Effects of Lactic Acid Bacterial Inoculants on Fermentation Quality, Bacterial Community, and Mycotoxins of Alfalfa Silage under Vacuum or Nonvacuum Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Silage Making
2.2. Chemical Composition and Fermentation Quality Analyses
2.3. Bacterial Community Analysis
2.4. Determination of AFB1 and DON in Alfalfa Silage
2.5. Statistical Analysis
3. Results
3.1. Forage Characteristics before Ensiling
3.2. Chemical Composition and Fermentation Characteristics of Alfalfa Silage
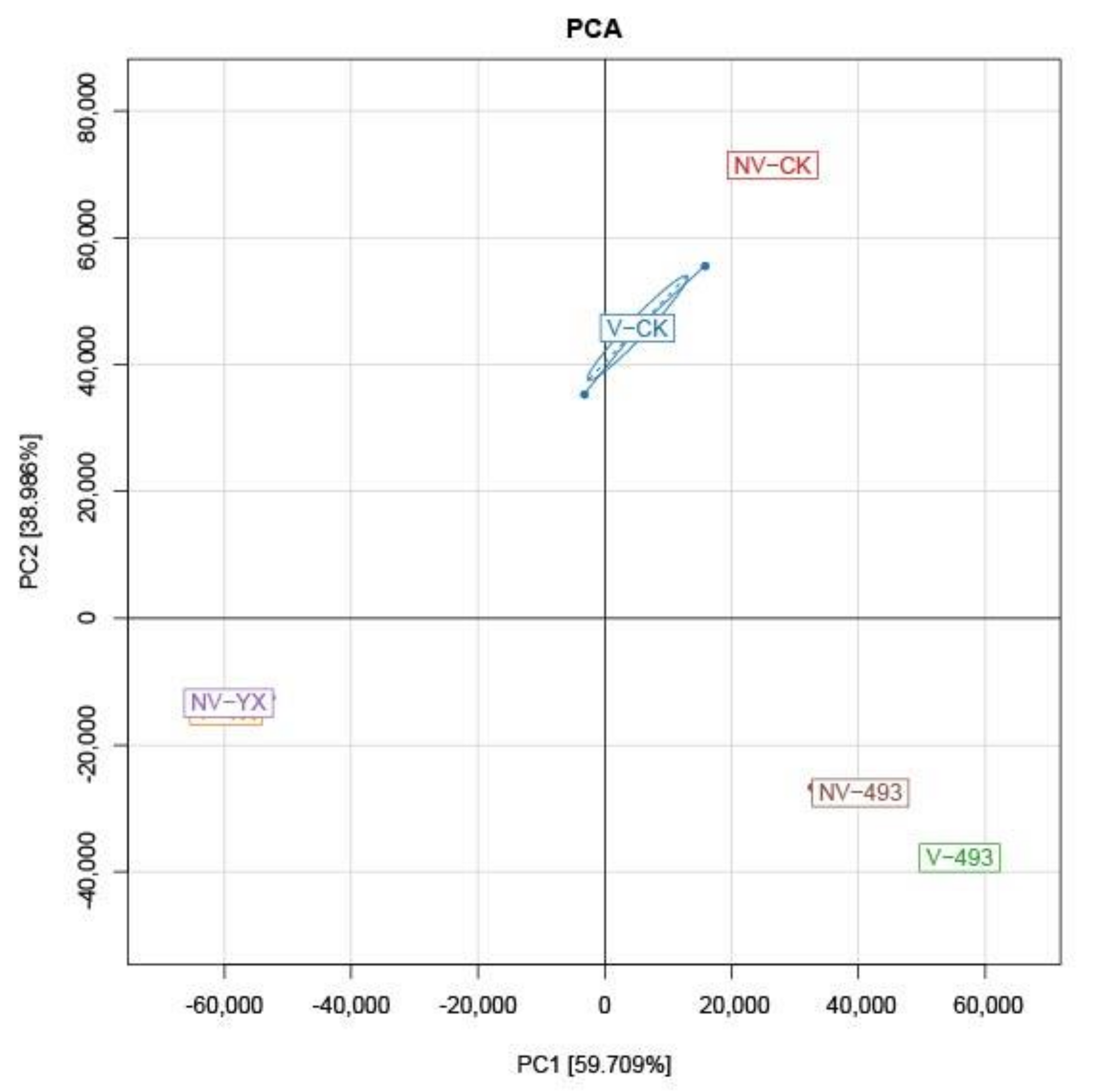
3.3. Bacterial Diversity and Community Analysis of Alfalfa Silage at 90 Days
3.3.1. Alpha and Beta Diversity Indices of Bacterial Communities
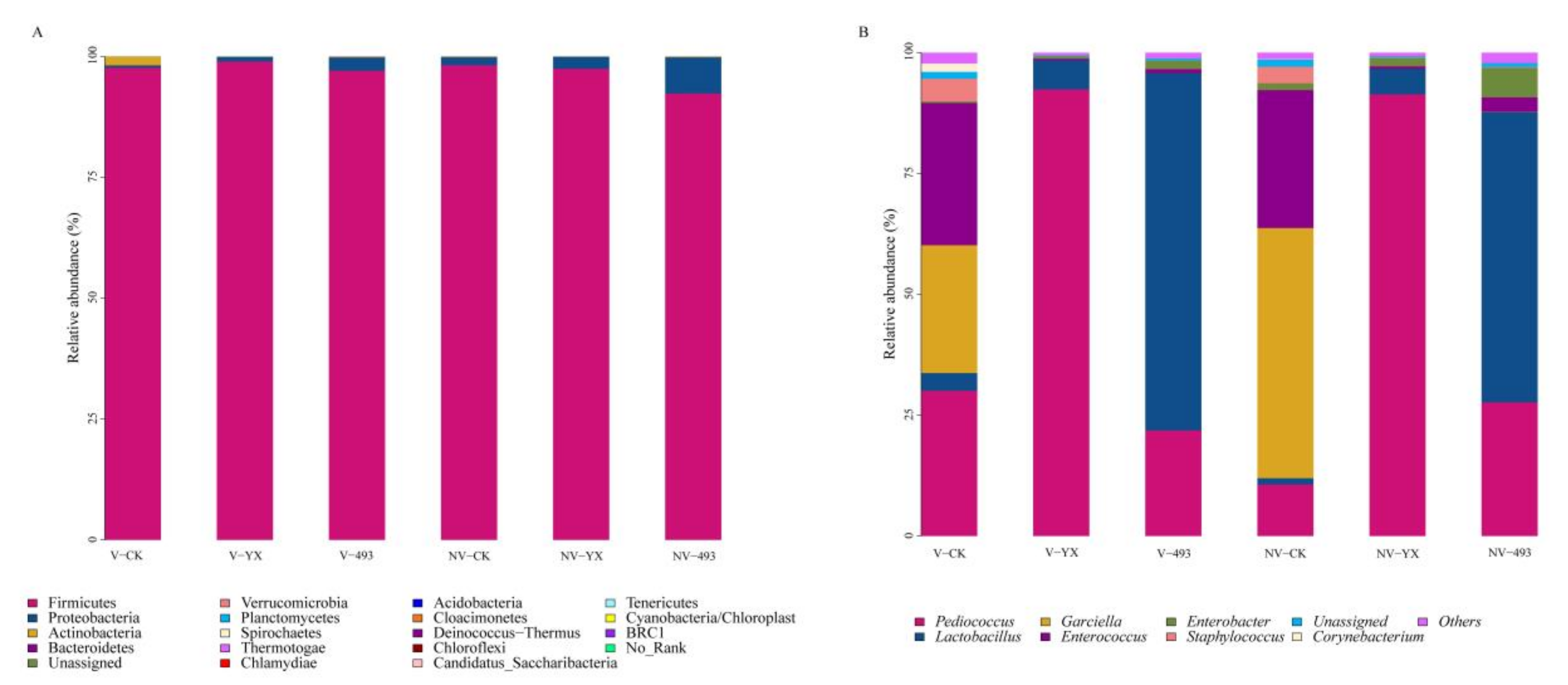
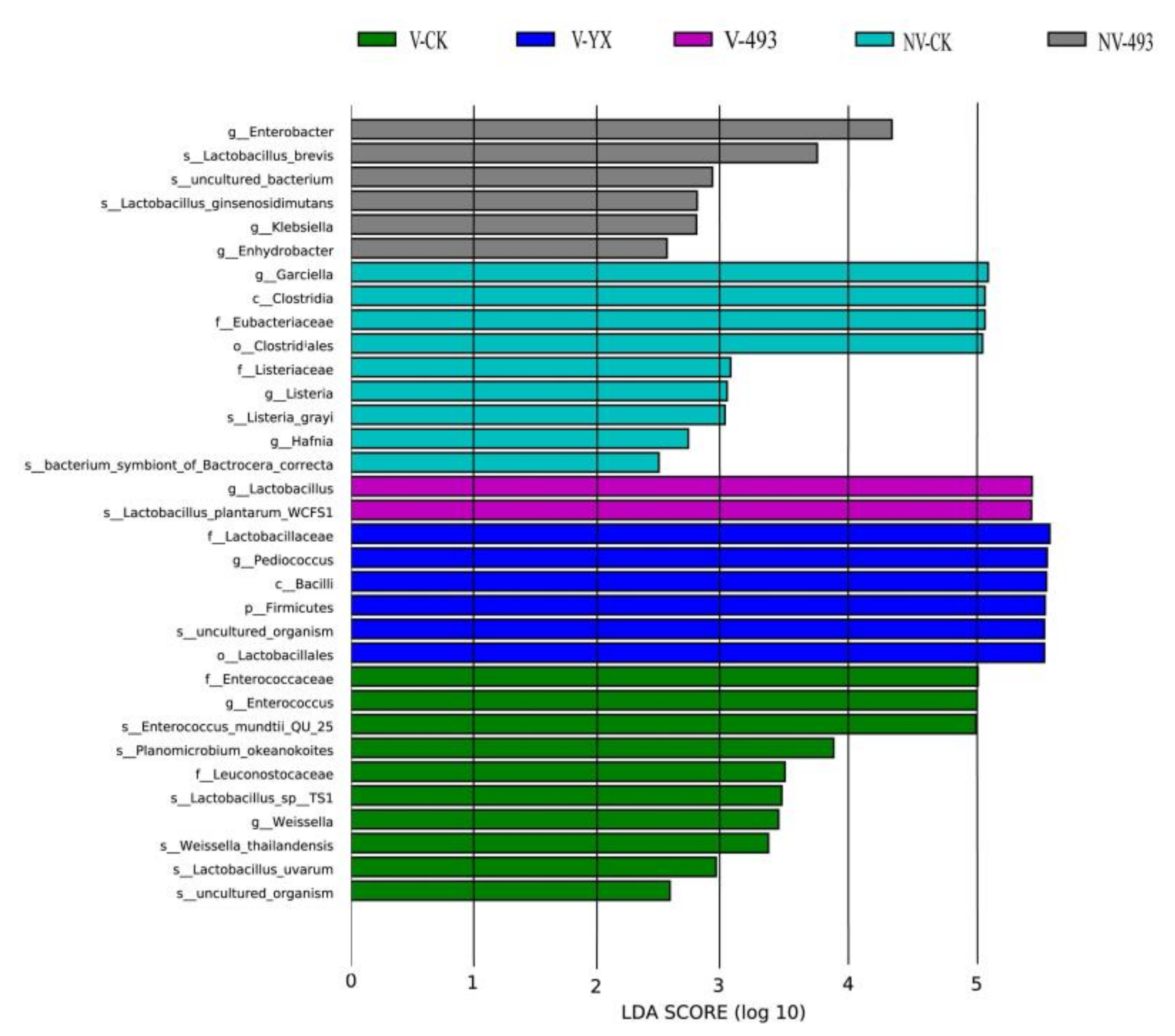
3.3.2. Abundance of Bacterial Community
3.3.3. Correlation Analysis between Bacterial Community and Fermentation Characteristics of Alfalfa Silage at 90 Days
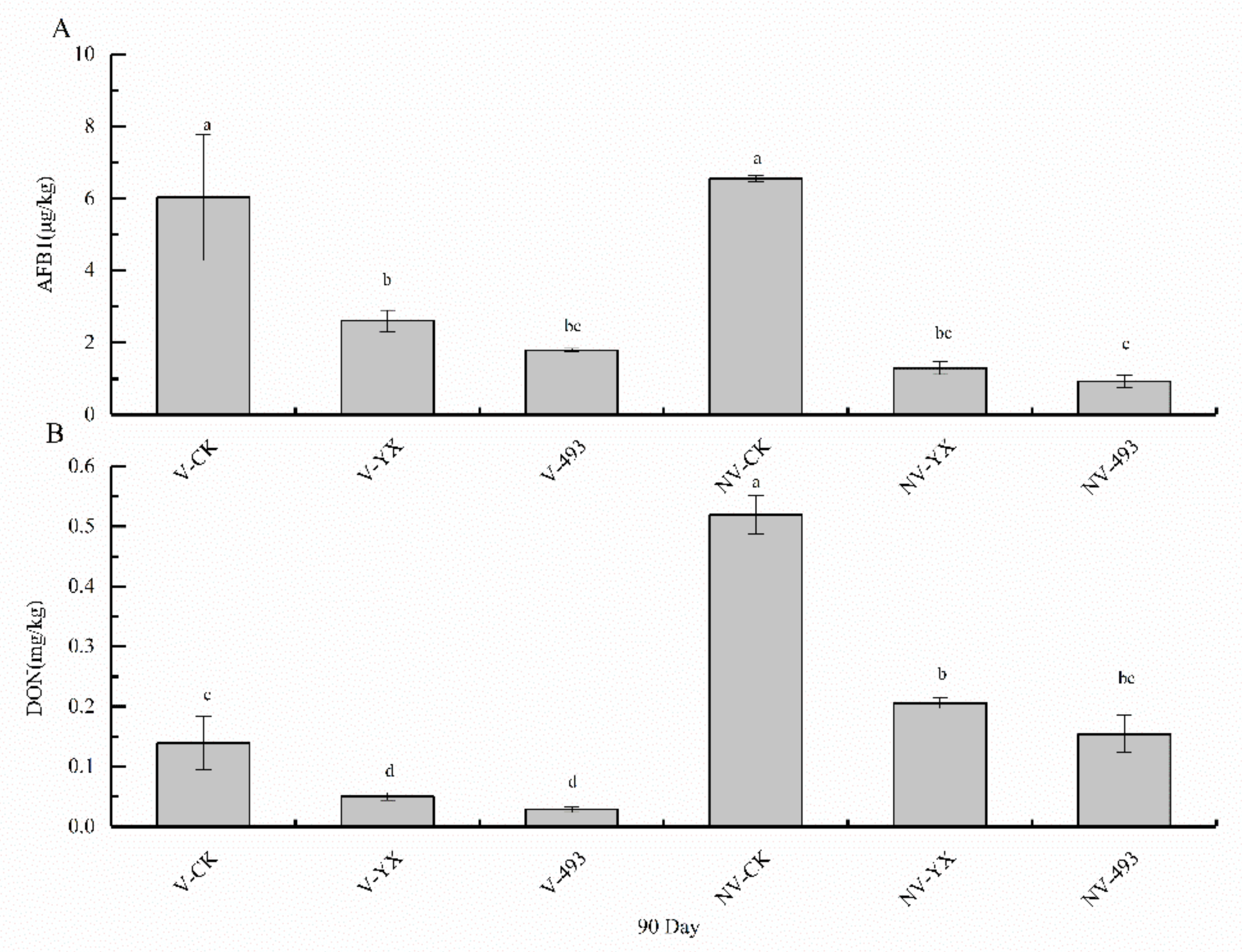
3.4. Effects of Treatments on AFB1 and DON Contents in Alfalfa Silage at 90 Days
4. Discussion
4.1. Characteristics of Pre-Ensiled Alfalfa
4.2. Effect of LAB Inoculants and V Treatment on Fermentation Characteristics of Alfalfa Silage
4.3. Effect of LAB Inoculants and V Treatment on Bacterial Community of Alfalfa Silage at 90 Days
4.4. Correlation Analysis between Bacterial Community and Fermentation Characteristics
4.5. Effect of Two LAB Inoculants and V Treatment on AFB1 and DON Contents of Alfalfa Silage
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, J.G.; Xie, Y.X.; Yu, Z.; Meng, G.; Wu, Z. Effect of Lactobacillus plantarum expressing multifunctional glycoside hydrolases on the characteristics of alfalfa silage. Appl. Microbiol. Biot. 2019, 103, 7983–7995. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhou, X.K.; Gu, Q.C.; Liang, M.Z.; Mu, S.L.; Zhou, B.; Huang, F.; Lin, B.; Zou, C.X. Analysis of the correlation between bacteria and fungi in sugarcane tops silage prior to and after aerobic exposure. Bioresource Technol. 2019, 291, 121835. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.W.; Feng, Y.P.; Liu, T.; Li, J.P.; Wang, Z.Y.; Fu, S.F.; Zheng, Y.; Peng, Z.P. Effects of different simulated seasonal temperatures on the fermentation characteristics and microbial community diversities of the maize straw and cabbage waste co-ensiling system. Sci. Total Environ. 2020, 708, 12. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.L.; Yuan, X.J.; Li, J.F.; Dong, Z.H.; Shao, T. Dynamics of microbial community and fermentation quality during ensiling of sterile and nonsterile alfalfa with or without Lactobacillus plantarum inoculant. Bioresource Technol. 