Reduction in Biogenic Amine Content in Baechu (Napa Cabbage) Kimchi by Biogenic Amine-Degrading Lactic Acid Bacteria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains Used
2.2. Determination of Histamine and Tyramine Degradation by LAB Strains
2.3. Identification of LAB Strains
2.4. Baechu Kimchi Fermentation with the Selected LAB Strains Capable of Degrading Toxic BAs
2.5. Measurements of Physicochemical and Microbial Properties
2.6. Analyses of BAs in Baechu Kimchi Samples and Assay Menstrua
2.7. Detection and Identification of Multicopper Oxidase Gene in LAB Strains
2.8. Statistical Analyses
3. Results and Discussion
3.1. Histamine and Tyramine Degradation Activity of LAB Strains Isolated from Kimchi Products
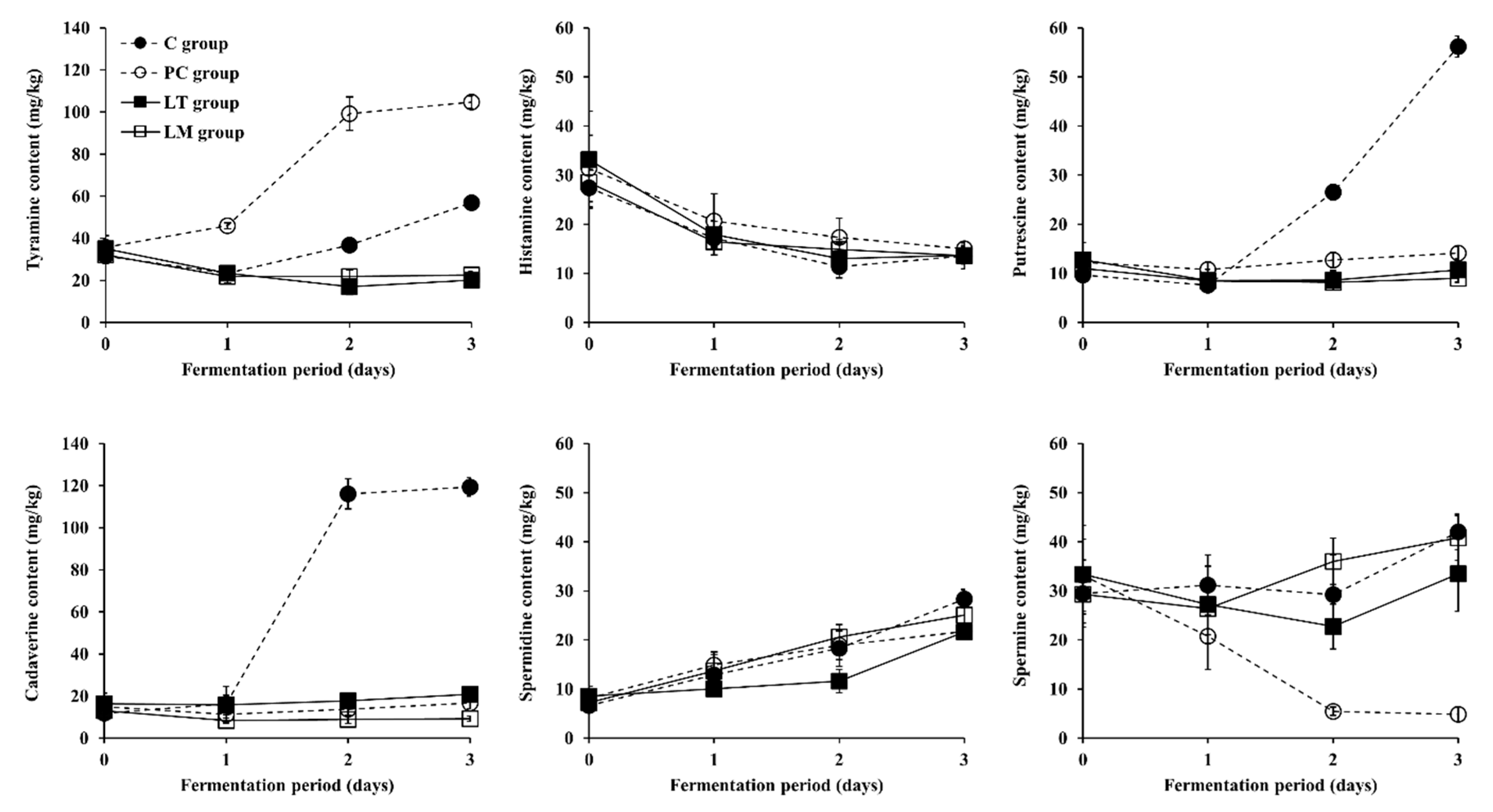
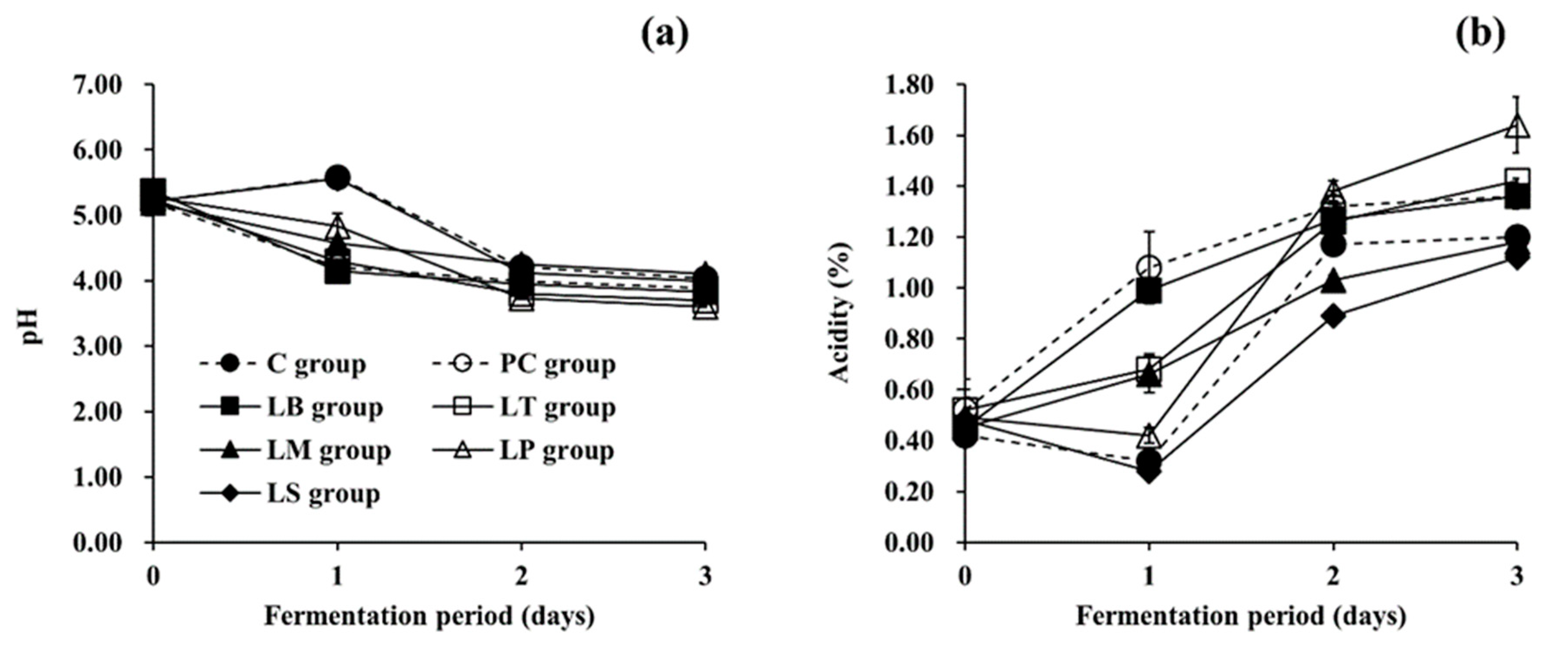
3.2. Changes in Physicochemical and Microbiological Parameters during Fermentation of Baechu Kimchi Inoculated with BA-Degrading LAB Strains

3.3. Changes in BA Content during Fermentation of Baechu Kimchi Inoculated with BA-Degrading LAB Strains
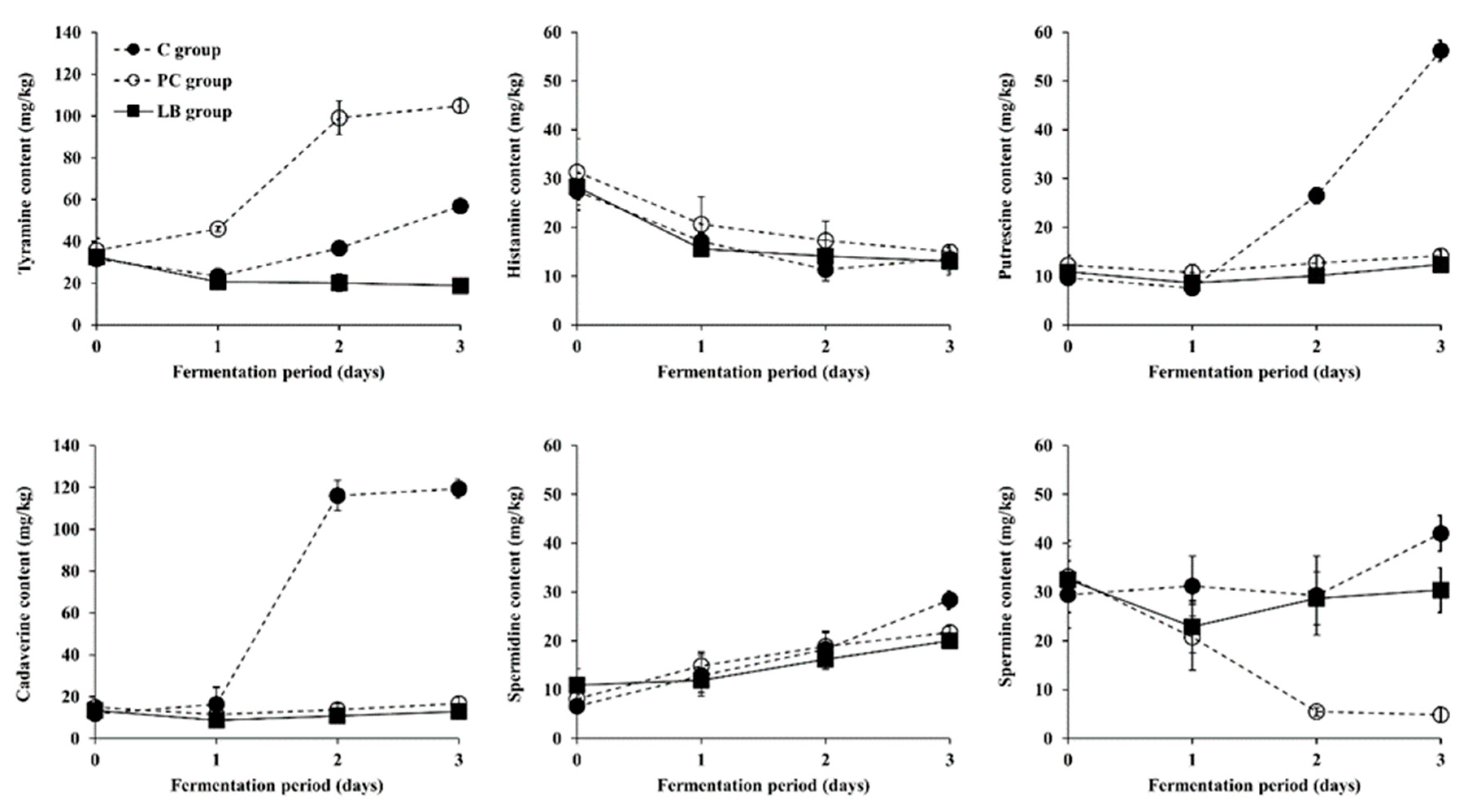
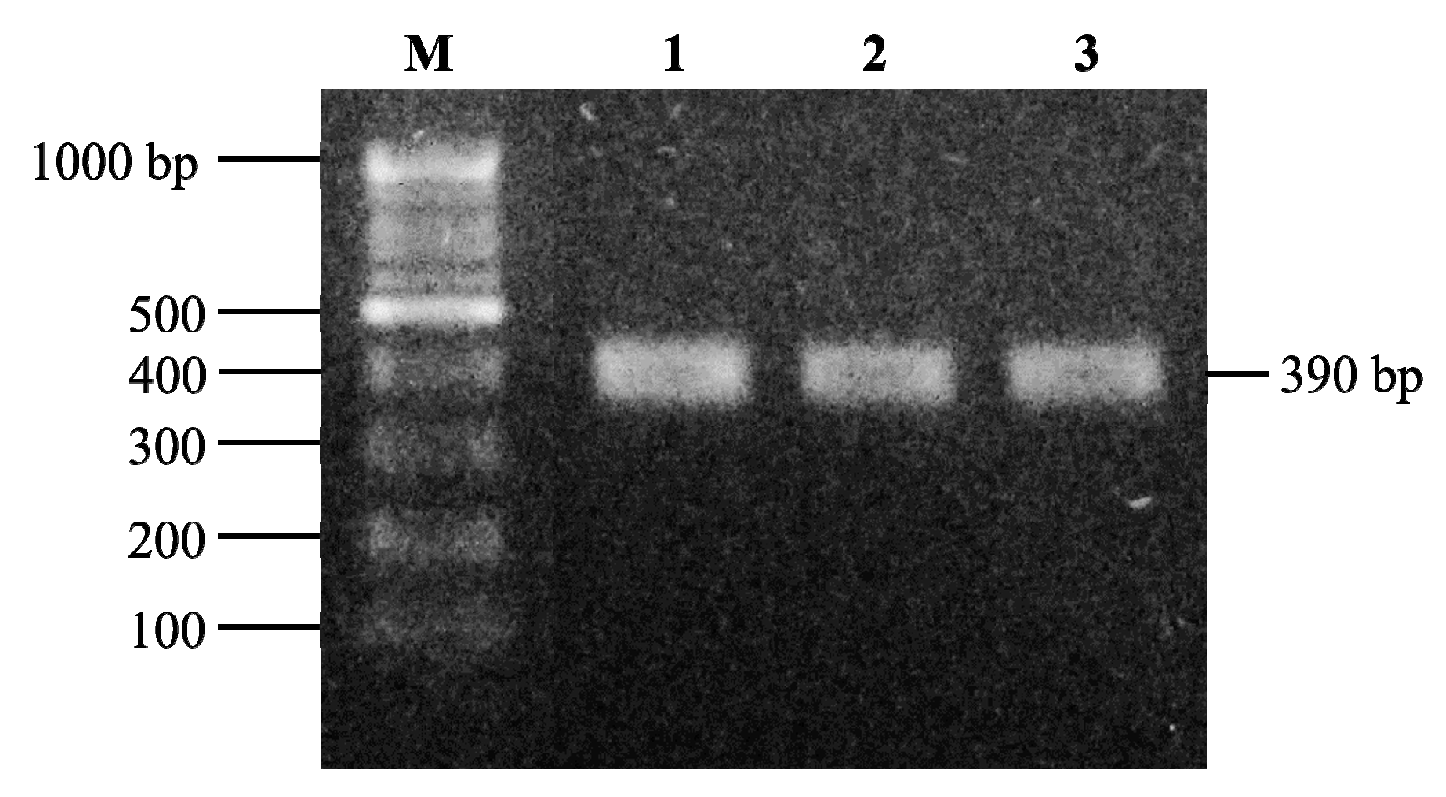
3.4. Contribution of Multicopper Oxidase Gene in L. brevis Strains to BA Degradation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Silla Santos, M.H. Biogenic amines: Their importance in foods. Int. J. Food Microbiol. 1996, 29, 213–231. [Google Scholar] [CrossRef]
- Ladero, V.; Calles-Enríquez, M.; Fernández, M.; Alvarez, M.A. Toxicological effects of dietary biogenic amines. Curr. Nutr. Food Sci. 2010, 6, 145–156. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Scientific opinion on risk based control of biogenic amine formation in fermented foods. EFSA J. 2011, 9, 2393–2486. [Google Scholar] [CrossRef] [Green Version]
- Smith, T.A. Amines in food. Food Chem. 1981, 6, 169–200. [Google Scholar] [CrossRef]
- Taylor, S.L.; Eitenmiller, R.R. Histamine food poisoning: Toxicology and clinical aspects. CRC Crit. Rev. Toxicol. 1986, 17, 91–128. [Google Scholar] [CrossRef] [PubMed]
- Bardócz, S. Polyamines in food and their consequences for food quality and human health. Trends Food Sci. Technol. 1995, 6, 341–346. [Google Scholar] [CrossRef]
