Plasmid DNA Production in Proteome-Reduced Escherichia coli
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Plasmid
2.2. Batch Cultures in Shake Flasks
2.3. Fed-Batch Cultures in Shake-Flasks
2.4. Analyses
2.5. Analyses
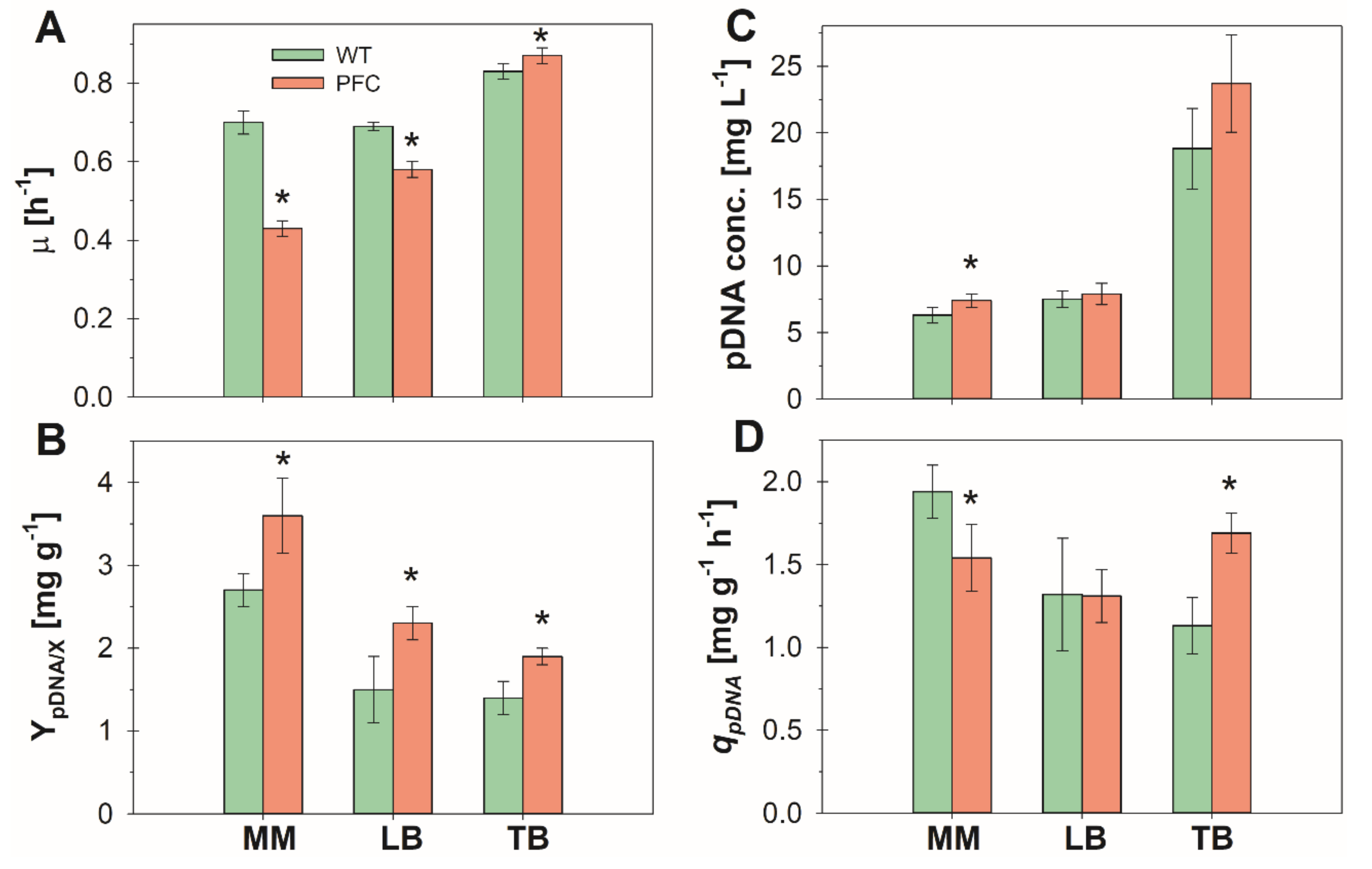
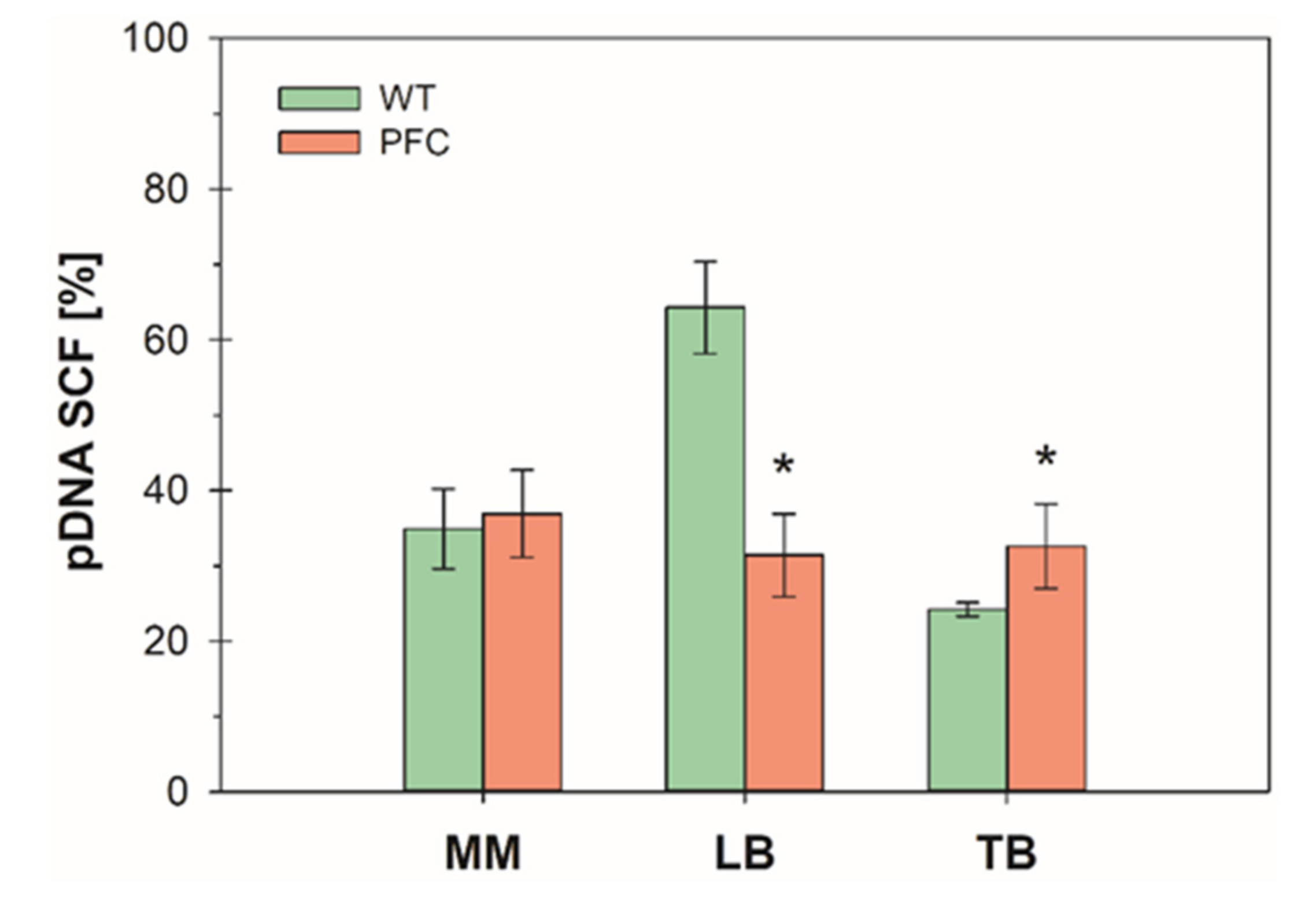
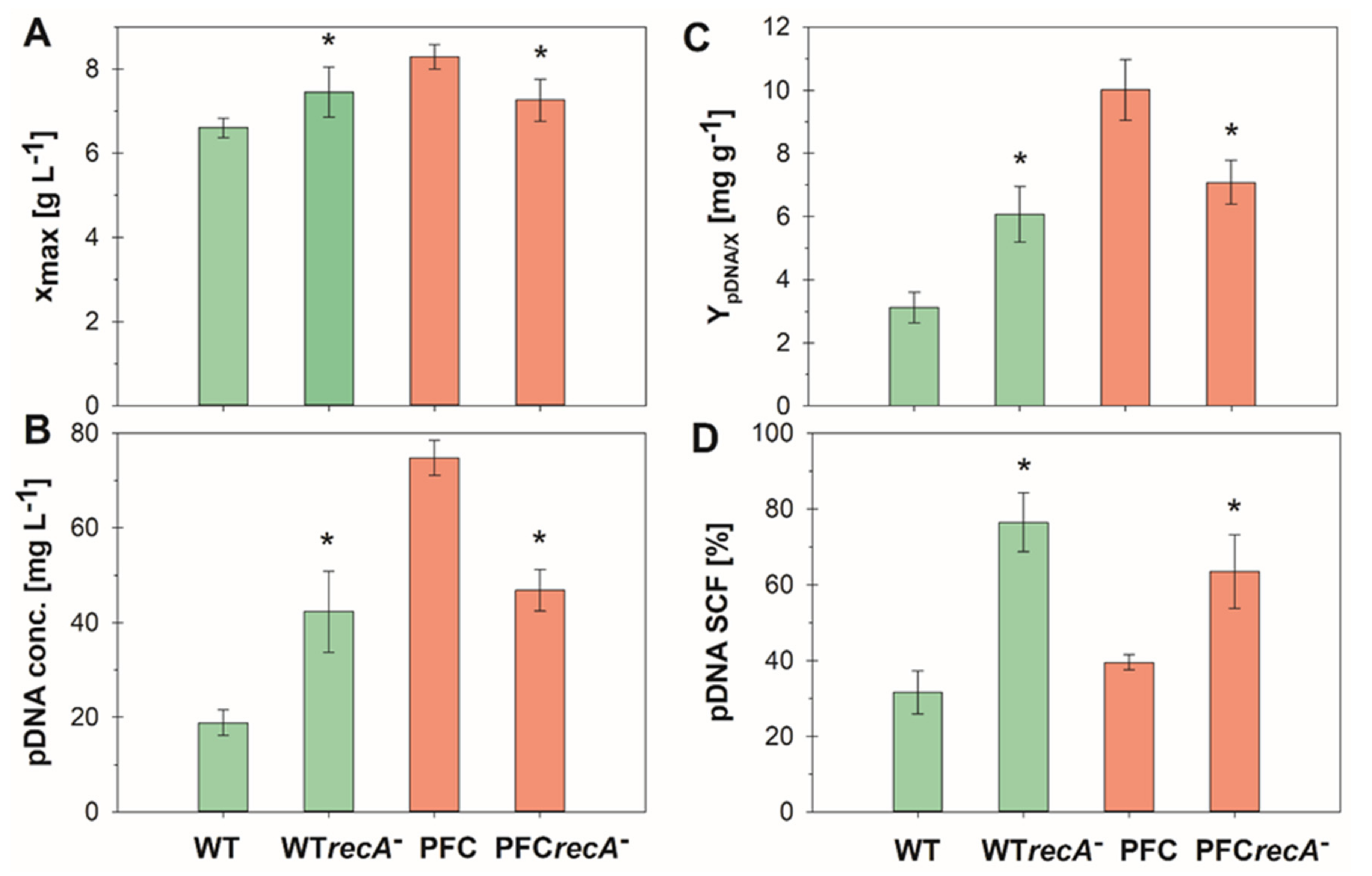
3. Results and Discussion
3.1. Batch Cultures
3.2. Fed-Batch Cultures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wu, G.; Yan, Q.; Jones, J.A.; Tang, Y.J.; Fong, S.S.; Koffas, M.A.G. Metabolic Burden: Cornerstones in Synthetic Biology and Metabolic Engineering Applications. Trends Biotechnol. 2016, 34, 652–664. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, M.; Takors, R. Reduced and Minimal Cell Factories in Bioprocesses: Towards a Streamlined Chassis. In Minimal Cells: Design, Construction, Biotechnological Applications; Lara, A.R., Gosset, G., Eds.; Springer Nature: Basel, Switzerland, 2020; pp. 1–44. [Google Scholar]
- Kurokawa, M.; Ying, B.W. Experimental challenges for reduced genomes: The cell model Escherichia coli. Microorganisms 2020, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Valgepea, K.; Peebo, K.; Adamberg, K.; Vilu, R. Lean-proteome strains–next step in metabolic engineering. Front. Bioeng. Biotechnol. 2015, 3, 11. [Google Scholar] [CrossRef]
- Hidalgo, D.; Utrilla, J. Resource Allocation Principles and Minimal Cell Design. In Minimal Cells: Design, Construction, Biotechnological Applications; Lara, A.R., Gosset, G., Eds.; Springer Nature: Basel, Switzerland, 2020; pp. 211–230. [Google Scholar]
