Beneficial Microorganisms to Control the Gray Mold of Grapevine: From Screening to Mechanisms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Sampling
2.2. Isolation, Purification, and Enrichment of Antagonistic Bacteria
2.3. In Vitro Screening of Potential Antagonistic Bacteria
2.4. Identification of Antagonistic Bacteria
2.5. Biochemical Characterization of Biocontrol Isolates
2.6. Cellular Fatty Acid Analysis
2.7. Whole Genome Sequencing, Assembly, and Annotation
2.8. Grapevine In Vitro Plantlets
2.9. Bacterial Isolates and Inoculum Preparation
2.10. Inoculation of In Vitro Plantlets with Antagonistic Bacteria and Infection by B. cinerea
2.11. IMAGING-PAM Analysis
2.12. Statistical Analysis
3. Results
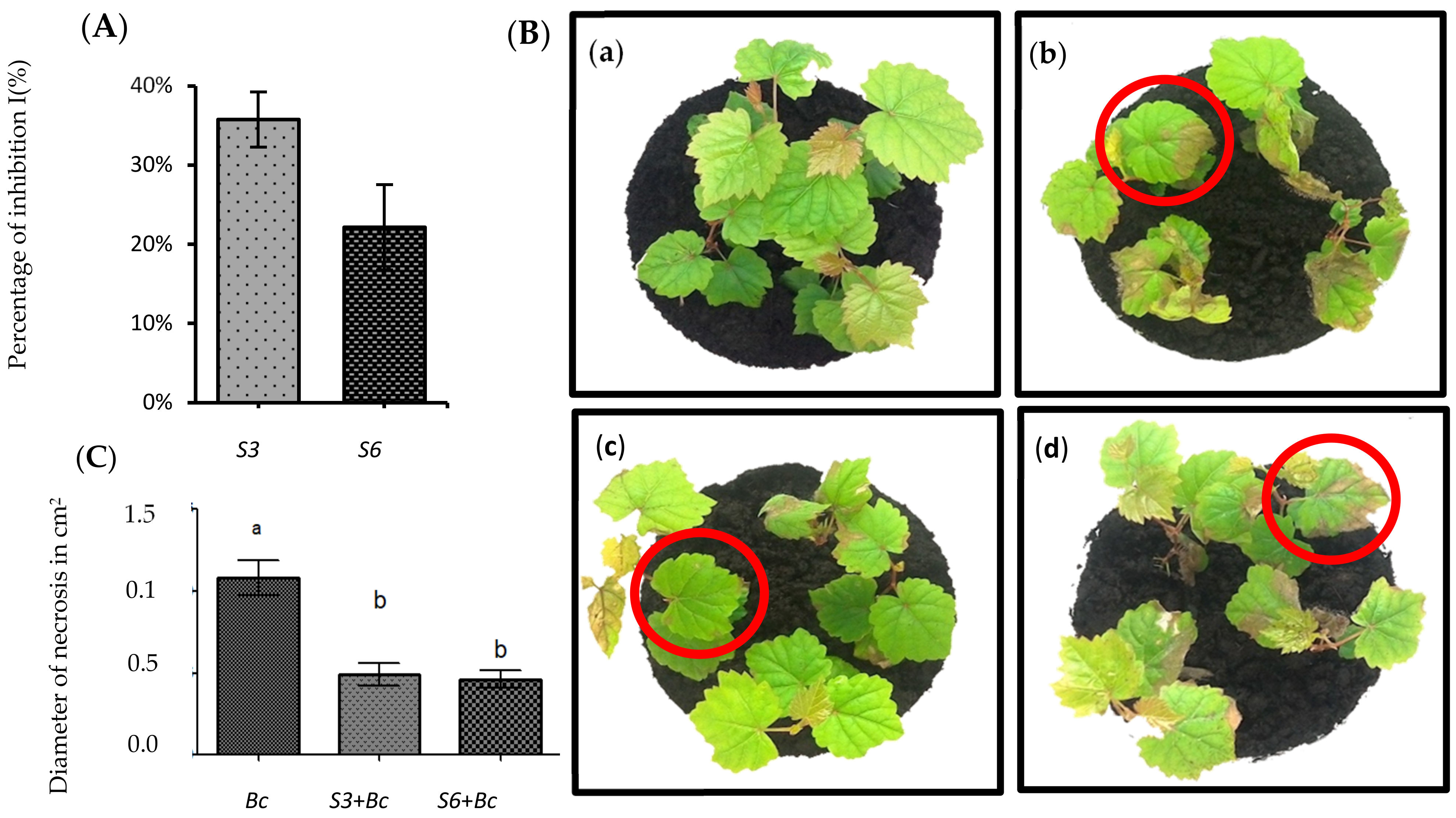
3.1. Isolation and In Vitro Screening of Antagonistic Bacteria
3.2. Disease Symptoms Were Significantly Reduced in Root-Bacterized Plantlets
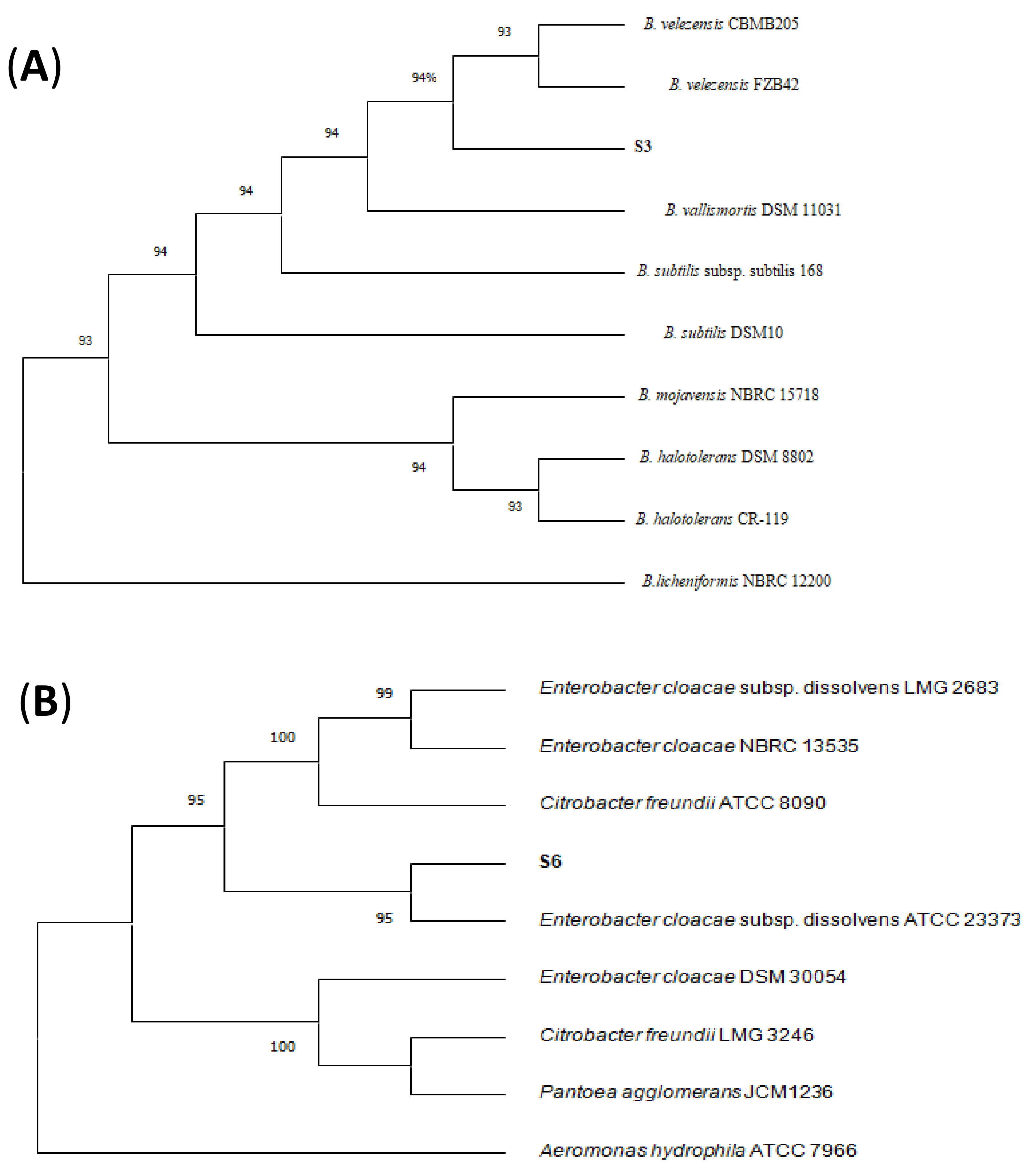
3.3. Identification of Antagonistic Bacteria
3.4. Characterization of Biocontrol Isolates
3.5. Genomic Feature and In Silico Analysis
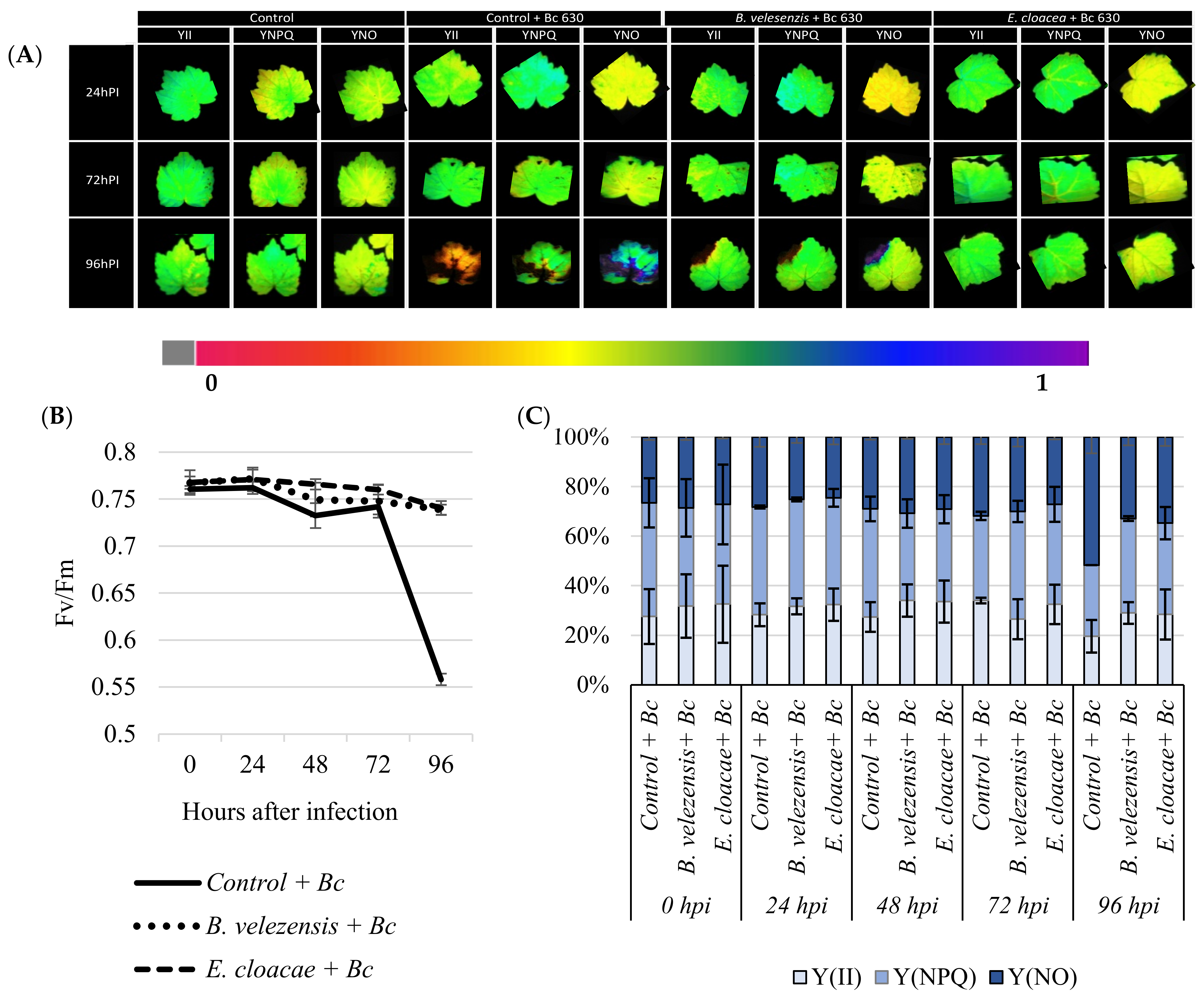
3.6. Antagonistic Strains Prevent Plants from Considerable Photo-Inhibition of PSII after Pathogen Challenge
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rojas, C.M.; Senthil-Kumar, M.; Tzin, V.; Mysore, K.S. Regulation of primary plant metabolism during plant-pathogen interactions and its contribution to plant defense. Front. Plant Sci. 2014, 5, 17. [Google Scholar] [CrossRef] [Green Version]
- Verdenal, T.; Dienes-Nagy, Á.; Spangenberg, J.E.; Zufferey, V.; Spring, J.; Viret, O.; Marin-Carbonne, J.; van Leeuwen, C. Understanding and managing nitrogen nutrition in grapevine: A review. OENO One 2021, 55, 1–43. [Google Scholar] [CrossRef]
- Júnior, A.F.N.; Tränkner, M.; Ribeiro, R.V.; von Tiedemann, A.; Amorim, L. Photosynthetic Cost Associated With Induced Defense to Plasmopara viticola in Grapevine. Front. Plant Sci. 2020, 11, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Bonfig, K.B.; Schreiber, U.; Gabler, A.; Roitsch, T.; Berger, S. Infection with virulent and avirulent P. syringae strains differentially affects photosynthesis and sink metabolism in Arabidopsis leaves. Planta 2006, 225, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, R.; Maia, M.; Ferreira, A.E.N.; Silva, A.B.; Freire, A.P.; Cordeiro, C.; Silva, M.S.; Figueiredo, A. Early stage metabolic events associated with the establishment of Vitis vinifera–Plasmopara viticola compatible interaction. Plant Physiol. Biochem. 2019, 137, 1–13. [Google Scholar] [CrossRef]
- Jones, D.J.L.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Windram, O.; Denby, K.J. Modelling signaling networks underlying plant defence. Curr. Opin. Plant Biol. 2015, 27, 165–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Lorenzo, G.; Ferrari, S.; Cervone, F.; Okun, E. Extracellular DAMPs in Plants and Mammals: Immunity, Tissue Damage and Repair. Trends Immunol. 2018, 39, 937–950. [Google Scholar] [CrossRef]
- Fillinger, S.; Yigal, E. Botrytis—The Fungus, the Pathogen and Its Management in Agricultural Systems; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Dean, R.; Van-Kan, J.A.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di-Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [Green Version]
- Weiberg, A.; Wang, M.; Lin, F.-M.; Zhao, H.; Zhang, Z.; Kaloshian, I.; Huang, H.; Jin, H. Fungal Small RNAs Suppress Plant Immunity by Hijacking Host RNA Interference Pathways. Science 2013, 342, 118–123. [Google Scholar] [CrossRef] [Green Version]
- Boddy, L. Pathogens of Autotrophs, 3rd ed.; Watkinson, S.C., Boddy, L., Money, N.P., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 245–292, Chapter 8. [Google Scholar]
- Fernández-Ortuño, D.; Torés, J.A.; Chamorro, M.; Pérez-García, A.; de Vicente, A. Characterization of resistance to six chemical classes of site-specific fungicides registered for gray mold control on strawberry in Spain. Plant Dis. 2016, 100, 2234–2239. [Google Scholar] [CrossRef] [Green Version]