- Warthesen, J.J.; Scanlan, R.A.; Bills, D.D.; Libbey, L.M. Formation of heterocyclic N-nitrosamines from the reaction of nitrite and selected primary diamines and amino acids. J. Agric. Food Chem. 1975, 23, 898–902. [Google Scholar] [CrossRef]
- Ten Brink, B.; Damink, C.; Joosten, H.M.L.J.; Huis in ’t Veld, J.H.J. Occurrence and formation of biologically active amines in foods. Int. J. Food Microbiol. 1990, 11, 73–84. [Google Scholar] [CrossRef]
- Del Rio, B.; Redruello, B.; Linares, D.M.; Ladero, V.; Fernandez, M.; Martin, M.C.; Ruas-Madiedo, P.; Alvarez, M.A. The dietary biogenic amines tyramine and histamine show synergistic toxicity towards intestinal cells in culture. Food Chem. 2017, 218, 249–255. [Google Scholar] [CrossRef]
- Del Rio, B.; Redruello, B.; Linares, D.M.; Ladero, V.; Ruas-Madiedo, P.; Fernandez, M.; Martin, M.C.; Alvarez, M.A. Spermine and spermidine are cytotoxic towards intestinal cell cultures, but are they a health hazard at concentrations found in foods? Food Chem. 2018, 269, 321–326. [Google Scholar] [CrossRef]
- Del Rio, B.; Redruello, B.; Linares, D.M.; Ladero, V.; Ruas-Madiedo, P.; Fernandez, M.; Martin, M.C.; Alvarez, M.A. The biogenic amines putrescine and cadaverine show in vitro cytotoxicity at concentrations that can be found in foods. Sci. Rep. 2019, 9, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Rio, B.; Redruello, B.; Fernandez, M.; Cruz Martin, M.; Ladero, V.; Alvarez, M.A. The biogenic amine tryptamine, unlike β-phenylethylamine, shows in vitro cytotoxicity at concentrations that have been found in foods. Food Chem. 2020, 331, 127303. [Google Scholar] [CrossRef] [PubMed]
- Linares, D.M.; Del Rio, B.; Redruello, B.; Ladero, V.; Martin, M.C.; Fernandez, M.; Ruas-Madiedo, P.; Alvarez, M.A. Comparative analysis of the in vitro cytotoxicity of the dietary biogenic amines tyramine and histamine. Food Chem. 2016, 197, 658–663. [Google Scholar] [CrossRef] [Green Version]
- European Commission (EC). Commission Regulation No. 2073/2005 of 15th November 2005 on microbiological criteria for foodstuffs. Off. J. Eur. Union 2005, L338, 1–25. [Google Scholar]
- U.S. Food and Drug Administration (FDA). Fish and Fishery Products Hazards and Controls Guidance, 4th ed.; Center for Food Safety and Applied Nutrition: Rockville, MD, USA, 2011. [Google Scholar]
