17, 5926. [Google Scholar]
- Hoelzer, K.; Puillot, R.; Dennis, S. Update on Listeria monocytogenes. In Advances in Microbial Food Safety Vol. 2; Sofos, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 149–194. [Google Scholar]
- Knudsen, G.M.; Nielsen, J.B.; Marvig, R.L.; Ng, Y.; Worning, P.; Westh, H.; Gram, L. Genome-wide-analyses of Listeria monocytogenes from food processing plants reveal clonal diversity and date the emergence of persisting sequence types. Environ. Microbiol. Rep. 2017, 9, 428–440. [Google Scholar] [CrossRef] [Green Version]
- Thévenot, D.; Dernburg, A.; Vernozy, C. An updated review of Listeria monocytogenes in the pork meat industry and its products. J. Appl. Microbiol. 2006, 101, 7–17. [Google Scholar] [CrossRef]
- Encinas, J.P.; Sanz, J.J.; Garcia-Lopez, M.L.; Otero, A. Behaviour of Listeria spp. in naturally contaminated chorizo (Spanish fermented sausage). Int. J. Food Microbiol. 1999, 46, 167–171. [Google Scholar] [CrossRef]
- Farber, J.M.; Peterkin, P.I. Listeria monocytogenes, a food-borne pathogen. Microbiol. Rev. 1991, 55, 476–551. [Google Scholar] [CrossRef]
- Gormley, F.J.; Little, C.L.; Grant, K.A.; De Pinna, E.; McLauchlin, J. The microbiological safety of ready-to-eat specialty meats from markets and specialty food shops: A UK wide study with a focus on Salmonella and Listeria monocytogenes. Food Microbiol. 2010, 27, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Roccato, A.; Uyttendaele, M.; Barrucci, F.; Cibin, V.; Favretti, M.; Cereser, A.; Cin, M.D.; Pezzuto, A.; Piovesana, A.; Longo, A.; et al. Artisanal Italian salami and soppresse: Identification of control strategies to manage microbiological hazards. Food Microbiol. 2017, 61, 5–13. [Google Scholar] [CrossRef]
- García-Díez, J.G.; Patarata, L. Behavior of Salmonella spp., Listeria monocytogenes, and Staphylococcus aureus in chouriço de vinho, a dry fermented sausage made from wine-marinated meat. J. Food Prot. 2013, 76, 588–594. [Google Scholar] [CrossRef]
- Possas, A.; Valdramidis, V.; García-Gimeno, R.M.; Pérez-Rodríguez, F. High hydrostatic pressure processing of sliced fermented sausages: A quantitative exposure assessment for Listeria monocytogenes. Innovative Food Sci. Emerg. Technol. 2019, 52, 406–419. [Google Scholar] [CrossRef]
- Martin, B.; Garriga, M.; Aymerich, T. Prevalence of Salmonella spp. and Listeria monocytogenes at small-scale Spanish factories producing traditional fermented sausages. J. Food Prot. 2011, 174, 812–815. [Google Scholar] [CrossRef] [PubMed]
- Gómez, D.; Iguácel, L.; Rota, M.; Carramiñana, J.; Ariño, A.; Yangüela, J. Occurrence of Listeria monocytogenes in ready-to-eat meat products and meat processing plants in Spain. Food 2015, 4, 271–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabedo, L.; Barrot, L.; Canelles, A. Prevalence of Listeria monocytogenes and Salmonella in ready-to-eat food in Catalonia, Spain. J. Food Protect. 2008, 71, 855–859. [Google Scholar] [CrossRef]
- Meloni, D. Presence of Listeria monocytogenes in Mediterranean style dry fermented sausages. Foods 2015, 4, 34–50. [Google Scholar] [CrossRef] [Green Version]
- Cartwright, E.J.; Jackson, K.A.; Johnson, S.D.; Graves, L.M.; Silk, B.J.; Mahon, B.E. Listeriosis outbreaks and associated food vehicles, United States, 1998–2008. Emerg. Infect. Dis. 2013, 19, 1. [Google Scholar] [CrossRef] [PubMed]
- EFSA; ECDC. Scientific opinion on the Listeria monocytogenes contamination of ready-to-eat foods and the risk for human health in the EU. EFSA J. 2018, 16, 5134. [Google Scholar]
- European Commission. Commission Regulation (EC) No. 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. Off. J. Eur. Union 2005, L338, 1–26. [Google Scholar]
