Mobile Colistin Resistance Gene mcr-1 Detected on an IncI2 Plasmid in Salmonella Typhimurium Sequence Type 19 from a Healthy Pig in South Korea
Abstract
1. Introduction
2. Materials and Methods
2.1. Identification of Colistin-Resistant and mcr-1 Carrying Salmonella Serotypes
2.2. Characterization of mcr-1-Carrying Isolate
2.3. Whole-Genome Sequencing and Annotation
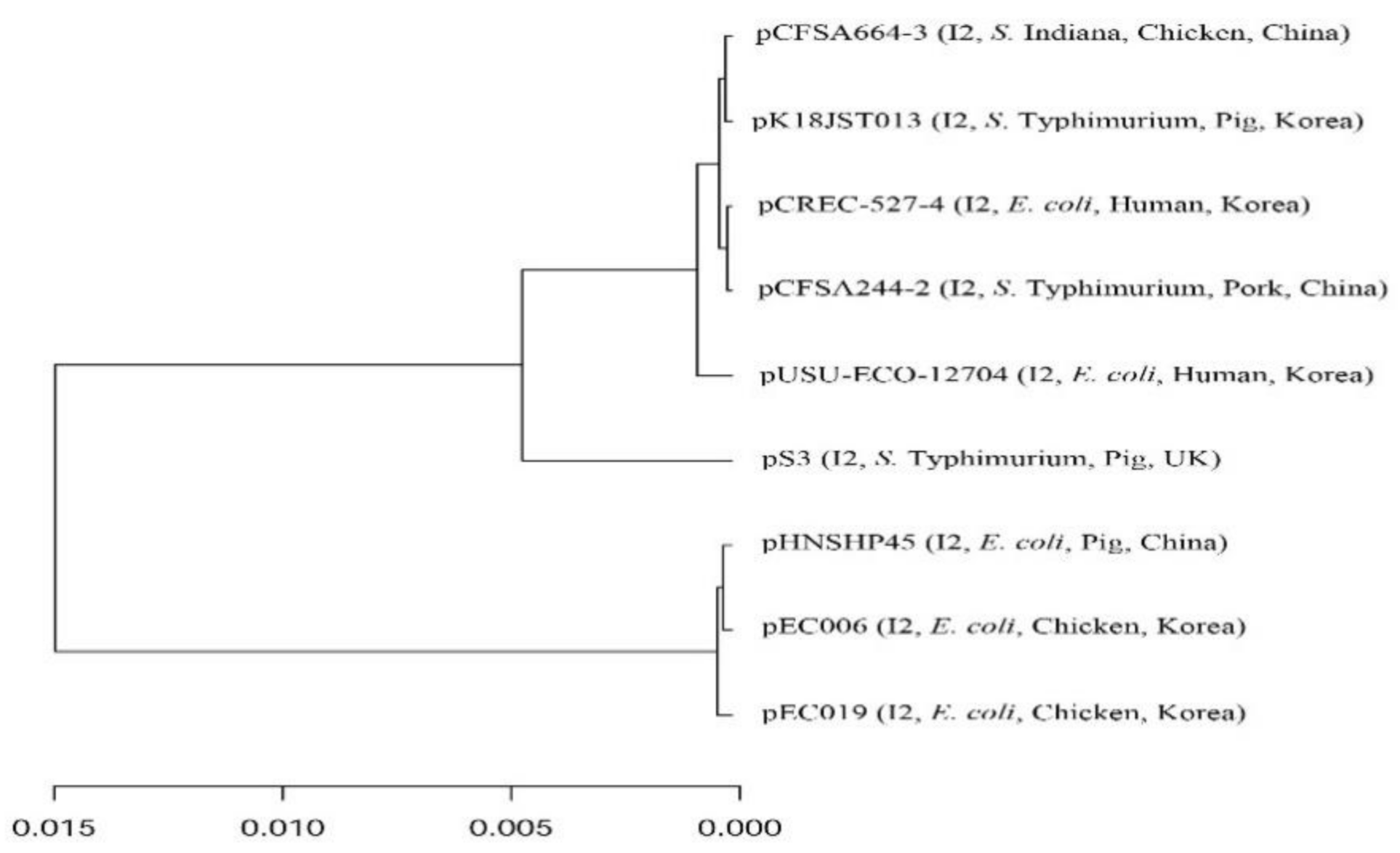
3. Results and Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lokken, K.L.; Walker, G.T.; Tsolis, R.M. Disseminated infections with antibiotic-resistant non-typhoidal Salmonella strains: Contributions of host and pathogen factors. Pathog. Dis. 2016, 74, 103. [Google Scholar] [CrossRef]
- GBD. The global burden of non-typhoidal Salmonella invasive disease: A Systematic analysis for the global burden of disease study 2017. Lancet 2019, 19, 1312–1324. [Google Scholar]
- Korean Center for Disease Control and Prevention (KCDC). Guideline for Control of Waterborne and Foodborne Disease; KCDC: Cheongju, Korea, 2018; pp. 6–8.