- Cheigh, H.-S.; Park, K.-Y.; Lee, C.Y. Biochemical, microbiological, and nutritional aspects of kimchi (Korean fermented vegetable products). Crit. Rev. Food Sci. Nutr. 1994, 34, 175–203. [Google Scholar] [CrossRef]
- Codex Alimentarius Commission. Codex Standard for Kimchi; Codex Stan 223-2001; Food and Agriculture Organization of the United Nations: Rome, Italy, 2001. [Google Scholar]
- Jung, M.Y.; Kim, T.-W.; Lee, C.; Kim, J.Y.; Song, H.S.; Kim, Y.B.; Ahn, S.W.; Kim, J.S.; Roh, S.W.; Lee, S.H. Role of jeotgal, a Korean traditional fermented fish sauce, in microbial dynamics and metabolite profiles during kimchi fermentation. Food Chem. 2018, 265, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Lee, D.; Yang, C.; Jeon, J.; Kim, J.; Han, H. Microbial population dynamics of kimchi, a fermented cabbage product. FEMS Microbiol. Lett. 2006, 257, 262–267. [Google Scholar] [CrossRef] [Green Version]
- Jung, J.Y.; Lee, S.H.; Jin, H.M.; Hahn, Y.; Madsen, E.L.; Jeon, C.O. Metatranscriptomic analysis of lactic acid bacterial gene expression during kimchi fermentation. Int. J. Food Microbiol. 2013, 163, 171–179. [Google Scholar] [CrossRef]
- Jeong, S.H.; Lee, S.H.; Jung, J.Y.; Choi, E.J.; Jeon, C.O. Microbial succession and metabolite changes during long-term storage of kimchi. J. Food Sci. 2013, 78, M763–M769. [Google Scholar] [CrossRef]
- Jung, J.Y.; Lee, S.H.; Jeon, C.O. Kimchi microflora: History, current status, and perspectives for industrial kimchi production. Appl. Microbiol. Biotechnol. 2014, 98, 2385–2393. [Google Scholar] [CrossRef]
- Hwang, S.-Y.; Hur, Y.-M.; Choi, Y.-H.; Rhee, S.-H.; Park, K.-Y.; Lee, W.-H. Inhibitory effect of kimchi extracts on mutagenesis of aflatoxin B1. Environ. Mutagens Carcinog. 1997, 17, 133–137. [Google Scholar]
- Islam, M.S.; Choi, H. Antidiabetic effect of Korean traditional Baechu (Chinese cabbage) kimchi in a type 2 diabetes model of rats. J. Med. Food 2009, 12, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; An, S.-Y.; Lee, M.-S.; Kim, T.H.; Lee, H.-K.; Hwang, W.S.; Choe, S.J.; Kim, T.-Y.; Han, S.J.; Kim, H.J.; et al. Fermented kimchi reduces body weight and improves metabolic parameters in overweight and obese patients. Nutr. Res. 2011, 31, 436–443. [Google Scholar] [CrossRef]