2019, 275, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Muthusamy, K.; Soundharrajan, I.; Srisesharam, S.; Kim, D.; Kuppusamy, P.; Lee, K.D.; Choi, K.C. Probiotic Characteristics and Antifungal Activity of Lactobacillus plantarum and Its Impact on Fermentation of Italian Ryegrass at Low Moisture. Appl. Sci.-Basel. 2020, 10, 1. [Google Scholar] [CrossRef] [Green Version]
- Fabiszewska, A.U.; Zielinska, K.J.; Wrobel, B. Trends in designing microbial silage quality by biotechnological methods using lactic acid bacteria inoculants: A minireview. World J Microb. Biot. 2019, 35, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; He, L.W.; Xing, Y.Q.; Zheng, Y.T.; Zhou, W.; Pian, R.Q.; Yang, F.Y.; Chen, X.Y.; Zhang, Q. Dynamics of Bacterial Community and Fermentation Quality during Ensiling of Wilted and Unwilted Moringa oleifera Leaf Silage with or without Lactic Acid Bacterial Inoculants. mSphere. 2019, 4, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, T.; Zhang, Z.X.; Shimojo, M.; Wang, T.; Masuda, Y. Comparison of fermentation characteristics of Italian ryegrass (Lolium multiflorum Lam.) and guineagrass (Panicum maximum Jacq.) during the early stage of ensiling. Asian. Austral. J. Anim. 2005, 18, 1727–1734. [Google Scholar] [CrossRef]
- Filya, I.; Muck, R.E.; Contreras-Govea, F.E. Inoculant effects on alfalfa silage: Fermentation products and nutritive value. J. Dairy Sci. 2007, 90, 5108–5114. [Google Scholar] [CrossRef] [PubMed]
- Graf, K.; Ulrich, A.; Idler, C.; Klocke, M. Bacterial community dynamics during ensiling of perennial ryegrass at two compaction levels monitored by terminal restriction fragment length polymorphism. J. Appl. Microbiol. 2016, 120, 1479–1491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keshri, J.; Chen, Y.R.; Pinto, R.; Kroupitski, Y.; Weinberg, Z.G.; Saldinger, S.S. Bacterial Dynamics of Wheat Silage. Front. Microbiol. 2019, 10, 1532. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Benno, Y.; Ogawa, M.; Ohmomo, S.; Kumai, S.; Nakase, T. Influence of Lactobacillus spp. from an inoculant and of Weissella and Leuconostoc spp. from forage crops on silage fermentation. Appl. Environ. Microb. 1998, 64, 2982–2987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, D.; Li, B.N.; Zheng, M.L.; Niu, D.Z.; Zuo, S.S.; Xu, C.H. Effects of Pediococcus pentosaceus on fermentation, aerobic stability and microbial communities during ensiling and aerobic spoilage of total mixed ration silage containing alfalfa (Medicago sativa L.). Grassl. Sci. 2019, 66, 215–224. [Google Scholar] [CrossRef]