- Grégoire, N.; Aranzana-Climent, V.; Magréault, S.; Marchand, S.; Couet, W. Clinical Pharmacokinetics and Pharmacodynamics of Colistin. Clin. Pharmacokinet. 2017, 56, 1441–1460. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, H.; Liu, Y.-H.; Feng, Y. Towards Understanding MCR-like Colistin Resistance. Trends Microbiol. 2018, 26, 794–808. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Wang, Y.; Walsh, T.R.; Yi, L.-X.; Zhang, R.; Spencer, J.D.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef]
- Ling, Z.; Yin, W.; Shen, Z.; Wang, Y.; Shen, J.; Walsh, T.R. Epidemiology of mobile colistin resistance genes mcr-1 to mcr-9. J. Antimicrob. Chemother. 2020, 75, 3087–3095. [Google Scholar] [CrossRef]
- Lim, S.K.; Kang, H.Y.; Lee, K.; Moon, D.C.; Lee, H.S.; Jung, S.C. First detection of the mcr-1 gene in Escherichia coli isolated from livestock between 2013 and 2015 in South Korea. Antimicrob. Agents Chemother. 2016, 60, 6991–6993. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mechesso, A.F.; Moon, D.C.; Kang, H.Y.; Song, H.-J.; Kim, S.-J.; Choi, J.-H.; Kim, M.H.; Na, S.H.; Kim, H.-Y.; Jung, B.Y.; et al. Emergence of mcr-3 carrying Escherichia coli in diseased Pigs in South Korea. Microorganisms 2020, 8, 1538. [Google Scholar] [CrossRef] [PubMed]
- Cha, M.H.; Woo, G.J.; Kim, S.K.; Woo, J.H.; Kim, J.; Ryu, J.G.; Kwak, H.S.; Chi, Y.M. Emergence of transferable mcr-9 gene carrying colistin-resistant Salmonella Enterica Dessu ST14 isolated from retail chicken meat in Korea. Foodborne Pathog. Dis. 2020, 17, 720–727. [Google Scholar]
- Chiou, C.-S.; Chen, Y.-T.; Wang, Y.-W.; Liu, Y.-Y.; Kuo, H.-C.; Tu, Y.-H.; Lin, A.-C.; Liao, Y.-S.; Hong, Y.-P. Dissemination of mcr-1-carrying plasmids among colistin-resistant Salmonella Strains from humans and food-producing animals in Taiwan. Antimicrob. Agents Chemother. 2017, 61, e00338-17. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Ohnishi, M.; Kawanishi, M.; Akiba, M.; Kuroda, M. Investigation of a plasmid genome database for colistin-resistance gene mcr-1. Lancet Infect. Dis. 2016, 16, 284–285. [Google Scholar] [CrossRef]
- Yang, Y.Q.; Zhang, A.Y.; Ma, S.Z.; Kong, L.H.; Li, Y.X.; Liu, J.X.; Davis, M.A.; Guo, X.Y.; Liu, B.H.; Lei, C.W.; et al. Co-occurrence of mcr-1 and ESBL on a single plasmid in Salmonella enterica. J. Antimicrob. Chemother. 2016, 71, 2336–2338. [Google Scholar] [CrossRef]
- Yi, L.; Wang, J.; Gao, Y.; Liu, Y.; Doi, Y.; Wu, R.; Zeng, Z.; Liang, Z.; Liu, J.-H. mcr-1-harboring Salmonella enterica Serovar Typhimurium Sequence Type 34 in Pigs, China. Emerg. Infect. Dis. 2017, 23, 291–295. [Google Scholar] [CrossRef]
- Figueiredo, R.; Card, R.M.; Nunez, J.; Pomba, C.; Mendonça, N.; Anjum, M.F.; Da Silva, G.J. Detection of an mcr-1-encoding plasmid mediating colistin resistance in Salmonella enterica from retail meat in Portugal. J. Antimicrob. Chemother. 2016, 71, 2338–2340. [Google Scholar] [CrossRef]
- Quesada, A.; Concepción Porrero, M.; Téllez, S.; Palomo, G.; García, M.; Domínguez, L. Polymorphism of genes encoding PmrAB in colistin-resistant strains of Escherichia coli and Salmonella enterica isolated from poultry and swine. J. Antimicrob. Chemother. 2015, 70, 71–74. [Google Scholar] [CrossRef]