- Rupp, S.; Weber, R.W.S.; Rieger, D.; Detzel, P.; Hahn, M. Spread of Botrytis cinerea strains with multiple fungicide resistance in german horticulture. Front. Microbiol. 2017, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, W.X.; Adnan, M.; Shang, Y.; Lin, Y.; Luo, C.X. Sensitivity of Botrytis cinerea from nectarine/cherry in China to six fungicides and characterization of resistant isolates. Plant Dis. 2018, 102, 2578–2585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sautua, F.J.; Baron, C.; Pérez-Hernández, O.; Carmona, M.A. First report of resistance to carbendazim and procymidone in Botrytis cinerea from strawberry, blueberry and tomato in Argentina. Crop Prot. 2019, 125, 2017–2020. [Google Scholar] [CrossRef]
- Reeves, W.R.; McGuire, M.K.; Stokes, M.; Vicini, J.L. Assessing the Safety of Pesticides in Food: How Current Regulations Protect Human Health. Adv. Nutr. 2019, 10, 80–88. [Google Scholar] [CrossRef]
- Liu, J.; Sui, Y.; Wisniewski, M.; Droby, S.; Liu, Y. Review: Utilization of antagonistic yeasts to manage postharvest fungal diseases of fruit. Int. J. Food Microbiol. 2013, 167, 153–160. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W.; Zhou, Y.; Yao, S.; Deng, L.; Zeng, K. Isolation, identification and in vitro screening of Chongqing orangery yeasts for the biocontrol of Penicillium digitatum on citrus fruit. Biol. Control 2017, 110, 18–24. [Google Scholar] [CrossRef]
- Romanazzi, G.; Feliziani, E.; Bautista-Baños, S.; Sivakumar, D. Shelf Life Extension of Fresh Fruit and Vegetables by Chitosan Treatment. Crit. Rev. Food Sci. Nutr. 2017, 57. [Google Scholar] [CrossRef]
- Li, T.; Li, H.; Liu, T.; Zhu, J.; Zhang, L.; Mu, W.; Liu, F. Evaluation of the antifungal and biochemical activities of mefentrifluconazole against Botrytis cinerea. Pestic. Biochem. Physiol. 2021, 104784. [Google Scholar] [CrossRef]
- Pertot, I.; Caffi, T.; Rossi, V.; Mugnai, L.; Hoffmann, C.; Grando, M.S.; Gary, C.; Lafond, D.; Duso, C.; Thiery, D.; et al. A critical review of plant protection tools for reducing pesticide use on grapevine and new perspectives for the implementation of IPM in viticulture. Crop Prot. 2017, 97, 70–84. [Google Scholar] [CrossRef]
- Deshwal, V.K.; Pandey, P.; Kang, S.C.; Maheshwari, D.K. Rhizobia as a biological control agent against soil borne plant pathogenic fungi. Indian J. Exp. Biol. 2003, 41, 1160–1164. [Google Scholar] [PubMed]
- Lugtenberg, B.; Kamilova, F. Plant-Growth-Promoting Rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef] [Green Version]
- Kumar, H.; Bajpai, V.K.; Dubey, R.C.; Maheshwari, D.K.; Kang, S.C. Wilt disease management and enhancement of growth and yield of Cajanus cajan (L) var. Manak by bacterial combinations amended with chemical fertilizer. Crop Prot. 2010, 29, 591–598. [Google Scholar] [CrossRef]
- Peian, Z.; Haifeng, J.; Peijie, G.; Sadeghnezhad, E.; Qianqian, P.; Tianyu, D.; Teng, L.; Huanchun, J.; Jinggui, F. Chitosan induces jasmonic acid production leading to resistance of ripened fruit against Botrytis cinerea infection. Food Chem. 2021, 337, 127772. [Google Scholar] [CrossRef] [PubMed]
- Platania, C.; Restuccia, C.; Muccilli, S.; Cirvilleri, G. Efficacy of killer yeasts in the biological control of Penicillium digitatum on Tarocco orange fruits (Citrus sinensis). Food Microbiol. 2012, 30, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Kloepper, J.W.; Ryu, C.M. Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci. 2009, 14, 1–4. [Google Scholar] [CrossRef]
- Nally, M.C.; Pesce, V.M.; Maturano, Y.P.; Muñoz, C.J.; Combina, M.; Toro, M.E.; de Figueroa, L.C.; Vazquez, F. Biocontrol of Botrytis cinerea in table grapes by non-pathogenic indigenous Saccharomyces cerevisiae yeasts isolated from viticultural environments in Argentina. Postharvest Biol. Technol. 2012, 64, 40–48. [Google Scholar] [CrossRef]