- Brüning, D.; Gerlach, K.; Weiß, K.; Südekum, K.H. Effect of compaction, delayed sealing and aerobic exposure on maize silage quality and on formation of volatile organic compounds. Grass Forage Sci. 2018, 73, 53–66. [Google Scholar] [CrossRef]
- Gallo, A.; Bernardes, T.F.; Copani, G.; Fortunati, P.; Giuberti, G.; Bruschi, S.; Bryan, K.A.; Nielsen, N.G.; Witt, K.L.; Masoero, F. Effect of inoculation with Lactobacillus buchneri LB1819 and Lactococcus lactis O224 on fermentation and mycotoxin production in maize silage compacted at different densities. Anim. Feed Sci. Tech. 2018, 246, 36–45. [Google Scholar] [CrossRef]
- Ogunade, I.M.; Martinez-Tuppia, C.; Queiroz, O.C.M.; Jiang, Y.; Drouin, P.; Wu, F.; Vyas, D.; Adesogan, A.T. Silage review: Mycotoxins in silage: Occurrence, effects, prevention, and mitigation. J. Dairy Sci. 2018, 101, 4034–4059. [Google Scholar] [CrossRef]
- Wang, M.S.; Xu, S.Y.; Wang, T.Z.; Jia, T.T.; Xu, Z.Z.; Wang, X.; Yu, Z. Effect of inoculants and storage temperature on the microbial, chemical and mycotoxin composition of corn silage. Asian. Austral. J. Anim. 2018, 31, 1903–1912. [Google Scholar] [CrossRef]
- Wambacq, E.; Vanhoutte, I.; Audenaert, K.; De Gelder, L.; Haesaert, G. Occurrence, prevention and remediation of toxigenic fungi and mycotoxins in silage: A review. J. Sci. Food Agr. 2016, 96, 2284–2302. [Google Scholar] [CrossRef]
- Ferrero, F.; Prencipe, S.; Spadaro, D.; Gullino, M.L.; Cavallarin, L.; Piano, S.; Tabacco, E.; Borreani, G. Increase in aflatoxins due to Aspergillus section Flavi multiplication during the aerobic deterioration of corn silage treated with different bacteria inocula. J. Dairy Sci. 2019, 102, 1176–1193. [Google Scholar] [CrossRef] [Green Version]
- Peles, F.; Sipos, P.; Gyori, Z.; Pfliegler, W.P.; Giacometti, F.; Serraino, A.; Pagliuca, G.; Gazzotti, T.; Pocsi, I. Adverse Effects, Transformation and Channeling of Aflatoxins into Food Raw Materials in Livestock. Front. Microbiol. 2019, 10, 2861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arthur Thomas, T. An automated procedure for the determination of soluble carbohydrates in herbage. J. Sci. Food Agric. 1977, 28, 639–642. [Google Scholar] [CrossRef]
- Broderick, G.A.; Kang, J.H. Automated Simultaneous Determination of Ammonia and Total Amino Acids in Ruminal Fluid and in Vitro Media. J. Dairy Sci. 1980, 63, 64–75. [Google Scholar] [CrossRef]