- Kim, B.; Park, K.Y.; Kim, H.Y.; Ahn, S.C.; Cho, E.J. Anti-aging effects and mechanisms of kimchi during fermentation under stress-induced premature senescence cellular system. Food Sci. Biotechnol. 2011, 20, 643–649. [Google Scholar] [CrossRef]
- Kim, B.K.; Choi, J.M.; Kang, S.A.; Park, K.Y.; Cho, E.J. Antioxidative effects of Kimchi under different fermentation stage on radical-induced oxidative stress. Nutr. Res. Pract. 2014, 8, 638–643. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-Y.; Song, J.-L.; Chang, H.-K.; Kang, S.-A.; Park, K.-Y. Kimchi protects against azoxymethane/dextran sulfate sodium–induced colorectal carcinogenesis in mice. J. Med. Food 2014, 17, 833–841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mah, J.-H.; Kim, Y.J.; No, H.-K.; Hwang, H.-J. Determination of biogenic amines in kimchi, Korean traditional fermented vegetable products. Food Sci. Biotechnol. 2004, 13, 826–829. [Google Scholar]
- Park, Y.K.; Lee, J.H.; Mah, J.-H. Occurrence and reduction of biogenic amines in kimchi and Korean fermented seafood products. Foods 2019, 8, 547. [Google Scholar] [CrossRef] [Green Version]
- Cho, T.-Y.; Han, G.-H.; Bahn, K.-N.; Son, Y.-W.; Jang, M.-R.; Lee, C.-H.; Kim, S.-H.; Kim, D.-B.; Kim, S.-B. Evaluation of biogenic amines in Korean commercial fermented foods. Korean J. Food Sci. Technol. 2006, 38, 730–737. [Google Scholar]
- Jin, Y.H.; Lee, J.H.; Park, Y.K.; Lee, J.-H.; Mah, J.-H. The occurrence of biogenic amines and determination of biogenic amine-producing lactic acid bacteria in Kkakdugi and Chonggak kimchi. Foods 2019, 8, 73. [Google Scholar] [CrossRef] [Green Version]
- Kang, K.H.; Kim, S.H.; Kim, S.-H.; Kim, J.G.; Sung, N.-J.; Lim, H.; Chung, M.J. Analysis and risk assessment of N-nitrosodimethylamine and its precursor concentrations in Korean commercial kimchi. J. Korean Soc. Food Sci. Nutr. 2017, 46, 244–250. [Google Scholar] [CrossRef]
- Lee, J.-H.; Jin, Y.H.; Park, Y.K.; Yun, S.J.; Mah, J.-H. Formation of biogenic amines in Pa (green onion) kimchi and Gat (mustard leaf) kimchi. Foods 2019, 8, 109. [Google Scholar] [CrossRef] [Green Version]