- Esmaeel, Q.; Jacquard, C.; Clément, C.; Sanchez, L.; Ait, E.; Barka, E.A. Genome sequencing and traits analysis of Burkholderia strains reveal a promising biocontrol effect against grey mould disease in grapevine (Vitis vinifera L.). World J. Microbiol. Biotechnol. 2019, 35. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef] [Green Version]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [Green Version]
- Aziz, R.K.; Bartels, D.; Best, A.A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The RAST Server: Rapid annotations using subsystems technology. BMC Genom. 2008, 9, 363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Disz, T.; Akhter, S.; Cuevas, D.; Olson, R.; Overbeek, R.; Vonstein, V.; Stevens, R.; Edwards, R.A. Accessing the SEED Genome Databases via Web Services API: Tools for Programmers. BMC Bioinform. 2010, 11, 319. [Google Scholar] [CrossRef] [Green Version]
- Weber, T.; Blin, K.; Duddela, S.; Krug, D.; Kim, H.U.; Bruccoleri, R.; Lee, S.Y.; Fischbach, M.A.; Müller, R.; Wohlleben, W.; et al. AntiSMASH 3.0-A comprehensive resource for the genome mining of biosynthetic gene clusters. Nucleic Acids Res. 2015, 43, W237–W243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barka, E.A.; Nowak, J.; Clément, C. Enhancement of chilling resistance of inoculated grapevine plantlets with a plant growth-promoting rhizobacterium, Burkholderia phytofirmans strain PsJN. Appl. Environ. Microbiol. 2006, 72, 7246–7252. [Google Scholar] [CrossRef] [Green Version]
- Miotto-Vilanova, L.; Jacquard, C.; Courteaux, B.; Wortham, L.; Michel, J.; Clément, C.; Barka, E.A.; Sanchez, L. Burkholderia phytofirmans PsJN Confers Grapevine Resistance against Botrytis cinerea via a Direct Antimicrobial Effect Combined with a Better Resource Mobilization. Front. Plant Sci. 2016, 7, 1236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genty, B.; Briantais, J.-M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta Gen. Subj. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Kramer, D.M.; Johnson, G.; Kiirats, O.; Edwards, G.E. New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosynth. Res. 2004, 79, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-R, L.M.; Gunturu, S.; Harvey, W.T.; Rosselló-Mora, R.; Tiedje, J.M.; Cole, J.R.; Konstantinidis, K.T. The Microbial Genomes Atlas (MiGA) webserver: Taxonomic and gene diversity analysis of Archaea and Bacteria at the whole genome level. Nucleic Acids Res. 2018, 46, W282–W288. [Google Scholar] [CrossRef]
- Meier-Kolthoff, J.P.; Göker, M. TYGS is an automated high-throughput platform for state-of-the-art genome-based taxonomy. Nat. Commun. 2019, 10, 2182. [Google Scholar] [CrossRef]
- Sasse, J.; Martinoia, E.; Northen, T. Feed Your Friends: Do Plant Exudates Shape the Root Microbiome? Trends Plant Sci. 2018, 23, 25–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Zhao, D.; Qi, G.; Mao, Z.; Hu, X.; Du, B.; Liu, K.; Ding, Y. Effects of Bacillus velezensis FKM10 for Promoting the Growth of Malus hupehensis Rehd. and Inhibiting Fusarium verticillioides. Front. Microbiol. 2020, 10, 2889. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Jiang, H.; Hao, J.J. Effects of Bacillus velezensis strain BAC03 in promoting plant growth. Biol. Control 2016, 98, 18–26. [Google Scholar] [CrossRef]
- Taghavi, S.; van der Lelie, D.; Hoffman, A.; Zhang, Y.; Walla, M.D.; Vangronsveld, J.; Newman, L.; Monchy, S. Genome sequence of the plant growth promoting endophytic bacterium Enterobacter sp. 638. PLoS Genet. 2010, 6, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macedo-Raygoza, G.M.; Valdez-Salas, B.; Prado, F.M.; Prieto, K.R.; Yamaguchi, L.F.; Kato, M.J.; Canto-Canché, B.B.; Carrillo-Beltrán, M.; di Mascio, P.; White, J.F.; et al. Enterobacter cloacae, an endophyte that establishes a nutrient-transfer symbiosis with banana plants and protects against the black sigatoka pathogen. Front. Microbiol. 2019, 10, 804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toral, L.; Rodríguez, M.; Béjar, V.; Sampedro, I. microorganisms Crop Protection against Botrytis cinerea by Rhizhosphere Biological Control Agent Bacillus velezensis XT1. Microorganisms 2020, 8, 992. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Ma, Q.; Bian, L.; Liu, X.; Xu, Y.; Zhang, H.; Shao, J.; Liu, Y. Bacillus velezensis CLA178-Induced Systemic Resistance of Rosa multiflora Against Crown Gall Disease. Front. Microbiol. 2020, 11, 587667. [Google Scholar] [CrossRef] [PubMed]