- Incze, K. Sausages, types of dry and semidry. In Encyclopedia of Meat Sciences; Jensen, W.K., Devine, C., Dikeman, E., Eds.; Academic Press: Amsterdam, The Netherlands, 2004; Volume 3, pp. 1207–1218. [Google Scholar]
- Encinas, J.P.; Lopez, T.; Garcia, M.L.; Otero, A.; Moreno, B. Yeast populations on Spanish fermented sausages. Meat Sci. 2000, 54, 203–208. [Google Scholar] [CrossRef]
- Al-Zeyara, S.A.; Jarvis, B.; Mackey, B.M. The inhibitory effect of natural microflora of food on growth of Listeria monocytogenes in enrichment broths. Int. J. Food Microbiol. 2011, 145, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Cornu, M.; Billoir, E.; Bergis, H.; Beaufort, A.; Zuliani, V. Modeling microbial competition in food: Application to the behavior of Listeria monocytogenes and lactic acid flora in pork meat products. Food Microbiol. 2011, 28, 639–647. [Google Scholar] [CrossRef] [PubMed]
- McMillin, K. Where is MAP Going? A review and future potential of modified atmosphere packaging for meat. Meat Sci. 2008, 80, 43–65. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, T.; Doyle, M.P. Transfer of foodborne pathogens during mechanical slicing and their inactivation by levulinic acid-based sanitizer on slicers. Food Microbiol. 2014, 38, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Ganan, M.; Hierro, E.; Hospital, X.F.; Barroso, E.; Fernández, M. Use of pulsed light to increase the safety of ready-to-eat cured meat products. Food Control 2013, 32, 512–517. [Google Scholar] [CrossRef]
- Lin, C.; Takeuchi, K.; Zhang, L.; Dohm, C.B.; Meyer, J.D.; Hall, P.A.; Doyle, M.P. Cross-Contamination between processing equipment and deli meats by Listeria monocytogenes. J. Food Protect. 2006, 69, 71–79. [Google Scholar] [CrossRef]
- Vorst, K.L.; Todd, E.C.D.; Ryser, E.T. Transfer of Listeria monocytogenes during mechanical slicing of turkey breast, bologna, and salami. J. Food Protect. 2006, 69, 619–696. [Google Scholar] [CrossRef]
- Cava, R.; García-Parra, J.; Ladero, L. Effect of high hydrostatic pressure processing and storage temperature on food safety, microbial counts, colour and oxidative changes of a traditional dry-cured sausage. LWT Food Sci. Technol. 2020, 28, 109462. [Google Scholar] [CrossRef]
- Abatcha, M.G.; Goni, M.D.; Abbas, M.A.; Jalo, I.M.; Mohammed, G. A Review of Listeria and Salmonella: An update on description, characteristics, incidence, and antibiotic susceptibility. Adv. Anim. Vet. Sci. 2020, 8, 1232–1248. [Google Scholar] [CrossRef]
- Nielsen, C.K.; Kjems, J.; Mygind, T.; Snabe, T.; Meyer, R.K. Effects of Tween 80 on Growth and Biofilm Formation in Laboratory Media. Front. Microbiol. 2016, 7, 1878. [Google Scholar] [CrossRef]
- Simón, A.; González-Fandos, E. Influence of modified atmosphere packaging and storage temperature on the sensory and microbiological quality of fresh peeled white asparagus. Food Control 2011, 22, 369–374. [Google Scholar] [CrossRef]
- González-Fandos, E.; Herrera, B. Efficacy of acetic acid against Listeria monocytogenes attached in poultry skin during refrigerated storage. Foods 2014, 3, 527–540. [Google Scholar] [CrossRef] [Green Version]
- Castaño, A.; Garcia-Fontan, M.C.; Fresno, J.M.; Tornadijo, M.E.; Carballo, J. Survival of Enterobacteriaceae during processing pf Chorizo de cebolla, a Spanish fermented sausage. Food Control 2002, 13, 107–115. [Google Scholar] [CrossRef]
- Cabeza, M.C.; de la Hoz, L.; Velasco, R.; Cambero, M.I.; Ordóñez, J.A. Safety and quality of ready-to-eat dry fermented sausages subjected to E-beam radiation. Meat Sci. 2009, 83, 320–327. [Google Scholar] [CrossRef]