- Yang, F.; Wang, Y.; Zhao, S.; Wang, Y. Lactobacillus plantarum Inoculants Delay Spoilage of High Moisture Alfalfa Silages by Regulating Bacterial Community Composition. Front. Microbiol. 2020, 11, 1989. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.; Yan, Y.H.; Li, X.L.; Li, X.M.; Shuai, Y.; Feng, G.Y.; Ran, Q.F.; Cai, Y.M.; Li, Y.; Zhang, X.Q. Microbial communities and natural fermentation of corn silages prepared with farm bunker-silo in Southwest China. Bioresour. Technol. 2018, 265, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.H.; Wu, J.X.; Dong, Z.H.; Wang, S.R.; Shao, T. Effects of overnight wilting and additives on the fatty acid profile, alpha-tocopherol and beta-carotene of whole plant oat silages. Anim. Feed Sci. Tech. 2020, 260. [Google Scholar] [CrossRef]
- Guo, X.S.; Undersander, D.J.; Combs, D.K. Effect of Lactobacillus inoculants and forage dry matter on the fermentation and aerobic stability of ensiled mixed-crop tall fescue and meadow fescue. J. Dairy Sci. 2013, 96, 1735–1744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, L.W.; Wang, C.; Xing, Y.Q.; Zhou, W.; Pian, R.Q.; Chen, X.Y.; Zhang, Q. Ensiling characteristics, proteolysis and bacterial community of high-moisture corn stalk and stylo silage prepared with Bauhinia variegate flower. Bioresour. Technol. 2020, 296, 122336. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.B.; Zhang, Y.D.; Wang, F.E.; Liu, K.Z.; Huang, G.X.; Zheng, N.; Wang, J.Q. Effects of Sugar Cane Molasses Addition on the Fermentation Quality, Microbial Community, and Tastes of Alfalfa Silage. Animals 2021, 11, 355. [Google Scholar] [CrossRef] [PubMed]
- Ni, K.K.; Wang, X.K.; Lu, Y.; Guo, L.N.; Li, X.M.; Yang, F.Y. Exploring the silage quality of alfalfa ensiled with the residues of astragalus and hawthorn. Bioresource Technol. 2020, 297, 122249. [Google Scholar] [CrossRef]
- Wang, S.; Sun, Y.; Zhao, J.; Dong, Z.; Li, J.; Nazar, M.; Shao, T. Assessment of inoculating various epiphytic microbiota on fermentative profile and microbial community dynamics in sterile Italian ryegrass. J. Appl. Microbiol. 2020, 129, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Kung, L., Jr.; Savage, R.M.; da Silva, E.B.; Polukis, S.A.; Smith, M.L.; Johnson, A.C.B.; Miller, M.A. The effects of air stress during storage and low packing density on the fermentation and aerobic stability of corn silage inoculated with Lactobacillus buchneri 40788. J. Dairy Sci. 2021, 104, 4206–4222. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Li, D.X.; Wang, X.K.; Lin, Y.L.; Zhang, Q.; Chen, X.Y.; Yang, F.Y. Fermentation dynamics and diversity of bacterial community in four typical woody forages. Ann. Microbiol. 2019, 69, 233–240. [Google Scholar] [CrossRef]