- Shastry, R.P.; Welch, M.; Rai, V.R.; Ghate, S.D.; Sandeep, K.; Rekha, P.D. The whole-genome sequence analysis of Enterobacter cloacae strain Ghats1: Insights into endophytic lifestyle-associated genomic adaptations. Arch. Microbiol. 2020, 202, 1571–1579. [Google Scholar] [CrossRef]
- Shi, J.F.; Sun, C.Q. Isolation, identification, and biocontrol of antagonistic bacterium against Botrytis cinerea after tomato harvest. Braz. J. Microbiol. 2017, 48, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.Q.; Li, Q.L.; Hu, J.C. The complete genome sequence of Bacillus velezensis 9912D reveals its biocontrol mechanism as a novel commercial biological fungicide agent. J. Biotechnol. 2017, 247, 25–28. [Google Scholar] [CrossRef]
- Wang, F.; Xiao, J.; Zhang, Y.; Li, R.; Liu, L.; Deng, J. Biocontrol ability and action mechanism of Bacillus halotolerans against Botrytis cinerea causing grey mould in postharvest strawberry fruit. Postharvest Biol. Technol. 2021, 174, 111456. [Google Scholar] [CrossRef]
- Gordon, R.E.; Haynes, W.C.; Pang, C.H.-N.; Smith, N.R. The Genus Bacillus; Agricultural Research Service, U.S. Department of Agriculture: Washington, DC, USA, 1973. Available online: http://books.google.com/books?id=lXMpXssWFD0C (accessed on 3 June 2021).
- Priest, F.G.; Goodfellow, M. Bacillus amyloliquefaciens sp. nov. norn. rev. Microbiol. Soc. 1987. [Google Scholar] [CrossRef]
- Ruiz-García, C.; Béjar, V.; Martínez-Checa, F.; Llamas, I.; Quesada, E. Bacillus velezensis sp. nov., a surfactant-producing bacterium isolated from the river Vélez in Málaga, southern Spain. Int. J. Syst. Evol. Microbiol. 2005, 55, 191–195. [Google Scholar] [CrossRef] [Green Version]
- Madhaiyan, M.; Poonguzhali, S.; Kwon, S.W.; Sa, T.M. Bacillus methylotrophicus sp. nov., a methanol-utilizing, plant-growth-promoting bacterium isolated from rice rhizosphere soil. Int. J. Syst. Evol. Microbiol. 2010, 60, 2490–2495. [Google Scholar] [CrossRef] [PubMed]
- Dunlap, C.A.; Bowman, M.J.; Schisler, D.A.; Rooney, A.P. Genome analysis shows Bacillus axarquiensis is not a later heterotypic synonym of Bacillus mojavensis; reclassification of Bacillus malacitensis and Brevibacterium halotolerans as heterotypic synonyms of Bacillus axarquiensis. Int. J. Syst. Evol. Microbiol. 2016, 66, 2438–2443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunlap, C.A.; Kim, S.J.; Kwon, S.W.; Rooney, A.P. Bacillus velezensis is not a later heterotypic synonym of Bacillus amyloliquefaciens; Bacillus methylotrophicus, Bacillus amyloliquefaciens subsp. Plantarum and ‘Bacillus oryzicola’ are later heterotypic synonyms of Bacillus velezensis based on phylogenom. Int. J. Syst. Evol. Microbiol. 2016, 66, 1212–1217. [Google Scholar] [CrossRef]
- Dunlap, C.A.; Kim, S.-J.; Kwon, S.-W.; Rooney, A.P. Phylogenomic analysis shows that Bacillus amyloliquefaciens subsp. plantarum is a later heterotypic synonym of Bacillus methylotrophicus. Int. J. Syst. Evol. Microbiol. 2015, 65, 2104–2109. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wu, H.J.; Qiao, J.; Gao, X.; Borriss, R. Novel routes for improving biocontrol activity of Bacillus based bioinoculants. Front. Microbiol. 2015, 6, 1395. [Google Scholar] [CrossRef] [Green Version]
- Fan, B.; Blom, J.; Klenk, H.P.; Borriss, R. Bacillus amyloliquefaciens, Bacillus velezensis, and Bacillus siamensis Form an ‘Operational Group B. amyloliquefaciens’ within the B. subtilis species complex. Front. Microbiol. 2017, 8, 22. [Google Scholar] [CrossRef] [Green Version]