- Lastiri-Pancardo, G.; Mercado-Hernández, J.S.; Kim, J.; Jiménez, J.I.; Utrilla, J. A quantitative method for proteome reallocation using minimal regulatory interventions. Nature Chem. Biol. 2020. [Google Scholar] [CrossRef]
- Ma, C.C.; Wang, Z.L.; Xu, T.; He, Z.Y.; Wei, Y.Q. The approved gene therapy drugs worldwide: From 1998 to 2019. Biotechnol. Adv. 2020, 40, 107502. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. DRAFT Landscape of COVID-19 Candidate Vaccines; World Health Organization: Geneva, Switezerland, 2020. [Google Scholar]
- Akeno, Y.; Ting, B.W.; Tsuru, S.; Yomo, T. A reduced genome decreases the host carrying capacity for foreign DNA. Microb. Cell Fact. 2014, 13, 49. [Google Scholar] [CrossRef] [PubMed]
- Datsenko, K.A.; Wanner, B.L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 2000, 97, 6640–6645. [Google Scholar] [CrossRef] [PubMed]
- Jaén, K.E.; Velazquez, D.; Delvigne, F.; Sigala, J.C.; Lara, A.R. Engineering, E. coli for improved microaerobic pDNA production. Bioproc. Biosyst. Eng. 2019, 42, 1457–1466. [Google Scholar] [CrossRef]
- Soto, R.; Caspeta, L.; Barrón, B.L.; Gosset, G.; Ramírez, O.T.; Lara, A.R. High cell-density cultivation in batch mode for plasmid DNA vaccine production by a metabolically engineered E. coli strain with minimized overflow metabolism. Biochem. Eng. J. 2011, 56, 165–171. [Google Scholar] [CrossRef]
- Schmidt, T.; Friehs, K.; Flaschel, E. Structures of plasmid DNA. In Plasmids for Therapy and Vaccination; Schleef, M., Ed.; Wiley-VCH Verlag GmBH: Weinheim, Germany, 2001; pp. 29–43. [Google Scholar]