- Rau, R.B.; De Lima-Morales, D.; Wink, P.L.; Ribeiro, A.R.; Martins, A.F.; Barth, A.L. Emergence of mcr-1 Producing Salmonella enterica serovar Typhimurium from retail meat: First detection in Brazil. Foodborne Pathog. Dis. 2018, 15, 58–59. [Google Scholar] [CrossRef]
- Saavedra, S.Y.; Diaz, L.; Wiesner, M.; Correa, A.; Arévalo, S.A.; Reyes, J.; Hidalgo, A.M.; De La Cadena, E.; Perenguez, M.; Montaño, L.A.; et al. Genomic and molecular characterization of clinical isolates of Enterobacteriaceae harboring mcr-1 in Colombia, 2002 to 2016. Antimicrob. Agents Chemother. 2017, 61, e00841-17. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.C.; Mechesso, A.F.; Kang, H.Y.; Kim, S.-J.; Choi, J.-H.; Kim, M.H.; Song, H.-J.; Yoon, S.-S.; Lim, S.-K. First report of an Escherichia coli strain carrying the colistin resistance determinant mcr-1 from a dog in South Korea. Antibiotics 2020, 9, 768. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.-S.; Song, J.; Kim, J.; Shin, J. Increasing prevalence of multidrug-resistant mcr-1-positive Escherichia coli isolates from fresh vegetables and healthy food animals in South Korea. Int. J. Infect. Dis. 2020, 92, 53–55. [Google Scholar] [CrossRef]
- Yoon, E.-J.; Hong, J.S.; Yang, J.W.; Lee, K.J.; Lee, H.; Jeong, S.H. Detection of mcr-1 plasmids in Enterobacteriaceae isolates from human specimens: Comparison with those in Escherichia coli isolates from livestock in Korea. Ann. Lab. Med. 2018, 38, 555–562. [Google Scholar] [CrossRef]
- Wang, R.; Dorp, L.V.; Shaw, L.P.; Bradely, P.; Wang, Q.; Wang, X.; Jin, L.; Zhang, Q.; Liu, Y.; Rieux, A.; et al. The global distribution and spread of the mobilized colistin resistance gene mcr-1. Nat. Commun. 2018, 9, 1179. [Google Scholar] [CrossRef] [PubMed]
- Mechesso, A.F.; Moon, D.C.; Kim, S.-J.; Song, H.-J.; Kang, H.Y.; Na, S.H.; Choi, J.-H.; Kim, H.-Y.; Yoon, S.-S.; Lim, S.-K. Nationwide surveillance on serotype distribution and antimicrobial resistance profiles of non-typhoidal Salmonella serovars isolated from food-producing animals in South Korea. Int. J. Food Microbiol. 2020, 335, 108893. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, A.R.; Bortolaia, V.; Kjeldgaard, J.S.; Pedersen, S.K.; Leekitcharoenphon, P.; Hansen, I.M.; Guerra, B.; Malorny, B.; Borowiak, M.; Hammerl, J.A.; et al. Multiplex PCR for detection of plasmid-mediated colistin resistance determinants, mcr-1, mcr-2, mcr-3, mcr-4, and mcr-5 for surveillance purposes. Eurosurveillance 2018, 23, 17-00672. [Google Scholar] [CrossRef]
- Borowiak, M.; Baumann, B.; Fischer, J.; Thomas, K.; Deneke, C.; Hammerl, J.A.; Szabo, I.; Malorny, B. Development of a novel mcr-6 to mcr-9 multiplex PCR and assessment of mcr-1 to mcr-9 occurrence in colistin-resistant Salmonella enterica isolates from environment, feed, animals and food (2011–2018) in Germany. Front. Microbiol. 2020, 11, 80. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; Twentieth Informational Supplement; CLSI Document M100: Wayne, PA, USA, 2018. [Google Scholar]
- European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. EUCAST, 2018, Version 8.1. Available online: http://www.eucast.org (accessed on 17 July 2020).