- Myo, E.M.; Liu, B.; Ma, J.; Shi, L.; Jiang, M.; Zhang, K.; Ge, B. Evaluation of Bacillus velezensis NKG-2 for bio-control activities against fungal diseases and potential plant growth promotion. Biol. Control 2019, 134, 23–31. [Google Scholar] [CrossRef]
- Jiang, C.H.; Liao, M.J.; Wang, H.K.; Zheng, M.Z.; Xu, J.J.; Guo, J.H. Bacillus velezensis, a potential and efficient biocontrol agent in control of pepper gray mold caused by Botrytis cinerea. Biol. Control 2018, 126, 147–157. [Google Scholar] [CrossRef]
- Gao, Z.; Zhang, B.; Liu, H.; Han, J.; Zhang, Y. Identification of endophytic Bacillus velezensis ZSY-1 strain and antifungal activity of its volatile compounds against Alternaria solani and Botrytis cinerea. Biol. Control 2017, 105, 27–39. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, H.; Sun, X.; Liu, Y.; Yan, W.; Xun, W.; Shen, Q.; Zhang, R. Bacillus velezensis wall teichoic acids are required for biofilm. Appl. Environ. Microbiol. 2019, 85, e02116-18. [Google Scholar] [CrossRef] [Green Version]
- Morales-Cedeño, L.R.; Orozco-Mosqueda, M.D.; Loeza-Lara, P.D.; Parra-Cota, F.I.; de los Santos-Villalobos, S.; Santoyo, G. Plant growth-promoting bacterial endophytes as biocontrol agents of pre- and post-harvest diseases: Fundamentals, methods of application and future perspectives. Microbiol. Res. 2021, 242, 12. [Google Scholar] [CrossRef]
- Lima, G.; de Curtis, F.; Piedimonte, D.; Spina, A.M.; de Cicco, V. Integration of biocontrol yeast and thiabendazole protects stored apples from fungicide sensitive and resistant isolates of Botrytis cinerea. Postharvest Biol. Technol. 2006, 40, 301–307. [Google Scholar] [CrossRef]
- Püttmann, M.; Ade, N.; Hof, H. Dependence of fatty acid composition of Listeria spp. on growth temperature. Res. Microbiol. 1993, 144, 279–283. [Google Scholar] [CrossRef]
- Annous, B.A.; Becker, L.A.; Bayles, D.O.; Labeda, D.P.; Wilkinson, B.J. Critical role of anteiso-C(15:0) fatty acid in the growth of Listeria monocytogenes at low temperatures. Appl. Environ. Microbiol. 1997, 63, 3887–3894. [Google Scholar] [CrossRef] [Green Version]
- Fernández-González, A.J.; Martínez-Hidalgo, P.; Cobo-Díaz, J.F.; Villadas, P.J.; Martínez-Molina, E.; Toro, N.; Tringe, S.G.; Fernández-López, M. The rhizosphere microbiome of burned holm-oak: Potential role of the genus Arthrobacter in the recovery of burned soils. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, K.; Kosaka, Y.; Tsuge, S.; Kubo, Y.; Horino, O. Evaluation of the Endophyte Enterobacter cloacae SM10 Isolated from Spinach Roots for Biological Control against Fusarium Wilt of Spinach. J. Gen. Plant Pathol. 2001, 67, 78–84. [Google Scholar] [CrossRef]
- Latha, P.; Karthikeyan, M.; Rajeswari, E. Plant Health under Biotic Stress; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Guo, D.J.; Singh, R.K.; Singh, P.; Li, D.P.; Sharma, A.; Xing, Y.X.; Song, X.P.; Yang, L.T.; Li, Y.R. Complete Genome Sequence of Enterobacter roggenkampii ED5, a Nitrogen Fixing Plant Growth Promoting Endophytic Bacterium with Biocontrol and Stress Tolerance Properties, Isolated From Sugarcane Root. Front. Microbiol. 2020, 11, 28. [Google Scholar] [CrossRef]
- Mohamed, B.F.F.; Sallam, N.M.A.; Alamri, S.A.M.; Abo-Elyousr, K.A.M.; Mostafa, Y.S.; Hashem, M. Approving the biocontrol method of potato wilt caused by Ralstonia solanacearum (Smith) using Enterobacter cloacae PS14 and Trichoderma asperellum T34. Egypt. J. Biol. Pest Control 2020, 30, 13. [Google Scholar] [CrossRef]
- Abdeljalil, N.O.; Vallance, J.; Gerbore, J.; Yacoub, A.; Daami-Remadi, M.; Rey, P. Combining potential oomycete and bacterial biocontrol agents as a tool to fight tomato Rhizoctonia root rot. Biol. Control 2021, 155, 104521. [Google Scholar] [CrossRef]