- Tsai, Y.-H.; Kung, H.-F.; Lin, Q.-L.; Hwang, J.-H.; Cheng, S.-H.; Wei, C.-I.; Hwang, D.-F. Occurrence of histamine and histamine-forming bacteria in kimchi products in Taiwan. Food Chem. 2005, 90, 635–641. [Google Scholar] [CrossRef]
- Gardini, F.; Özogul, Y.; Suzzi, G.; Tabanelli, G.; Özogul, F. Technological factors affecting biogenic amine content in foods: A review. Front. Microbiol. 2016, 7, 1218. [Google Scholar] [CrossRef] [Green Version]
- Mah, J.-H.; Park, Y.K.; Jin, Y.H.; Lee, J.-H.; Hwang, H.J. Bacterial production and control of biogenic amines in Asian fermented soybean foods. Foods 2019, 8, 85. [Google Scholar] [CrossRef] [Green Version]
- Callejón, S.; Sendra, R.; Ferrer, S.; Pardo, I. Identification of a novel enzymatic activity from lactic acid bacteria able to degrade biogenic amines in wine. Appl. Microbiol. Biotechnol. 2012, 98, 185–198. [Google Scholar] [CrossRef]
- Dapkevicius, M.L.E.; Nout, M.R.; Rombouts, F.M.; Houben, J.H.; Wymenga, W. Biogenic amine formation and degradation by potential fish silage starter microorganisms. Int. J. Food Microbiol. 2000, 57, 107–114. [Google Scholar] [CrossRef]
- Tosukhowong, A.; Visessanguan, W.; Pumpuang, L.; Tepkasikul, P.; Panya, A.; Valyasevi, R. Biogenic amine formation in Nham, a Thai fermented sausage, and the reduction by commercial starter culture, Lactobacillus plantarum BCC 9546. Food Chem. 2011, 129, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Guarcello, R.; De Angelis, M.; Settanni, L.; Formiglio, S.; Gaglio, R.; Minervini, F.; Moschetti, G.; Gobbetti, M. Selection of amine-oxidizing dairy lactic acid bacteria and identification of the enzyme and gene involved in the decrease of biogenic amines. Appl. Environ. Microbiol. 2016, 82, 6870–6880. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Wen, X.; Wen, Z.; Chen, S.; Wang, L.; Wei, X. Evaluation of the biogenic amines formation and degradation abilities of Lactobacillus curvatus from Chinese bacon. Front. Microbiol. 2018, 9, 1015. [Google Scholar] [CrossRef] [Green Version]
- Leuschner, R.G.; Heidel, M.; Hammes, W.P. Histamine and tyramine degradation by food fermenting microorganisms. Int. J. Food Microbiol. 1998, 39, 1–10. [Google Scholar] [CrossRef]