- Kidgell, C.; Reichard, U.; Wain, J.; Linz, B.; Torpdahl, M.; Dougan, G.; Achtman, M. Salmonella typhi, the causative agent of typhoid fever, is approximately 50,000 years old. Infect. Genet. Evol. 2002, 2, 39–45. [Google Scholar] [CrossRef]
- Skyberg, J.A.; Logue, C.M.; Nolan, L.K. Virulence genotyping of Salmonella spp. with multiplex PCR. Avian Dis. 2006, 50, 77–81. [Google Scholar] [CrossRef]
- Na, S.H.; Moon, D.C.; Kang, H.Y.; Song, H.J.; Kim, S.J.; Choi, J.H.; Yoon, J.W.; Yoon, S.S.; Lim, S.K. Molecular characteristics of extended-spectrum β-lactamase/AmpC-producing Salmonella enterica serovar Virchow isolated from food-producing animals during 2010–2017 in South Korea. Int. J. Food Microbiol. 2020, 322, 108572. [Google Scholar]
- Delcher, A.L.; Bratke, K.A.; Powers, E.C.; Salzberg, S.L. Identifying bacterial genes and endosymbiont DNA with Glimmer. Bioinformatics 2007, 23, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Altermann, E.; Lu, J.; McCulloch, A. GAMOLA2, a comprehensive software package for the annotation and curation of draft and complete microbial genomes. Front. Microbiol. 2017, 8, 346. [Google Scholar] [CrossRef][Green Version]
- Sullivan, M.J.; Petty, N.K.; Beatson, S.A. Easyfig: A genome comparison visualizer. Bioinformatics 2011, 27, 1009–1010. [Google Scholar] [CrossRef]
- Pritchard, L.; Glover, R.H.; Humphris, S.; Elphinstone, J.G.; Toth, I.K. Genomics and taxonomy in diagnostics for food security: Soft-rotting enterobacterial plant pathogens. Anal. Methods 2016, 8, 12–24. [Google Scholar] [CrossRef]
- El Garch, F.; De Jong, A.; Bertrand, X.; Hocquet, D.; Sauget, M. mcr-1-like detection in commensal Escherichia coli and Salmonella spp. from food-producing animals at slaughter in Europe. Vet. Microbiol. 2018, 213, 42–46. [Google Scholar] [CrossRef]
- Esaki, H.; Morioka, A.; Ishihara, K.; Kojima, A.; Shiroki, S.; Tamura, Y.; Takahashi, T. Antimicrobial susceptibility of Salmonella isolated from cattle, swine, and poultry (2001–2002): Report from the Japanese Veterinary Antimicrobial Resistance Monitoring Program. J. Antimicrob. Chemother. 2004, 53, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.-K.; Lee, H.-S.; Nam, H.-M.; Jung, S.-C.; Koh, H.-B.; Roh, I.-S. Antimicrobial resistance and phage types of Salmonella isolates from healthy and diarrheic pigs in Korea. Foodborne Pathog. Dis. 2009, 6, 981–987. [Google Scholar] [CrossRef]
- European Center for Disease Prevention and Control. The European Union summary report on antimicrobial resistance in zoonotic bacteria from humans, animals, and food in 2014. EFSA J. 2016, 14, 4380. [Google Scholar]
- Ricci, V.; Zhang, D.; Teale, C.; Piddock, L.J.V. The O-antigen epitope governs susceptibility to colistin in Salmonella enterica. mBio 2020, 11, e02831-19. [Google Scholar] [CrossRef]
- Cui, M.; Zhang, J.; Gu, Z.; Li, R.; Chan, E.W.-C.; Yan, M.; Wu, C.; Xu, X.; Chen, S. Prevalence and molecular characterization of mcr-1-positive Salmonella strains recovered from clinical specimens in China. Antimicrob. Agents Chemother. 2017, 61, e02471-16. [Google Scholar] [CrossRef]