- Chen, P.-S.; Peng, Y.-H. Inhibition of Penicillium digitatum and Citrus Green Mold by Volatile Compounds Produced by Enterobacter cloacae. J. Plant Pathol. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.P.; Nalwaya, S.; Jha, P.N. The draft genome sequence of the plant growth promoting rhizospheric bacterium Enterobacter cloacae SBP-8. Genom. Data 2017, 12, 81–83. [Google Scholar] [CrossRef]
- Cho, S.T.; Chang, H.H.; Egamberdieva, D.; Kamilova, F.; Lugtenberg, B.; Kuo, C.H. Genome analysis of Pseudomonas fluorescens PCL1751: A rhizobacterium that controls root diseases and alleviates salt stress for its plant host. PLoS ONE 2015, 10, e0140231. [Google Scholar] [CrossRef]
- Luo, Y.; Cheng, Y.; Yi, J.; Zhang, Z.; Luo, Q.; Zhang, D.; Li, Y. Complete genome sequence of industrial biocontrol strain Paenibacillus polymyxa HY96-2 and further analysis of its biocontrol mechanism. Front. Microbiol. 2018, 9, 1520. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.; Sun, L.; Ding, X.; Sun, D.; Liu, J.; Wang, W. Complete genome sequence of Caulobacter flavus RHGG3T, a type species of the genus Caulobacter with plant growth-promoting traits and heavy metal resistance. 3 Biotech 2019, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Chaouachi, M.; Marzouk, T.; Jallouli, S.; Elkahoui, S.; Gentzbittel, L.; Ben, C.; Djébali, N. Activity assessment of tomato endophytic bacteria bioactive compounds for the postharvest biocontrol of Botrytis cinerea. Postharvest Biol. Technol. 2021, 172, 18. [Google Scholar] [CrossRef]
- Gao, H.; Li, P.; Xu, X.; Zeng, Q.; Guan, W. Research on volatile organic compounds from Bacillus subtilis CF-3: Biocontrol effects on fruit fungal pathogens and dynamic changes during fermentation. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef]
- Patel, P.; Shah, R.; Modi, K. Isolation and characterization of plant growth promoting potential of Acinetobacter sp. RSC7 isolated from Saccharum officinarum cultivar Co 671. J. Exp. Biol. Agric. Sci. 2017, 5, 483–491. [Google Scholar] [CrossRef]
- Agbodjato, N.A.; Noumavo, P.A.; Baba-Moussa, F.; Salami, H.A.; Sèzan, A.; Bankolé, H.; Adjanohoun, A.; Baba-Moussa, L. Characterization of potential plant growth promoting rhizobacteria isolated from Maize (Zea mays L.) in central and Northern Benin (West Africa). Appl. Environ. Soil Sci. 2015, 2015. [Google Scholar] [CrossRef] [Green Version]
- Bashan, Y.; Kamnev, A.A.; de-Bashan, L.E. Tricalcium phosphate is inappropriate as a universal selection factor for isolating and testing phosphate-solubilizing bacteria that enhance plant growth: A proposal for an alternative procedure. Biol. Fertil. Soils 2013, 49, 465–479. [Google Scholar] [CrossRef]
- Romero, D.; de Vicente, A.; Rakotoaly, R.H.; Dufour, S.E.; Veening, J.W.; Arrebola, E.; Cazorla, F.M.; Kuipers, O.P.; Paquot, M.; Pérez-García, A. The iturin and fengycin families of lipopeptides are key factors in antagonism of Bacillus subtilis toward Podosphaera fusca. Mol. Plant Microbe Interact. 2007, 20, 430–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Shi, H.; Heng, J.; Wang, D.; Bian, K. Antimicrobial, plant growth-promoting and genomic properties of the peanut endophyte Bacillus velezensis LDO2. Microbiol. Res. 2019, 218, 41–48. [Google Scholar] [CrossRef]
- Xu, T.; Zhu, T.; Li, S. β-1,3-1,4-glucanase gene from Bacillus velezensis ZJ20 exerts antifungal effect on plant pathogenic fungi. World J. Microbiol. Biotechnol. 2016, 32, 26. [Google Scholar] [CrossRef]
- Cao, Y.; Pi, H.; Chandrangsu, P.; Li, Y.; Wang, Y.; Zhou, H.; Xiong, H.; Helmann, J.D.; Cai, Y. Antagonism of Two Plant-Growth Promoting Bacillus velezensis Isolates Against Ralstonia solanacearum and Fusarium oxysporum. Sci. Rep. 2018, 8, 4360. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.P.; Dietel, K.; Rändler, M.; Schmid, M.; Junge, H.; Borriss, R.; Hartmann, A.; Grosch, R. Effects of Bacillus amyloliquefaciens FZB42 on Lettuce Growth and Health under Pathogen Pressure and Its Impact on the Rhizosphere Bacterial Community. PLoS ONE 2013, 8, e68818. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.Y.; Song, H.; Sang, M.K.; Weon, H.Y.; Song, J. The complete genome sequence of Bacillus velezensis strain GH1-13 reveals agriculturally beneficial properties and a unique plasmid. J. Biotechnol. 2017, 259, 221–227. [Google Scholar] [CrossRef]