- Bai, J.; Ding, Z.; Ke, W.; Xu, D.; Wang, M.; Huang, W.; Zhang, Y.; Liu, F.; Guo, X. Different lactic acid bacteria and their combinations regulated the fermentation process of ensiled alfalfa: Ensiling characteristics, dynamics of bacterial community and their functional shifts. Microb. Biotechnol. 2021, 14, 1171–1182. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.S.; Wang, Y.P.; Yang, F.Y.; Wang, Y.; Zhang, H. Screening a Lactobacillus plantarum strain for good adaption in alfalfa ensiling and demonstrating its improvement of alfalfa silage quality. J. Appl. Microbiol. 2020, 129, 233–242. [Google Scholar] [CrossRef]
- Wang, S.; Dong, Z.; Li, J.; Chen, L.; Shao, T. Pediococcus acidilactici strains as silage inoculants for improving the fermentation quality, nutritive value and in vitro ruminal digestibility in different forages. J. Appl. Microbiol. 2019, 126, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, X.; Zhao, M.; Yu, Z. Lactic acid bacteria strains for enhancing the fermentation quality and aerobic stability of Leymus chinensis silage. Grass Forage Sci. 2016, 71, 472–481. [Google Scholar] [CrossRef]
- Ali, N.; Wang, S.R.; Zhao, J.; Dong, Z.H.; Li, J.F.; Nazar, M.; Shao, T. Microbial diversity and fermentation profile of red clover silage inoculated with reconstituted indigenous and exogenous epiphytic microbiota. Bioresource Technol. 2020, 314, 123606. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhao, S.; Wang, Y.; Fan, X.; Wang, Y.; Feng, C. Assessment of Bacterial Community Composition and Dynamics in Alfalfa Silages with and without Lactobacillus plantarum Inoculation Using Absolute Quantification 16S rRNA Sequencing. Front. Microbiol. 2021, 11, 3646. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, L.W.; Xing, Y.Q.; Zhou, W.; Pian, R.Q.; Yang, F.Y.; Chen, X.Y.; Zhang, Q. Bacterial diversity and fermentation quality of Moringa oleifera leaves silage prepared with lactic acid bacteria inoculants and stored at different temperatures. Bioresour. Technol. 2019, 284, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Ogunade, I.M.; Jiang, Y.; Cervantes, A.A.P.; Kim, D.H.; Oliveira, A.S.; Vyas, D.; Weinberg, Z.G.; Jeong, K.C.; Adesogan, A.T. Bacterial diversity and composition of alfalfa silage as analyzed by Illumina MiSeq sequencing: Effects of Escherichia coli O157:H7 and silage additives. J. Dairy Sci. 2018, 101, 2048–2059. [Google Scholar] [CrossRef]