- Food and Drugs Administration of the United States of America. Guidance for Industry: Considerations for Plasmid DNA Vaccines for Infectious Disease Indications; Food and Drugs Administration of the United States of America: Washington, DC, USA, 2007.
- Yau, S.Y.; Keshavarz-Moore, E.; Ward, J. Host strain influences on supercoiled plasmid DNA production in Escherichia coli: Implications for efficient design of large-scale processes. Biotechnol. Bioeng. 2008, 101, 529–544. [Google Scholar] [CrossRef]
- Hassan, S.; Keshavarz-Moore, E.; Ward, J. A cell engineering strategy to enhance supercoiled plasmid DNA production for gene therapy. Biotechnol. Bioeng. 2016, 113, 2064–2071. [Google Scholar] [CrossRef] [PubMed]
- Rozkov, A.; Larsson, B.; Gillström, S.; Björnestedt, R.; Schmidt, S.R. Large-scale production of endotoxin-free plasmids for transient expression in mammalian cell culture. Biotechnol. Bioeng. 2008, 99, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.A.; Luke, J.; Langtry, S.; Anderson, S.; Hodgson, C.P.; Carnes, A.E. Generic plasmid DNA production platform incorporating low metabolic burden seed-stock and fed-batch fermentation processes. Biotechnol. Bioeng. 2009, 103, 1129–1143. [Google Scholar] [CrossRef] [PubMed]
- Dorward, A.; O’Kennedy, R.O.; Folarin, O.; Ward, J.M.; Keshavarz-Moore, E. The role of amino acids in the amplification and quality of DNA vectors for industrial applications. Biotechnol. Prog. 2019, 35, e2883. [Google Scholar] [CrossRef] [PubMed]
- Panulla-Perällä, S.J.; Vasala, A.; Wilmanowski, R.; Casteleijn, M.G.; Neubauer, P. Enzyme controlled glucose auto-delivery for high cell density cultivations in microplates and shake flasks. Microb. Cell Fact. 2009, 7, 31. [Google Scholar] [CrossRef]