- Kim, S.-H.; Kim, S.H.; Kang, K.H.; Lee, S.; Kim, S.J.; Kim, J.G.; Chung, M.J. Kimchi probiotic bacteria contribute to reduced amounts of N-nitrosodimethylamine in lactic acid bacteria-fortified kimchi. LWT Food Sci. Technol. 2017, 84, 196–203. [Google Scholar] [CrossRef]
- Jeong, S.H.; Lee, H.J.; Jung, J.Y.; Lee, S.H.; Seo, H.-Y.; Park, W.-S.; Jeon, C.O. Effects of red pepper powder on microbial communities and metabolites during kimchi fermentation. Int. J. Food Microbiol. 2013, 160, 252–259. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Yoon, H.; Park, J.H.; Choi, A.; Hwang, H.-J.; Mah, J.-H. Validation of an HPLC analytical method for determination of biogenic amines in agricultural products and monitoring of biogenic amines in Korean fermented agricultural products. Toxicol. Res. 2015, 31, 299–305. [Google Scholar] [CrossRef]
- NCBI. Primer-BLAST. Available online: https://www.ncbi.nlm.nih.gov/tools/primer-blast/ (accessed on 17 November 2021).
- NCBI. BLAST. Available online: http://www.ncbi.nlm.nih.gov/BLAST/ (accessed on 17 November 2021).
- Kim, M.-J.; Kim, K.-S. Tyramine production among lactic acid bacteria and other species isolated from kimchi. LWT Food Sci. Technol. 2014, 56, 406–413. [Google Scholar] [CrossRef]
- Lee, C.-H. Lactic acid fermented foods and their benefits in Asia. Food Control 1997, 8, 259–269. [Google Scholar] [CrossRef]
- Alvarez, M.A.; Moreno-Arribas, M.V. The problem of biogenic amines in fermented foods and the use of potential biogenic amine-degrading microorganisms as a solution. Trends Food Sci. Technol. 2014, 39, 146–155. [Google Scholar] [CrossRef] [Green Version]
- Herrero-Fresno, A.; Martínez, N.; Sánchez-Llana, E.; Díaz, M.; Fernández, M.; Martin, M.C.; Ladero, V.; Alvarez, M.A. Lactobacillus casei strains isolated from cheese reduce biogenic amine accumulation in an experimental model. Int. J. Food Microbiol. 2012, 157, 297–304. [Google Scholar] [CrossRef]
- Mheen, T.-I.; Kwon, T.-W. Effect of temperature and salt concentration on kimchi fermentation. Korean J. Food Sci. Technol. 1984, 16, 443–450. [Google Scholar]
- Dierick, N.; Vandekerckhove, P.; Demeyer, O. Changes in nonprotein nitrogen compounds during dry sausage ripening. J. Food Sci. 1974, 39, 301–304. [Google Scholar] [CrossRef]