- Lai, C.-C.; Lin, Y.-T.; Lin, Y.-T.; Lu, M.-C.; Shi, Z.-Y.; Chen, Y.-S.; Wang, L.-S.; Tseng, S.-H.; Lin, C.-N.; Chen, Y.-H.; et al. Clinical characteristics of patients with bacteraemia due to the emergence of mcr-1-harbouring Enterobacteriaceae in humans and pigs in Taiwan. Int. J. Antimicrob. Agents 2018, 52, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Torpdahl, M.; Hasman, H.; Litrup, E.; Skov, R.L.; Nielsen, E.M.; Hammerum, A.M. Detection of mcr-1-encoding plasmid-mediated colistin-resistant Salmonella isolates from human infection in Denmark. Int. J. Antimicrob. Agents. 2017, 49, 261–262. [Google Scholar] [PubMed]
- Ma, S.; Lei, C.; Kong, L.; Jiang, W.; Liu, B.; Men, S.; Yang, Y.; Cheng, G.; Chen, Y.; Wang, H. Prevalence, antimicrobial resistance, and relatedness of Salmonella isolated from chickens and pigs on farms, abattoirs, and markets in Sichuan Province, China. Foodborne Pathog. Dis. 2017, 14, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Lima, T.; Domingues, S.; Da Silva, G.J. Plasmid-mediated colistin resistance in Salmonella enetrica: A review. Microorganisms 2019, 7, 55. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Wang, Y.; Xiao, Y. Prevalence and transmission of mobilized colistin resistance (mcr) gene in bacteria common to animals and humans. Biosaf. Health 2020, 2, 71–78. [Google Scholar] [CrossRef]
- Antunes, P.; Mourão, J.; Pestana, N.; Peixe, L. Leakage of emerging clinically relevant multidrug-resistant Salmonella clones from pig farms. J. Antimicrob. Chemother. 2011, 66, 2028–2032. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, K.L.; Kirchner, M.; Guerra, B.; A Granier, S.; Lucarelli, C.; Porrero, M.C.; Jakubczak, A.; Threlfall, E.J.; Mevius, D.J. Multiresistant Salmonella enterica serovar 4,[5],12:i:- in Europe: A new pandemic strain? Eurosurveillance. 2010, 15, 19580. [Google Scholar]
- García, P.; Guerra, B.; Bances, M.; Mendoza, M.C.; Rodicio, M.R. IncA/C plasmids mediate antimicrobial resistance linked to virulence genes in the Spanish clone of the emerging Salmonella enterica serotype 4,[5],12:i:-. J. Antimicrob. Chemother. 2010, 66, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Quan, J.; Zhao, D.; Wang, Y.; Yu, Y.; Zhu, J. Prevalence and molecular characteristics of mcr-1 gene in Salmonella typhimurium in a tertiary hospital of Zhejiang Province. Infect. Drug Resist. 2018, 12, 105–110. [Google Scholar] [CrossRef]
- Suh, D.K.; Song, J.C. Characterization of multi-drug resistant Salmonella enterica serovar Typhimurium isolated from swine sources. J. Exp. Biomed. Sci. 2005, 11, 115–119. [Google Scholar]
- Hua, M.; Huang, W.; Chen, A.; Rehmet, M.; Jin, C.; Huang, Z. Comparison of Antimicrobial Resistance Detected in Environmental and Clinical Isolates from Historical Data for the US. BioMed Res. Int. 2020, 2020, e4254530-11. [Google Scholar] [CrossRef]
- Leite, E.L.; Araújo, W.J.; Vieira, T.R.; Zenato, K.S.; Vasconcelos, P.C.; Cibulski, S.; Givisiez, P.E.; Cardoso, M.R.; Oliveira, C.J. First reported genome of an mcr-9-mediated colistin-resistant Salmonella Typhimurium isolate from Brazilian livestock. J. Glob. Antimicrob. Resist. 2020, 23, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Borges, K.A.; Furian, T.Q.; Borsoi, A.; Moraes, H.L.; Salle, C.T.P.; Nascimento, V.P. Detection of virulence-associated genes in Salmonella Enteritidis isolates from chicken in South of Brazil. Pesqui. Veterinária Bras. 2013, 33, 1416–1422. [Google Scholar] [CrossRef]