- Yuan, X.; Li, J.; Dong, Z.; Shao, T. The reconstitution mechanism of napier grass microiota during the ensiling of alfalfa and their contributions to fermentation quality of silage. Bioresour. Technol. 2020, 297, 122391. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.N.; Yao, D.D.; Li, D.X.; Lin, Y.L.; Bureenok, S.; Ni, K.K.; Yang, F.Y. Effects of Lactic Acid Bacteria Isolated from Rumen Fluid and Feces of Dairy Cows on Fermentation Quality, Microbial Community, and in vitro Digestibility of Alfalfa Silage. Front. Microbiol. 2020, 10, 2998. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Ma, D.; Niu, H.; Chang, J.; Yu, J.; Tong, Q.; Li, S. Enzyme additives influence bacterial communities of Medicago sativa silage as determined by Illumina sequencing. AMB Express 2021, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
| Items | Treatments | Ensiling Days (d) | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| 10 | 30 | 60 | 90 | T | D | T × D | |||
| DM (g/kg FM) | V-CK | 40.08 | 40.02 | 39.84 | 40.12 | 0.14 | NS | NS | NS |
| V-YX | 40.38 | 40.63 | 40.30 | 40.60 | |||||
| V-493 | 40.06 | 39.95 | 40.45 | 40.25 | |||||
| NV-CK | 40.34 | 40.09 | 41.12 | 40.44 | |||||
| NV-YX | 39.15 | 39.77 | 40.10 | 40.26 | |||||
| NV-493 | 39.82 | 39.71 | 40.44 | 39.73 | |||||
| WSC (g/kg DM) | V-CK | 1.41 B | 1.11 | 1.09 | 1.10 | 0.10 | <0.01 | <0.01 | <0.05 |
| V-YX | 1.92 Aa | 1.41 b | 1.48 b | 1.22 b | |||||
| V-493 | 1.85 ABa | 1.43 b | 1.31 b | 1.32 b | |||||
| NV-CK | 1.42 B | 1.40 | 1.14 | 1.08 | |||||
| NV-YX | 1.82 ABa | 1.27 b | 1.12 b | 1.17 b | |||||
| NV-493 | 1.72 ABa | 1.48 ab | 1.08 b | 1.27 b | |||||
| Items | Treatments | Ensiling Days (d) | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| 10 | 30 | 60 | 90 | T | D | T × D | |||
| pH | V-CK | 6.63 Aa | 6.56 Aab | 6.43 Ab | 6.40 Ab | 0.05 | <0.01 | <0.01 | <0.01 |
| V-YX | 5.30 Ba | 5.16 Cab | 5.12 Dab | 5.02 Cb | |||||
| V-493 | 5.47 Ba | 5.35 BCab | 5.27 CDb | 5.13 BCb | |||||
| NV-CK | 6.65 Aa | 6.43 Ab | 6.03 Bc | 6.34 Ab | |||||
| NV-YX | 5.48 Ba | 5.33 BCab | 5.41 Ca | 5.18 BCb | |||||
| NV-493 | 5.46 B | 5.39 B | 5.33 CD | 5.28 B | |||||
| LA (g/kg DM) | V-CK | 15.33 Cb | 22.23 Ba | 20.04 Bab | 13.77 Db | 1.05 | <0.01 | <0.01 | <0.01 |
| V-YX | 21.15 Bc | 32.89 Aa | 25.76 ABb | 31.93 Aa | |||||
| V-493 | 27.19 A | 26.99 AB | 28.73 A | 29.79 AB | |||||
| NV-CK | 19.37 BCa | 22.93 Ba | 22.64 Ba | 12.33 Db | |||||
| NV-YX | 21.51 Bb | 28.63 Aa | 24.09 Bb | 26.75 Bab | |||||
| NV-493 | 25.07 ABab | 28.35 Aa | 26.12 ABa | 21.69 Cb | |||||
| AA (g/kg DM) | V-CK | 13.88 b | 18.01 a | 19.48 ABa | 18.41 Ba | 1.06 | <0.01 | <0.05 | <0.05 |
| V-YX | 12.29 c | 16.99 b | 21.82 Aa | 23.67 Aa | |||||
| V-493 | 13.10 c | 17.44 b | 20.61 ABab | 22.54 ABa | |||||
| NV-CK | 12.88 | 15.34 | 16.64 B | 15.73 B | |||||
| NV-YX | 12.77 b | 16.93 a | 21.44 Aa | 21.37 ABa | |||||
| NV-493 | 11.68 b | 17.99 a | 20.07 ABa | 20.93 ABa | |||||