- Barbieri, F.; Montanari, C.; Gardini, F.; Tabanelli, G. Biogenic amine production by lactic acid bacteria: A review. Foods 2019, 8, 17. [Google Scholar] [CrossRef] [Green Version]
- Bonnin-Jusserand, M.; Grandvalet, C.; Rieu, A.; Weidmann, S.; Alexandre, H. Tyrosine-containing peptides are precursors of tyramine produced by Lactobacillus plantarum strain IR BL0076 isolated from wine. BMC Microbiol. 2012, 12, 199. [Google Scholar] [CrossRef] [Green Version]
- Costantini, A.; Pietroniro, R.; Doria, F.; Pessione, E.; Garcia-Moruno, E. Putrescine production from different amino acid precursors by lactic acid bacteria from wine and cider. Int. J. Food Microbiol. 2013, 165, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Ladero, V.; Cañedo, E.; Pérez, M.; Martín, M.C.; Fernández, M.; Alvarez, M.A. Multiplex qPCR for the detection and quantification of putrescine-producing lactic acid bacteria in dairy products. Food Control 2012, 27, 307–313. [Google Scholar] [CrossRef]
- Poveda, J.M.; Ruiz, P.; Sesena, S.; Palop, M.L. Occurrence of biogenic amine-forming lactic acid bacteria during a craft brewing process. LWT Food Sci. Technol. 2017, 85, 129–136. [Google Scholar] [CrossRef]
- Shin, S.-W.; Kim, Y.-S.; Kim, Y.-H.; Kim, H.-T.; Eum, K.-S.; Hong, S.-R.; Kang, H.-J.; Park, K.-H.; Yoon, M.-H. Biogenic-amine contents of Korean commercial salted fishes and cabbage kimchi. Korean J. Fish. Aquat. Sci. 2019, 52, 13–18. [Google Scholar]
- Jin, H.S.; Kim, J.B.; Yun, Y.J.; Lee, K.J. Selection of kimchi starters based on the microbial composition of kimchi and their effects. J. Korean Soc. Food Sci. Nutr. 2008, 37, 671–675. [Google Scholar] [CrossRef]
- Kim, S.J. Field difficulties in modernization of kimchi industry. Food Ind. Nutr. 2001, 6, 34–37. [Google Scholar]
- Pištěková, H.; Jančová, P.; Berčíková, L.; Buňka, F.; Sokolová, I.; Šopík, T.; Maršálková, K.; de Amaral, O.M.R.P.; Buňková, L. Application of qPCR for multicopper oxidase gene (MCO) in biogenic amines degradation by Lactobacillus casei. Food Microbiol. 2020, 91, 103550. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Jiang, D.; Fan, M.; Li, H.; Jin, C.; Liu, W. Selection of a versatile Lactobacillus plantarum for wine production and identification and preliminary characterisation of a novel histamine-degrading enzyme. Int. J. Food Sci. Technol. 2020, 55, 2608–2618. [Google Scholar] [CrossRef]