- Lu, X.; Zeng, M.; Xu, J.; Zhou, H.; Gu, B.; Li, Z.; Jin, H.; Wang, X.; Zhang, W.; Hu, Y.; et al. Epidemiologic and genomic insights on mcr-1-harbouring Salmonella from diarrhoeal outpatients in Shanghai, China, 2006–2016. EBioMedicine 2019, 42, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Anjum, M.F.; Duggett, N.A.; AbuOun, M.; Randall, L.; Nunez-Garcia, J.; Ellis, R.J.; Rogers, J.; Horton, R.; Brena, C.; Williamson, S.; et al. Colistin resistance in Salmonella and Escherichia coli isolates from a pig farm in Great Britain. J. Antimicrob. Chemother. 2016, 71, 2306–2313. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.H.Y.; Liu, L.; Yan, M.; Chan, E.W.C.; Chen, S. Dissemination of IncI2 plasmids that harbor the blaCTX-M element among clinical Salmonella isolates. Antimicrob. Agents Chemother. 2015, 59, 5026–5028. [Google Scholar] [CrossRef] [PubMed][Green Version]

| Serogroups | Prevalence (%) (no. of Resistance/no of Tested) |
|---|---|
| B | 0.1 (1/867) |
| C1 | 0 (0/996) |
| C2–C3 | 0 (0/402) |
| D1 | 56.1 (274/488) |
| E1 | 0 (0/125) |
| E2 | 0 (0/1) |
| E4 | 0 (0/74) |
| G | 0 (0/1) |
| H | 0 (0/1) |
| K | 0 (0/1) |
| L | 0 (0/1) |
| M | 0 (0/1) |
| NT | 3.3 (2/60) |
| Total | 9.2 (277/3018) |
| Isolate | Source | Isolation Year | Resistance Pattern of Donor | Sequence Type | Conjugation Efficiency | Resistance Pattern of Recipient | Replicon Type of Transconjugant | Virulence Factors |
|---|---|---|---|---|---|---|---|---|
| 18-A02-013 | Healthy Pig | 2018 | AMP CHL GEN STR FIS TET | ST19 | 3.0 ± 0.8 × 10−3 | COL | I2 | spiA, pagC, msgA, invA, sipB, prgH, spaN, orgA, tolC, lpfC, sifA, sopB, iroN, sitC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, D.C.; Kim, S.-J.; Mechesso, A.F.; Kang, H.Y.; Song, H.-J.; Choi, J.-H.; Yoon, S.-S.; Lim, S.-K. Mobile Colistin Resistance Gene mcr-1 Detected on an IncI2 Plasmid in Salmonella Typhimurium Sequence Type 19 from a Healthy Pig in South Korea. Microorganisms 2021, 9, 398. https://doi.org/10.3390/microorganisms9020398
Moon DC, Kim S-J, Mechesso AF, Kang HY, Song H-J, Choi J-H, Yoon S-S, Lim S-K. Mobile Colistin Resistance Gene mcr-1 Detected on an IncI2 Plasmid in Salmonella Typhimurium Sequence Type 19 from a Healthy Pig in South Korea. Microorganisms. 2021; 9(2):398. https://doi.org/10.3390/microorganisms9020398
Chicago/Turabian StyleMoon, Dong Chan, Su-Jeong Kim, Abraham Fikru Mechesso, Hee Young Kang, Hyun-Ju Song, Ji-Hyun Choi, Soon-Seek Yoon, and Suk-Kyung Lim. 2021. "Mobile Colistin Resistance Gene mcr-1 Detected on an IncI2 Plasmid in Salmonella Typhimurium Sequence Type 19 from a Healthy Pig in South Korea" Microorganisms 9, no. 2: 398. https://doi.org/10.3390/microorganisms9020398
APA StyleMoon, D. C., Kim, S.-J., Mechesso, A. F., Kang, H. Y., Song, H.-J., Choi, J.-H., Yoon, S.-S., & Lim, S.-K. (2021). Mobile Colistin Resistance Gene mcr-1 Detected on an IncI2 Plasmid in Salmonella Typhimurium Sequence Type 19 from a Healthy Pig in South Korea. Microorganisms, 9(2), 398. https://doi.org/10.3390/microorganisms9020398