| LA/AA | V-CK | 1.19 Ba | 1.24 Ba | 1.03 ab | 0.76 Bb | 0.14 | <0.05 | <0.05 | <0.05 |
| V-YX | 1.72 ABab | 1.84 Aa | 1.20 b | 1.37 Ab | |||||
| V-493 | 2.01 Aa | 1.55 ABb | 1.28 b | 1.33 Ab | |||||
| NV-CK | 1.50 Ba | 1.51 ABa | 1.36 a | 0.79 Bb | |||||
| NV-YX | 1.52 Bab | 1.55 ABa | 1.14 b | 1.26 Aab | |||||
| NV-493 | 2.15 Aa | 1.58 ABb | 1.23 bc | 1.04 ABc | |||||
| NH3-N (g/kg DM) | V-CK | 4.24 Ac | 6.05 Ab | 6.50 Ab | 7.27 Aa | 0.18 | <0.01 | <0.01 | <0.01 |
| V-YX | 2.56 Bb | 3.04 Cab | 3.15 Dab | 3.69 BCa | |||||
| V-493 | 3.05 Bb | 3.49 Cb | 4.01 Cab | 4.28 Ba | |||||
| NV-CK | 4.17 Ac | 4.68 Bbc | 5.33 Bb | 6.82 Aa | |||||
| NV-YX | 2.74 B | 3.05 C | 3.15 D | 3.36 C | |||||
| NV-493 | 2.60 Bc | 3.37 Cb | 4.01 Cab | 4.42 Ba | |||||
| PA (g/kg DM) | V-CK | ND | ND | ND | 13.11 | − | − | – | – |
| V-YX | ND | ND | ND | ND | |||||
| V-493 | ND | ND | ND | ND | |||||
| NV-CK | ND | ND | ND | 12.44 | |||||
| NV-YX | ND | ND | ND | ND | |||||
| NV-493 | ND | ND | ND | ND | |||||
| BA (g/kg DM) | V-CK | ND | ND | ND | 11.53 | – | – | – | − |
| V-YX | ND | ND | ND | ND | |||||
| V-493 | ND | ND | ND | ND | |||||
| NV-CK | ND | ND | ND | 12.17 | |||||
| NV-YX | ND | ND | ND | ND | |||||
| NV-493 | ND | ND | ND | ND | |||||
| Samples | Observed | Chao1 | ACE | Shannon | Simpson | Coverage |
|---|---|---|---|---|---|---|
| V-CK | 182.00 | 265.92 | 307.92 | 1.73 a | 0.26 d | 0.9994 |
| V-YX | 151.33 | 335.73 | 324.88 | 0.36 d | 0.86 a | 0.9993 |
| V-493 | 204.67 | 371.37 | 373.85 | 0.85 c | 0.58 b | 0.9992 |
| NV-CK | 180.50 | 254.22 | 268.60 | 1.45 b | 0.36 c | 0.9995 |
| NV-YX | 151.67 | 257.28 | 286.22 | 0.43 d | 0.84 a | 0.9994 |
| NV-493 | 208.00 | 366.84 | 369.41 | 1.29 b | 0.42 a | 0.9992 |
| Relative Abundance (%) | Vacuum | Nonvacuum | SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| V-CK | V-YX | V-493 | NV-CK | NV-YX | NV-493 | V | T | V × T | ||
| Pediococcus | 30.06 b | 92.39 a | 21.85 b | 10.67 c | 91.39 a | 27.67 b | 0.03 | NS | <0.01 | NS |
| Lactobacillus | 3.67 cd | 6.04 c | 73.90 a | 1.31 d | 5.25 cd | 60.06 b | 0.12 | <0.01 | <0.01 | <0.01 |
| Garciella | 26.43 b | <0.001 c | < 0.001 c | 51.73 a | <0.001 c | 0.07 c | 0.23 | <0.01 | <0.01 | <0.01 |
| Enterococcus | 29.43 a | 0.33 b | 0.84 b | 28.55 a | 0.52 b | 2.95 b | 0.12 | NS | <0.01 | NS |
| Enterobacter | 0.32 c | 0.51 c | 1.77 b | 1.41 b | 1.73 b | 6.13 a | 0.06 | <0.01 | <0.01 | <0.05 |
| Staphylococcus | 4.69 a | 0.04 b | 0.04 b | 3.43 a | 0.10 b | 0.18 b | 0.09 | NS | <0.01 | NS |
| Corynebacterium | 1.74 | <0.001 | ND | 0.08 | ND | <0.001 | 0.07 | NS | NS | NS |