- Menéndez, R.A.; Rendueles, E.; Sanz, J.J.; Santos, J.A.; García-Fernández, M.C. Physicochemical and microbiological characteristics of diverse Spanish cured meat products. CyTA J. Food 2018, 16, 199–204. [Google Scholar] [CrossRef]
- Stollewerk, K.; Jofré, A.; Comaposada, J.; Ferrini, G.; Garriga, M. Ensuring food safety by an innovative fermented sausage manufacturing system. Food Control 2011, 22, 1984–1991. [Google Scholar] [CrossRef]
- Thevenot, D.; Delignette-Muller, M.L.; Christieans, S.; Vernozy-Rozand, C. Prevalence of Listeria monocytogenes in 13 dried sausage processing plants and their products. Int. J. Food Microbiol. 2005, 102, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Menendez, R.; Rendueles, E.; Sanz, J.; Capita, R.; Garcia-Fernandez, C. Behavior of Listeria monocytogenes in Sliced Ready-to-Eat Meat Products Packaged under Vacuum or Modified Atmosphere Conditions. J. Food Prot. 2015, 78, 1891–1895. [Google Scholar] [CrossRef]
- Chevallier, I.; Ammor, S.; Laguet, A.; Labayle, S.; Castanet, V.; Dufour, E.; Talon, R. Microbial ecology of a small-scale facility producing traditional dry sausage. Food Control 2006, 17, 446–453. [Google Scholar] [CrossRef]
- Marcos, B.; Aymerich, T.; Garriga, M. Evaluation of high pressure processing as an additional hurdle to control Listeria monocytogenes and Salmonella enterica in low-acid fermented sausages. J. Food Sci. 2005, 70, M339–M344. [Google Scholar] [CrossRef]
- Prado, N.; Sampayo, M.; González, P.; Lombó, F.; Díaz, J. Physicochemical, sensory and microbiological characterization of Asturian Chorizo, a traditional fermented sausage manufactured in Northern Spain. Meat Sci. 2019, 156, 118–124. [Google Scholar] [CrossRef]
- Tirloni, E.; Di Pietro, V.; Rizzi, G.; Pomilio, F.; Cattaneo, P.; Bernardi, C.; Stella, S. Non-thermal inactivation of Listeria spp. in a typical dry-fermented sausage. Ital. J. Food Saf. 2019, 8, 168–173. [Google Scholar]
- Mataragas, M.; Bellio, A.; Rovetto, F.; Astegiano, S.; Greci, C.; Hertel, C.; Decastelli, L.; Cocolin, L. Quantification of persistence of the food-borne pathogens Listeria monocytogenes and Salmonella enterica during manufacture of Italian fermented sausages. Food Control 2015, 47, 552–559. [Google Scholar] [CrossRef]
- Byelashov, O.A.; Carlson, B.A.; Geornaras, I.; Kendall, P.A.; Scanga, J.A.; Sofos, J.N. Fate of postprocessing inoculated Listeria monocytogenes on vacuum-packaged pepperoni stored at 4, 12 or 25 °C. Food Microbiol. 2009, 26, 77–81. [Google Scholar] [CrossRef]
- García-Díez, J.; Patarata, L. Influence of salt level, starter culture, fermentable carbohydrates, and temperature on the behaviour of L. monocytogenes in sliced chouriço during storage. Acta Aliment. 2017, 46, 206–213. [Google Scholar] [CrossRef] [Green Version]
- Fontana, C.; Cocconcelli, P.S.; Vignolo, G. Monitoring the bacterial population dynamics during fermentation of artisanal Argentinean sausages. Int. J. Food Microbiol. 2005, 103, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Jácome, S.L.; Fonseca, S.; Pinheiro, R.; Todorov, D.S.; Noronhad, L.; Silva, J.; Gomes, A.; Pintado, M.; Morais, A.M.M.B.; Teixeira, P.; et al. Effect of Lactic Acid Bacteria on Quality and Safety of Ready-to-eat Sliced Cured/Smoked Meat Products. Chem. Eng. Trans. 2014, 38, 403–408. [Google Scholar]
- Papamanoli, E.; Kotzekidou, P.; Tzanetakis, N.; Litopoulou-Tzanetaki, E. Characterization of Micrococcaceae isolated from dry fermented sausage. Food Microbiol. 2002, 19, 441–449. [Google Scholar] [CrossRef]
- González-Fandos, M.E.; Sierra, M.L.; García-López, M.L.; Otero, A.; Sanz, J.; Moreno, B. Staphylococcal growth and enterotoxin production in the presence of meat cultures (non LAB). Meat Sci. 1996, 43, 255–263. [Google Scholar] [CrossRef]