- Ramírez, E.A.; Velazquez, D.; Lara, A.R. Enhancing plasmid DNA production in shake flask by enzyme-mediated glucose release and engineered E. coli. Biotechnol. Lett. 2016, 38, 651–657. [Google Scholar] [CrossRef]
- Galindo, J.E.; Barrón, B.L.; Lara, A.R. Plasmid DNA production in shake flasks is improved by enzyme-controlled glucose release. Ann. Microbiol. 2016, 66, 1337–1342. [Google Scholar] [CrossRef]
- Phue, J.N.; Lee, S.J.; Trinh, L.; Shiloach, J. Modified Escherichia coli B (BL21), a superior producer of plasmid DNA compared with Escherichia coli K (DH5α). Biotechnol. Bioeng. 2008, 101, 831–836. [Google Scholar] [CrossRef]
- Borja, M.G.; Meza, E.; Gosset, G.; Ramírez, O.T.; Lara, A.R. Engineering, E. coli to increase plasmid DNA production in high cell-density cultivations in batch mode. Microb. Cell Fact. 2012, 11, 132. [Google Scholar] [CrossRef]
- Reckinger, A.R.; Jeong, K.S.; Khodursky, A.B.; Hiasa, H. RecA can stimulate the relaxation activity of topoisomerase I: Molecular basis of topoisomerase-mediated genome-wide transcriptional responses in Escherichia coli. Nucleic. Acids Res. 2007, 35, 79–86. [Google Scholar] [CrossRef][Green Version]
- Carnes, A.E.; Luke, J.M.; Vincent, J.M.; Schukar, A.; Anderson, S.; Hodgson, C.P.; Williams, J.A. Plasmid DNA fermentation strain and process-specific effects on vector yield, quality, and transgene expression. Biotechnol. Bioeng. 2011, 108, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.M.; Oppolzer, P.D.; Sousa, F.; Queiroz, J.A.; Passarinha, L.A. Enhanced biosynthesis of plasmid DNA from Escherichia coli VH33 using Box–Behnken design associated to aromatic amino acids pathway. Biochem. Eng. J. 2015, 98, 117–126. [Google Scholar] [CrossRef]