| pH | LA | AA | LA/AA | WSC | NH3-N | BA | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | r | p | r | p | r | p | |
| Mantel test | ||||||||||||||
| Pediococcus | −0.500 | 0.043 | 0.137 | 0.599 | 0.123 | 0.639 | 0.199 | 0.443 | 0.168 | 0.518 | −0.752 | 0.001 | −0.323 | 0.206 |
| Lactobacillus | −0.539 | 0.028 | 0.392 | 0.120 | 0.630 | 0.008 | 0.012 | 0.966 | 0.741 | 0.001 | −0.238 | 0.357 | −0.553 | 0.021 |
| Garciella | 0.783 | 0.000 | −0.420 | 0.093 | −0.424 | 0.090 | −0.117 | 0.655 | −0.610 | 0.009 | 0.629 | 0.007 | 0.624 | 0.007 |
| Enterococcus | 0.914 | 0.000 | −0.201 | 0.438 | −0.392 | 0.120 | −0.025 | 0.928 | −0.282 | 0.274 | 0.863 | 0.000 | 0.724 | 0.001 |
| Enterobacter | 0.056 | 0.831 | −0.436 | 0.082 | 0.216 | 0.404 | −0.510 | 0.039 | 0.278 | 0.280 | −0.027 | 0.921 | −0.491 | 0.046 |
| Staphylococcus | 0.824 | 0.000 | −0.350 | 0.168 | −0.429 | 0.087 | −0.056 | 0.831 | −0.483 | 0.049 | 0.725 | 0.001 | 0.629 | 0.007 |
| Corynebacterium | 0.620 | 0.008 | −0.072 | 0.783 | −0.234 | 0.366 | 0.041 | 0.875 | −0.496 | 0.043 | 0.746 | 0.001 | 0.661 | 0.004 |
| Spearman’s correlation analysis | ||||||||||||||
| Chao1 | −0.328 | 0.198 | 0.549 | 0.024 | 0.184 | 0.479 | 0.353 | 0.165 | −0.574 | 0.016 | −0.002 | 0.996 | −0.296 | 0.248 |
| Shannon | 0.816 | 0.000 | −0.037 | 0.891 | −0.279 | 0.276 | 0.103 | 0.694 | −0.269 | 0.296 | 0.941 | 0.000 | 0.718 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, X.; Zhao, S.; Yang, F.; Wang, Y.; Wang, Y. Effects of Lactic Acid Bacterial Inoculants on Fermentation Quality, Bacterial Community, and Mycotoxins of Alfalfa Silage under Vacuum or Nonvacuum Treatment. Microorganisms 2021, 9, 2614. https://doi.org/10.3390/microorganisms9122614
Fan X, Zhao S, Yang F, Wang Y, Wang Y. Effects of Lactic Acid Bacterial Inoculants on Fermentation Quality, Bacterial Community, and Mycotoxins of Alfalfa Silage under Vacuum or Nonvacuum Treatment. Microorganisms. 2021; 9(12):2614. https://doi.org/10.3390/microorganisms9122614
Chicago/Turabian StyleFan, Xiaomiao, Shanshan Zhao, Fengyuan Yang, Yuan Wang, and Yanping Wang. 2021. "Effects of Lactic Acid Bacterial Inoculants on Fermentation Quality, Bacterial Community, and Mycotoxins of Alfalfa Silage under Vacuum or Nonvacuum Treatment" Microorganisms 9, no. 12: 2614. https://doi.org/10.3390/microorganisms9122614
APA StyleFan, X., Zhao, S., Yang, F., Wang, Y., & Wang, Y. (2021). Effects of Lactic Acid Bacterial Inoculants on Fermentation Quality, Bacterial Community, and Mycotoxins of Alfalfa Silage under Vacuum or Nonvacuum Treatment. Microorganisms, 9(12), 2614. https://doi.org/10.3390/microorganisms9122614