- Adeniji, A.A.; Loots, D.T.; Babalola, O.O. Bacillus velezensis: Phylogeny, useful applications, and avenues for exploitation. Appl. Microbiol. Biotechnol. 2019, 103, 3669–3682. [Google Scholar] [CrossRef]
- Fan, B.; Wang, C.; Song, X.; Ding, X.; Wu, L.; Wu, H.; Gao, X.; Borriss, R. Bacillus velezensis FZB42 in 2018: The Gram-Positive Model Strain for Plant Growth Promotion and Biocontrol. Front. Microbiol. 2018, 9, 2491. [Google Scholar] [CrossRef] [Green Version]
- Calatayud, A.; Roca, D.; Martínez, P.F. Spatial-temporal variations in rose leaves under water stress conditions studied by chlorophyll fluorescence imaging. Plant Physiol. Biochem. 2006, 44, 564–573. [Google Scholar] [CrossRef]
- Sperdouli, I.; Moustakas, M. A better energy allocation of absorbed light in photosystem II and less photooxidative damage contribute to acclimation of Arabidopsis thaliana young leaves to water deficit. J. Plant Physiol. 2014, 171, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Gawroński, P.; Witoń, D.; Vashutina, K.; Bederska, M.; Betliński, B.; Rusaczonek, A.; Karpiński, S. Mitogen-activated protein kinase 4 is a salicylic acid-independent regulator of growth but not of photosynthesis in Arabidopsis. Mol. Plant 2014, 7, 1151–1166. [Google Scholar] [CrossRef] [Green Version]
- Moustaka, J.; Ouzounidou, G.; Sperdouli, I.; Moustakas, M. Photosystem II Is More Sensitive than Photosystem I to Al3+ Induced Phytotoxicity. Materials 2018, 11, 1772. [Google Scholar] [CrossRef] [Green Version]
- Berger, S.; Sinha, A.K.; Roitsch, T. Plant physiology meets phytopathology: Plant primary metabolism and plant-pathogen interactions. J. Exp. Bot. 2007, 58, 4019–4026. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W. Photoprotection in an ecological context: The remarkable complexity of thermal energy dissipation. New Phytol. 2006, 172, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Jusović, M.; Velitchkova, M.Y.; Misheva, S.P.; Börner, A.; Apostolova, E.L.; Dobrikova, A.G. Photosynthetic Responses of a Wheat Mutant (Rht-B1c) with Altered DELLA Proteins to Salt Stress. J. Plant Growth Regul. 2018, 37, 645–656. [Google Scholar] [CrossRef]
- Guo, J.; Sun, K.; Zhang, Y.; Hu, K.; Zhao, X.; Liu, H.; Wu, S.; Hu, Y.; Zhang, Y.; Wang, Y. SlMAPK3, a key mitogen-activated protein kinase, regulates the resistance of cherry tomato fruit to Botrytis cinerea induced by yeast cell wall and β-glucan. Postharvest Biol. Technol. 2021, 171, 111350. [Google Scholar] [CrossRef]
| Oxidation of | Oxidation of | Oxidation of | ||||||
| S6 | S3 | S6 | S3 | S6 | S3 | |||
| 3-methylglucose | − | − | D-melibiose | + | + | L-lacticacid | + | + |
| Aceticacid | + | − | D-raffinose | + | − | L-malicacid | + | + |
| acetoaceticacid | − | − | D-saccharicacid | + | ± | L-pyroglutamicacid | ± | ± |
| bromo-succinicacid | + | − | D-salicin | ± | − | L-rhamnose | + | − |
| Citricacid | + | ± | D-serine | − | − | L-serine | + | − |
| D-arabitol | ± | − | D-sorbitol | + | + | Methylpyruvate | + | ± |
| D-asparticacid | − | − | D-trehalose | + | + | mucicacid | + | ± |
| D-cellobiose | + | + | D-turanose | ± | ± | myo-inositol | + | ± |
| Dextrin | ± | + | Formicacid | ± | − | N-acetylneuraminicacid | − | ± |
| D-fructose | + | + | Gelatin | − | − | N-acetyl-D-galactosamine | + | ± |
| D-fructose-6-PO4 | + | ± | Gentiobiose | + | − | N-acetyl-D-glucosamine | + | + |
| D-fucose | ± | − | Glucuronamide | + | + | N-acetyl-β-D-mannosamine | + | + |
| D-galactose | + | − | Glycerol | + | ± | Pectin | ± | ± |
| D-galacturonicacid | + | + | Glycyl-L-proline | + | − | p-hydroxyphenylaceticacid | ± | − |