- Lebert, I.; Leroy, S.; Talon, R. Microorganims in traditional fermented sausages: In Mediterranean products. In Handbook of Fermented Meat and Poultry; Toldrá, F., Ed.; Blackwell Publishing: Oxford, UK, 2007; pp. 113–124. [Google Scholar]
- Cardinali, F.; Milanović, V.; Osimani, A.; Aquilanti, L.; Taccari, M.; Garofalo, C.; Polverigiani, S.; Clementi, F.; Franciosi, E.; Tuohy, K.; et al. Microbial dynamics of model Fabriano-like fermented sausages as affected by starter cultures, nitrates and nitrites. Int. J. Food Microbiol. 2018, 278, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Drosinos, E.H.; Mataragas, M.; Xiraphi, N.; Moschonas, G.; Gaitis, F.; Metaxopoulos, J. Characterization of the microbial flora from a traditional Greek fermented sausage. Meat Sci. 2005, 69, 307–317. [Google Scholar] [CrossRef]
- Meloni, D.; Consolati, S.G.; Mazza, R.; Mureddu, A.; Fois, F.; Piras, F.; Mazette, R. Presence and molecular characterization of the major serovars of Listeria monocytogenes in ten Sardinian fermented sausage processing plants. Meat Sci. 2014, 97, 443–450. [Google Scholar] [CrossRef]
- Christieans, S.; Picgirard, L.; Parafita, E.; Lebert, A.; Gregoric, T. Impact of reducing nitrate/nitrite levels on the behavior of Salmonella Typhimurium and Listeria monocytogenes in French dry fermented sausages. Meat Sci. 2018, 137, 160–167. [Google Scholar] [CrossRef]
- Nikodinoska, I.; Baffonia, L.; Gioia, D.; Manso, B.; García-Sánchez, L.; Melero, B.; Rovira, J. Protective ultures against foodborne pathogens in a nitrite reduced fermented meat product. LWT Food Sci. Technol. 2019, 101, 293–299. [Google Scholar] [CrossRef]
- Branciari, R.; Ortenzi, R.; Roila, R.; Miraglia, D.; Ranucci, D.; Valiani, A. Listeria monocytogenes in soft spreadable salami: Study of- the pathogen behavior and growth prediction during manufacturing process and shelf life. Appl. Sci. 2020, 10, 4438. [Google Scholar] [CrossRef]
- Martínez, L.; Pedro Bastida, P.; Castillo, J.; Ros, G.; Gema Nieto, G. Green Alternatives to Synthetic Antioxidants, Antimicrobials, Nitrates, and Nitrites in Clean Label Spanish Chorizo. Antioxidants 2019, 8, 184. [Google Scholar] [CrossRef] [Green Version]
- Amadoro, C.; Rossi, F.; Piccirilli, M.; Colavita, G. Features of Lactobacillus sakei isolated from Italian sausages: Focus on strains from Ventricina del Vastese. Ital. J. Food Saf. 2015, 4, 5449. [Google Scholar] [CrossRef] [Green Version]
- Nyquist, O.L.; McLeod, A.; Brede, D.A.; Snipen, L.; Aakra, Å.; Nes, I.F. Comparative genomics of Lactobacillus sakei with emphasis on strains from meat. Mol. Genet. Genom. 2011, 285, 297–311. [Google Scholar] [CrossRef]
- Goerges, S.; Aigner, U.; Silakowski, B.; Scherer, S. Inhibition of Listeria monocytogenes by food-borne yeasts. Appl. Environ. Microbiol. 2006, 72, 313–318. [Google Scholar] [CrossRef] [Green Version]
- Goerges, S.; Koslowsky, M.; Velagic, S.; Borst, N.; Bockelmann, W.; Heller, K.J.; Scherer, S. Anti-listerial potential of food-borne yeasts in red smear cheese. Int. Dairy J. 2011, 21, 83–89. [Google Scholar] [CrossRef]
- Meisel, C.; Gehlen, K.H.; Fischer, A.; Hammes, W.P. Inhibition of the growth of Staphylococcus aureus in dry sausages by Lactobacillus curvatus, Micrococcus varians and Debaryomyces hansenii. Food Biotechnol. 1989, 3, 145–168. [Google Scholar] [CrossRef]
- Alia, A.; Cordoba, J.J.; Rodriguez, A.; Garcia, C.; Andrade, M.J. Evaluation of the efficacy of Debaryomyces hansenii as protective culture for controlling Listeria monocytogenes in sliced dry-cured ham. LWT Food Sci. Technol. 2020, 119, 108886. [Google Scholar] [CrossRef]
- Dura, A.; Flores, M.; Toldra, F. Effect of Debaryomyces spp. on the proteolysis of dry fermented sausage. Meat Sci. 2004, 68, 319–328. [Google Scholar] [CrossRef]