| Kimchi Isolate No. 1 | Buffer | Media | Identification | ||
|---|---|---|---|---|---|
| Degradation (%) | Degradation (%) | ||||
| HIS 2 | TYR 2 | HIS | TYR | ||
| PK08 | 3.65–8.58 3 (6.12 ± 3.49) a | 3.50–11.54 (7.52 ± 5.69) a | 5.26–8.56 (6.91 ± 2.33) a | 1.82–11.68 (6.75 ± 6.97) a | Levilactobacillus brevis |
| PK05 | 7.47–10.81 (9.14 ± 2.36) a | 5.25–7.43 (6.34 ± 1.54) a | 1.17–1.59 (1.38 ± 0.30) b | 2.97–13.13 (8.05 ± 7.18) a | Lactiplantibacillus pentosus |
| YM20 | ND–1.39 (0.70 ± 0.98) b | 3.25–11.90 (7.58 ± 6.12) a | 4.09–4.28 (4.19 ± 0.13) a | 2.11–14.36 (8.24 ± 8.66) a | Leuconostoc mesenteroides |
| YM21 | 0.21–10.54 (5.38 ± 7.30) a | 3.47–11.41 (7.44 ± 5.61) a | 0.58–7.96 (4.27 ± 5.22) a | 2.39–12.11 (7.25 ± 6.87) a | Latilactobacillus sakei |
| KD15 | 3.64–5.67 (4.66 ± 1.44) a | 4.03–14.97 (9.50 ± 7.74) a | ND–4.34 (2.17 ± 3.07) a | 2.39–14.88 (8.64 ± 8.83) a | Lactiplantibacillus plantarum |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Jin, Y.H.; Pawluk, A.M.; Mah, J.-H. Reduction in Biogenic Amine Content in Baechu (Napa Cabbage) Kimchi by Biogenic Amine-Degrading Lactic Acid Bacteria. Microorganisms 2021, 9, 2570. https://doi.org/10.3390/microorganisms9122570
Lee J, Jin YH, Pawluk AM, Mah J-H. Reduction in Biogenic Amine Content in Baechu (Napa Cabbage) Kimchi by Biogenic Amine-Degrading Lactic Acid Bacteria. Microorganisms. 2021; 9(12):2570. https://doi.org/10.3390/microorganisms9122570
Chicago/Turabian StyleLee, Junsu, Young Hun Jin, Alixander Mattay Pawluk, and Jae-Hyung Mah. 2021. "Reduction in Biogenic Amine Content in Baechu (Napa Cabbage) Kimchi by Biogenic Amine-Degrading Lactic Acid Bacteria" Microorganisms 9, no. 12: 2570. https://doi.org/10.3390/microorganisms9122570
APA StyleLee, J., Jin, Y. H., Pawluk, A. M., & Mah, J.-H. (2021). Reduction in Biogenic Amine Content in Baechu (Napa Cabbage) Kimchi by Biogenic Amine-Degrading Lactic Acid Bacteria. Microorganisms, 9(12), 2570. https://doi.org/10.3390/microorganisms9122570