| D-gluconicacid | + | + | Inosine | + | ± | propionicacid | − | − |
| D-glucose-6-PO4 | + | ± | L-alanine | + | + | Quinicacid | − | ± |
| D-glucuronicacid | + | + | L-arginine | ± | + | Stachyose | + | − |
| D-lacticacidmethylester | ± | ± | L-asparticacid | + | + | Sucrose | + | + |
| D-malicacid | − | − | L-fucose | ± | − | Tween 40 | − | ± |
| D-maltose | + | + | L-galactonicacid lactone | + | + | A-D-glucose | + | + |
| D-mannitol | + | + | L-glutamicacid | + | + | |||
| D-mannose | + | + | L-histidine | + | + | |||
| Growth in the presence of | S6 | S3 | Growth in the presence of | S6 | S3 | Growth in the presence of | S6 | S3 |
| 1% Nacl | + | + | Lincomycin | + | − | Rifamycin SV | + | − |
| 4% Nacl | + | + | Lithiumchloride | + | + | Sodium bromate | − | − |
| 8% Nacl | ± | ± | Minocycline | ± | − | Sodium butyrate | + | + |
| 1% sodium lactate | + | + | Nalidixicacid | ± | − | Tetrazoliumblue | + | − |
| Aztreonam | ± | − | Niaproof 4 | + | − | Tetrazolium violet | + | ± |
| D-serine | − | − | pH 5 | ± | − | Troleandomycin | + | − |
| Fusidicacid | ± | − | pH 6 | + | + | Vancomycin | + | − |
| Guanidinehcl | + | + | Potassium tellurite | + | + | |||
| Structure | Fatty Acid | Systematic Name Saturated | % In Isolated Strains | |
|---|---|---|---|---|
| S3 | S6 | |||
| Saturated | C12: 0 | Dodecanoic | 4.23 | 7.52 |
| C13: 0 | Tridecanoic | - | 1.68 | |
| C13: 0 ANTEISO | 0.40 | - | ||
| C14: 0 | Tetradecanoic | 1.01 | 8.23 | |
| C14: 0 ISO | 1,11 | - | ||
| C15: 0 ISO | 11.95 | - | ||
| C15: 0 ANTEISO | 37.18 | - | ||
| C16: 0 | Hexadecanoic | 17.16 | 18.07 | |
| C16: 0 ISO | 2.41 | - | ||
| C17: 0 | Heptadecanoic | 0.57 | 2.38 | |
| C17: 0 ISO | 9.44 | - | ||
| C17: 0 ANTEISO | 11.64 | - | ||
| C18: 0 | Octadecenoic | 1.21 | - | |
| Hydroxy Unsaturated | C15: 0 3-OH | 3- Hydroxy- pentadecenoic | - | 0.58 |
| C16: 1 ω 5c | cis-11-Hexadecenoic | - | - | |
| C16: 1 ω 11c | 1.69 | - | ||
| C17:1 ω 8c | - | 0.60 | ||
| C18: 1 ω 7c | cis-11- Octadecenoic | - | 13.49 | |
| Cyclopropane | C17: 0 cyclo | Cyclo-heptadecanoic | - | 15.04 |
| C19: 0 cyclo ω 8c | 9-(2-eptylcyclopropyl) Nonanoic | - | 1.86 | |
| Branched chain | Summed feature 1 | C 15:1 ISO H/ C 13:0 3OH C 15:1 ISO I/ C 13:0 3OH | - | 3.28 |
| Summed feature 2 | C12: 0 aldehyde, C 16:1 ISO I/ C 14:0 3OH and/or unknown ECL 10.928 | - | 17.85 | |
| Summed feature 3 | C 16:1 ω7c/ 15 iso 2OH | - | 8.38 | |
| Attribute | S3 | S6 |
|---|---|---|
| Size (bp) | 4.15 Mb | 4.60 Mb |
| G+C content (%) | 46.35 | 55.90 |
| RNA genes | 98 | 101 |
| Protein-coding genes | 3983 | 4258 |
| N50 | 959,830 | 319,447 |
| L50 | 2 | 4 |
| Number of Subsystems | 328 | 560 |
| Most frequently species | B. velezensis | E. cloacae |
| Number of contigs | 21 | 30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amarouchi, Z.; Esmaeel, Q.; Sanchez, L.; Jacquard, C.; Hafidi, M.; Vaillant-Gaveau, N.; Ait Barka, E. Beneficial Microorganisms to Control the Gray Mold of Grapevine: From Screening to Mechanisms. Microorganisms 2021, 9, 1386. https://doi.org/10.3390/microorganisms9071386
Amarouchi Z, Esmaeel Q, Sanchez L, Jacquard C, Hafidi M, Vaillant-Gaveau N, Ait Barka E. Beneficial Microorganisms to Control the Gray Mold of Grapevine: From Screening to Mechanisms. Microorganisms. 2021; 9(7):1386. https://doi.org/10.3390/microorganisms9071386
Chicago/Turabian StyleAmarouchi, Zakaria, Qassim Esmaeel, Lisa Sanchez, Cédric Jacquard, Majida Hafidi, Nathalie Vaillant-Gaveau, and Essaid Ait Barka. 2021. "Beneficial Microorganisms to Control the Gray Mold of Grapevine: From Screening to Mechanisms" Microorganisms 9, no. 7: 1386. https://doi.org/10.3390/microorganisms9071386
APA StyleAmarouchi, Z., Esmaeel, Q., Sanchez, L., Jacquard, C., Hafidi, M., Vaillant-Gaveau, N., & Ait Barka, E. (2021). Beneficial Microorganisms to Control the Gray Mold of Grapevine: From Screening to Mechanisms. Microorganisms, 9(7), 1386. https://doi.org/10.3390/microorganisms9071386