- Mellefont, L.A.; McMeekin, T.A.; Ross, T. Effect of relative inoculum concentration on Listeria monocytogenes growth in co-culture. Int. J. Food Microbiol. 2008, 121, 157–168. [Google Scholar] [CrossRef]
- Lindqvist, R.; Lindblad, M. Inactivation of Escherichia coli, Listeria monocytogenes and Yersinia enterocolitica in fermented sausages during maturation/storage. Int. J. Food Microbiol. 2009, 129, 59–67. [Google Scholar] [CrossRef]
- Gounadaki, A.S.; Skandamis, P.N.; Drosinos, E.H.; Nychas, G.J.E. Effect of packaging and storage temperature on the survival of Listeria monocytogenes inoculated postprocessing on sliced salami. J. Food Prot. 2007, 70, 2313–2320. [Google Scholar] [CrossRef] [PubMed]
- Esturk, O.; Ayhan, Z. Effect of modified atmosphere packaging and Storage time on physical and sensory properties of Sliced salami. J. Food Process. Preserv. 2009, 33, 114–125. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.S.; Stratakos, A.C. Application of Modified Atmosphere Packaging and Active/Smart Technologies to Red Meat and Poultry: A Review. Food Bioprocess Technol. 2012, 5, 1423–1446. [Google Scholar] [CrossRef]
- Comi, G.; Urso, R.; Paiani, M.; Ottaviani, S. Additive and aw influence on L. monocytogenes behaviour in dry-cured ham packaged both under vacuum and under MAP conditions. Ind. Aliment. 2005, 44, 272–278. [Google Scholar]
- Saraiva, C.; Fontes, M.C.; Patarata, L.; Martins, C.; Cadavez, V.; Gonzales-Barron, U. Modelling the kinetics of Listeria monocytogenes in refrigerated fresh beef under different packaging atmospheres. LWT Food Sci. Technol. 2016, 66, 664–671. [Google Scholar] [CrossRef] [Green Version]
- Talon, R.; Lebert, I.; Lebert, A.; Leroy, S.; Garriga, M.; Aymerich, T.; Drosinos, E.H.; Zanardi, E.; Ianieri, A.; Fraqueza, M.J.; et al. Traditional dry fermented sausages produced in smallscale processing units in Mediterranean countries and Slovakia. 1: Microbial ecosystems of processing environments. Meat Sci. 2007, 77, 570–579. [Google Scholar] [CrossRef]
- Zwirzitz, B.; Wetzels, S.U.; Dixon, E.D.; Fleischmann, S.; Selberherr, E.; Thalguter, S.; Quijada, N.M.; Dzieciol, M.; Wagner, M.; Stessl, B. Co-Occurrence of Listeria spp. and spoilage associated Microbiota During Meat Processing Due to Cross-Contamination Events. Front. Microbiol. 2021, 12, 632935. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez-Fandos, E.; Vazquez de Castro, M.; Martinez-Laorden, A.; Perez-Arnedo, I. Behavior of Listeria monocytogenes and Other Microorganisms in Sliced Riojano Chorizo (Spanish Dry-Cured Sausage) during Storage under Modified Atmospheres. Microorganisms 2021, 9, 1384. https://doi.org/10.3390/microorganisms9071384
Gonzalez-Fandos E, Vazquez de Castro M, Martinez-Laorden A, Perez-Arnedo I. Behavior of Listeria monocytogenes and Other Microorganisms in Sliced Riojano Chorizo (Spanish Dry-Cured Sausage) during Storage under Modified Atmospheres. Microorganisms. 2021; 9(7):1384. https://doi.org/10.3390/microorganisms9071384
Chicago/Turabian StyleGonzalez-Fandos, Elena, Maria Vazquez de Castro, Alba Martinez-Laorden, and Iratxe Perez-Arnedo. 2021. "Behavior of Listeria monocytogenes and Other Microorganisms in Sliced Riojano Chorizo (Spanish Dry-Cured Sausage) during Storage under Modified Atmospheres" Microorganisms 9, no. 7: 1384. https://doi.org/10.3390/microorganisms9071384
APA StyleGonzalez-Fandos, E., Vazquez de Castro, M., Martinez-Laorden, A., & Perez-Arnedo, I. (2021). Behavior of Listeria monocytogenes and Other Microorganisms in Sliced Riojano Chorizo (Spanish Dry-Cured Sausage) during Storage under Modified Atmospheres. Microorganisms, 9(7), 1384. https://doi.org/10.3390/microorganisms9071384