- Gonçalves, G.A.L.; Bower, D.M.; Prazeres, D.M.F.; Monteiro, G.A.; Prather, K.L.J. De novo creation of MG1655-derived E. coli strains specifically designed for plasmid DNA production. Appl. Microbiol. Biotechnol. 2013, 97, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, G.A.L.; Bower, D.M.; Prazeres, D.M.F.; Monteiro, G.A.; Prather, K.L.J. Rational engineering of Escherichia coli strains for plasmid biopharmaceutical manufacturing. Biotechnol. J. 2012, 7, 251–261. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de la Cruz, M.; Ramírez, E.A.; Sigala, J.-C.; Utrilla, J.; Lara, A.R. Plasmid DNA Production in Proteome-Reduced Escherichia coli. Microorganisms 2020, 8, 1444. https://doi.org/10.3390/microorganisms8091444
de la Cruz M, Ramírez EA, Sigala J-C, Utrilla J, Lara AR. Plasmid DNA Production in Proteome-Reduced Escherichia coli. Microorganisms. 2020; 8(9):1444. https://doi.org/10.3390/microorganisms8091444
Chicago/Turabian Stylede la Cruz, Mitzi, Elisa A. Ramírez, Juan-Carlos Sigala, José Utrilla, and Alvaro R. Lara. 2020. "Plasmid DNA Production in Proteome-Reduced Escherichia coli" Microorganisms 8, no. 9: 1444. https://doi.org/10.3390/microorganisms8091444
APA Stylede la Cruz, M., Ramírez, E. A., Sigala, J.-C., Utrilla, J., & Lara, A. R. (2020). Plasmid DNA Production in Proteome-Reduced Escherichia coli. Microorganisms, 8(9), 1444. https://doi.org/10.3390/microorganisms8091444





